Iodine is an essential micronutrient required for the synthesis of thyroid hormones(Reference Rousset, Dupuy, Miot, De Groot, Chrousos and Dungan1), which are important for optimal growth and brain development(2,Reference Bernal3) . Iodine deficiency can affect thyroid hormone production, as indicated by altered levels of thyroxine, triiodothyronine and thyroid-stimulating hormone (TSH)(Reference Rousset, Dupuy, Miot, De Groot, Chrousos and Dungan1). Newborn TSH concentration is an indicator of fetal iodine nutrition prior to birth as iodine deficiency during fetal life can lead to an increase in TSH concentration in the newborn(Reference Rohner, Zimmermann and Jooste4,Reference Nazeri, Mirmiran and Kabir5) .
Methods of monitoring iodine status of populations include measuring TSH concentration in newborns, median urinary iodine concentration (UIC) in school-aged children and prevalence of goitre in school-aged children(6). According to the joint criteria of the WHO, UNICEF and the International Council for Control of Iodine Deficiency Disorders, populations are classified as iodine deficient if more than 3 % of newborns have TSH > 5 mIU/l in whole blood, or median UIC < 100 μg/l, or goitre prevalence is >5 % in school-aged children(6). When TSH is used to classify population iodine status, the recent WHO recommendation(7) specified to use neonatal blood samples taken 48–96 h after birth because newborn TSH concentrations in the first 24–48 h are higher due to the neonatal TSH surge(Reference Li and Eastman8) and are not recommended to be used to classify population iodine status.
In Australia, newborn TSH concentration is routinely assessed in whole-blood spot samples taken by heel-prick as part of the Newborn Screening Test(9). The test aims to detect disorders such as congenital hypothyroidism and newborn errors of metabolism. Iodine deficiency re-emerged in Australia(Reference Li, Eastman and Waite10–Reference Wilcken and Wiley13) and, as a result, mandatory iodine fortification of bread was introduced in October 2009(14). Pregnant and lactating women in Australia are also recommended to take an iodine supplement of 150 μg/d since January 2010(15). Following iodine fortification, the 2011–2012 National Health Survey and other surveys showed Australians are iodine sufficient as assessed by median UIC(16–Reference Huynh, Condo and Gibson18). Data on iodine status of the population in Australia using newborn TSH as a marker are scarce. A recent study conducted in the Western Australia showed mild iodine deficiency using this marker(Reference Clapin, Lewis and Greed19) but the population was iodine sufficient based on UIC(16). There is a lack of national data assessing population iodine status using newborn TSH as a marker in the post-fortification period, including in South Australia.
Monitoring the iodine status of the population post-fortification using multiple markers is important to evaluate the efficacy and safety of the iodine fortification programme, as disagreements between the TSH marker v. median UIC or goitre prevalence in classifying the iodine status of populations have been reported(Reference Li and Eastman8,Reference Vandevijvere, Coucke and Vanderpas20) . In the present study we used an interrupted time-series design to investigate newborn TSH levels before, during and after implementation of the mandatory iodine fortification programme(Reference Soumerai, Starr and Majumdar21). We also explored the impact of the intervention on the iodine status of the population using different TSH cut-offs to classify iodine status. Based on available evidence, we hypothesised that there will be a reduction in the proportion of newborns with TSH > 5 mIU/l following the fortification programme.
Methods
Design, setting and participants
The present interrupted time-series study used population-level data collected across the entire state of South Australia from 2005 to 2016 (n 254 156). The TSH concentration of all neonates who underwent newborn screening, a routine test in South Australia with a screening rate of 98 %(Reference Metz, Ranieri and Gerace22), was extracted from the newborn screening database in de-identified format. As part of the newborn screening process, newborn whole-blood samples were taken from neonates by heel-prick. Newborn TSH concentration was determined using dissociation-enhanced fluoroimmunoassay (DELFIA). The same method was used for the assessment of TSH over the entire study period. Newborns with congenital hypothyroidism, defined as newborn TSH ≥ 13 mIU/l, were excluded from the study (n 388).
Outcome
The primary outcome of the present study was iodine status of the population as defined by the percentage of newborns with TSH > 5 mIU/l based on the WHO criteria(6). These criteria classify a population as iodine sufficient if the percentage of newborns with TSH > 5 mIU/l is<3 %, and as mildly, moderately and severely iodine deficient if the percentage of newborns with TSH > 5 mIU/l is 3–19·9, 20–39·9 and >40 %, respectively(6). Newborn TSH concentration declines over the first few days after birth(Reference Fisher and Klein23,Reference Lee24) and newborns sampled between 48 and 72 h after birth had a higher median TSH concentration than those sampled 72–96 h after birth(Reference Clapin, Lewis and Greed19). We considered the percentage of newborns with TSH > 6 mIU/l as a secondary outcome(Reference Evans, Barry Nix and Hillier25), since the majority of the samples were collected early at 48–72 h after birth when TSH concentrations are higher.
Exposure
Newborns were classified into three groups based on their exposure to the mandatory iodine fortification during their fetal growth period assuming a full-term pregnancy of 9 months. Since the fortification programme was implemented in October 2009, newborns born before October 2009 formed the pre-fortification group. Newborns born between October 2009 and June 2010 formed the transitional group, as the fetal growth of these children occurred crossing both the pre- and post-fortification periods. Newborns born after June 2010 formed the post-fortification group, as these children would have been conceived after the fortification programme was mandated.
Time-varying confounders
In the present study, time-varying confounders are population characteristics that change over time. The timing of blood sample collection, birth weight and season at birth were adjusted in the analysis as potential time-varying confounders. The timing of blood sample collection for TSH assessment has been shown to affect the newborn TSH concentration(Reference Clapin, Lewis and Greed19,Reference Vanderpump26,Reference Gruneiro-Papendieck, Chiesa and Mendez27) , with early blood samples collected in the first 24 h of birth having higher TSH concentration due to the surge in neonatal TSH(Reference Li and Eastman8). Low-birth-weight infants have been shown to have higher TSH concentration(Reference Clapin, Lewis and Greed19,Reference Rashmi and Sekhri28) and infants born in the spring and winter seasons had a higher TSH concentration due to cold atmospheric temperature(Reference Trumpff, Vandevijvere and Moreno-Reyes29). The percentage of low-birth-weight infants may vary across time(Reference Dobbins, Sullivan and Roberts30). Use of iodine-containing antiseptics during delivery(Reference Li and Eastman8), gestational age at birth(Reference Korada, Pearce and Avis31), sex of the newborn(Reference Clapin, Lewis and Greed19,Reference Korada, Pearce and Avis31) , organochlorine pesticides exposure in the perinatal period(Reference Freire, Lopez-Espinosa and Fernandez32) and mode of delivery(Reference Li and Eastman8,Reference Lao and Panesar33) have been shown to affect TSH concentration, but there was little change in these factors over the study period in South Australia(34).
Statistical analysis
The data were analysed using the statistical software package Stata version 14. Median and interquartile range of TSH concentration and the percentage of newborns with TSH > 5 mIU/l were calculated for the overall sample as well as separately by fortification group, newborn characteristics and year of sampling. Descriptive analyses were repeated for the percentage of newborns with TSH > 6 mlU/l. The χ 2 test was used to compare newborn characteristics (for categorical variables) and the percentage of newborns with TSH > 5 mIU/l between fortification groups. The Kruskal–Wallis test was used to compare the median TSH concentration between sub-populations defined by newborn characteristics.
A segmented regression analysis(Reference Bernal, Cummins and Gasparrini35) was employed to assess the effect of mandatory iodine fortification on the percentage of newborns with TSH > 5 mIU/l. Newborns with heel-prick blood sampled outside the WHO recommendation of 3 to 4 d (48–96 h) after birth were excluded from the segmented regression analysis. Newborns with missing information on time of blood sampling, or birth weight, or improbable values for birth weight (defined as <300 g or >10 kg), were also excluded from the segmented regression analysis. Sex and gestational age were not included in the segmented regression analysis because data on sex were incomplete for post-fortification groups, while data on gestational age were incomplete for pre-fortification groups.
The primary analysis was an unadjusted analysis performed using a Poisson regression model with the number of newborns with TSH > 5 mIU/l per month as the outcome, the fortification groups as the primary predictor variable and the natural logarithm of the total number of newborns per month as an offset. Overdispersion was handled by estimating the scale parameter by Pearson’s χ 2 statistic divided by df. Several exploratory adjusted analyses were also performed. First, birth weight and infants’ age at sampling were adjusted in the model to control for possible time-varying confounding. To allow for changes over time in birth weight, we used the mean or median birth weight or the percentage of infants with low birth weight per month. Both linear and quadratic effects were considered. To examine potential changes over time in the infants’ age at sampling, we tested the median age at sampling or the percentage of infants sampled in each 6 h period after birth for each month. The Akaike information criterion was used to choose the best way to model the effects of birth weight and time of sampling in these exploratory analyses, and the lowest value was taken for a better model fit. Second, season of birth was included to model any seasonal trends in the data. Finally, time in months was included as a predictor to allow for a linear trend over time and an interaction between time in months and fortification group was included to test for possible slope changes between fortification periods.
Global tests were performed to assess whether there was any evidence of a jump in the percentage of newborns with TSH > 5 mIU/l (fortification group effect) or, in exploratory analyses, a slope change (time in months by fortification group interaction effect) between the fortification periods. The effect of fortification group on the percentage of newborns with TSH > 5 mIU/l was reported as the incidence rate ratio (IRR) with a 95 % CI. A P value less than 0·05 was considered statistically significant.
Results
Characteristics of the study population
Of the 254 156 newborns for whom TSH data were available, 388 were diagnosed with congenital hypothyroidism (15 per 10 000 births) and were excluded from the analysis. A heel-prick blood sample was collected between 48 and 96 h after birth from 213 642 (84·0 % ) newborns. Of these, 211 033 (98·8 % ) had data on birth weight and were included in the segmented regression analysis (Fig. 1).
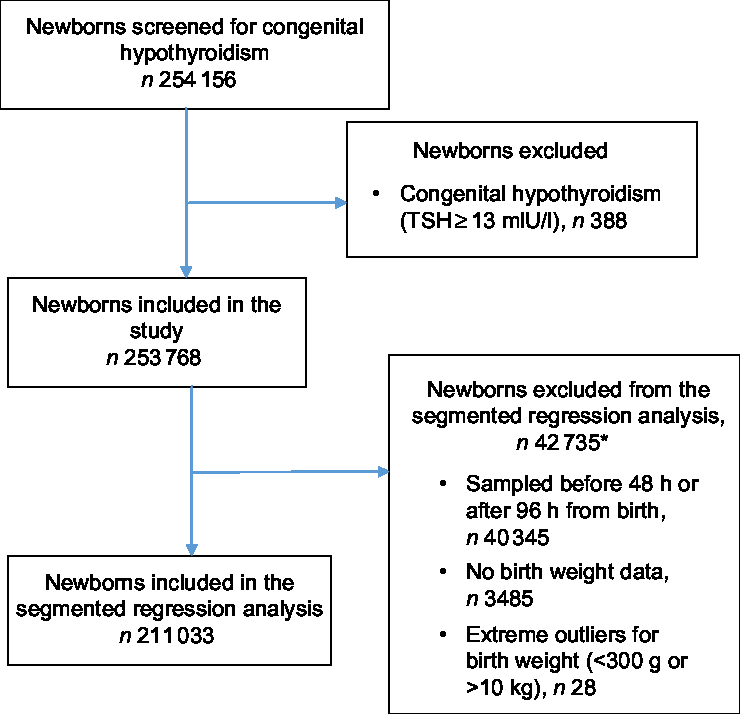
Fig. 1 Flowchart of participants in the study. *Newborns could be excluded under multiple exclusion criteria (TSH, thyroid-stimulating hormone)
Table 1 shows the characteristics of the study population. The majority (91 % ) of newborns were sampled 48–72 h after birth, both in the pre- and post-fortification periods. The percentage of newborns with TSH > 5 mIU/l was higher in males than females, higher in lower-birth-weight than normal-birth-weight infants, higher in preterm than term infants, and higher in winter and spring than summer and autumn (Table 2). The percentage of newborns with TSH > 5 mIU/l and median TSH concentration were highest in newborns blood sampled at 2 d of age and declined afterwards (Fig. 2).
Table 1 Characteristics of the study population, 2005–2016, South Australia (n 211 033)
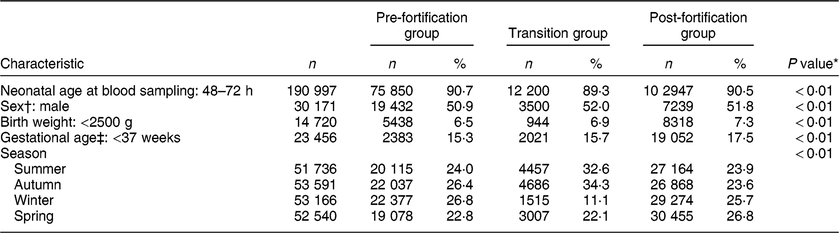
n, number of samples.
* P values were from the χ 2 test for all three groups.
† Sample size for sex: n 58 858.
‡ Sample size for gestational age: n 136 958.
Table 2 Comparison of the percentage of thyroid-stimulating hormone (TSH) concentration >5 mIU/l and median TSH concentration of newborns by potential time-varying confounders, 2005–2016, South Australia (n 211 033)
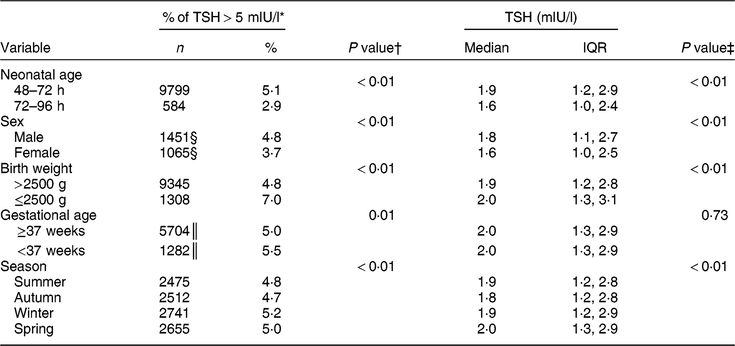
n, number of samples; IQR, interquartile range.
* Denominator was n 211 033 unless otherwise specified.
† P values were from the χ 2 test.
‡ P values were from the Kruskal–Wallis test.
§ Denominator was n 58 858.
║ Denominator was n 136 958.
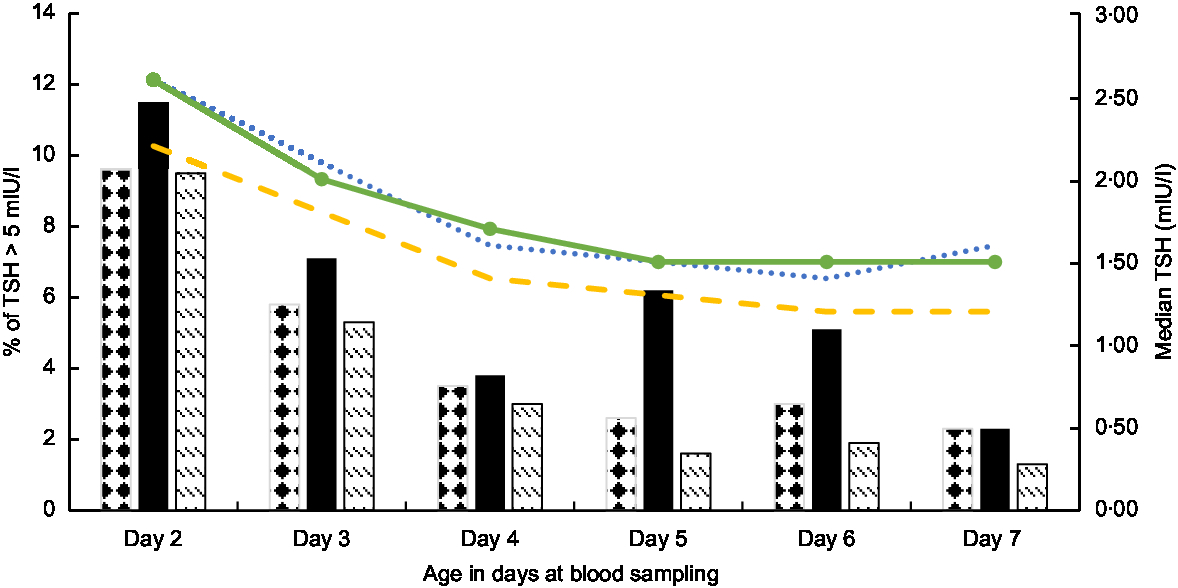
Fig. 2 Median thyroid-stimulating hormone (TSH) concentration and the percentage of newborn TSH concentration >5 mIU/l by neonatal age at blood sampling and fortification period (![]() , median, pre-fortification period;
, median, pre-fortification period; ![]() , median, transition period;
, median, transition period; ![]() , median, post-fortification period;
, median, post-fortification period; ![]() , percentage, pre-fortification period;
, percentage, pre-fortification period; ![]() , percentage, transition period;
, percentage, transition period; ![]() , percentage, post-fortification period), 2005–2016, South Australia (n 239 182)
, percentage, post-fortification period), 2005–2016, South Australia (n 239 182)
Iodine status in the pre-fortification, transition and post-fortification periods
The median (interquartile range) TSH concentration was 2·0 (1·3–2·9) mIU/l in the post-fortification period, 2·0 (1·2–3·1) mIU/l in the transitional period and 1·7 (1·0–2·7) mIU/l in pre-fortification period. Figure 3 presents the percentage of newborns with TSH concentration >5 and >6 mIU/l. The percentage of newborns with TSH > 5 mIU/l was lower in the post-fortification (4·6 % ) period than the pre-fortification (5·1 % ) and transition (6·2 % ) periods (P < 0·01; Fig. 3). Using samples collected at 72–96 h after birth only, the percentage of TSH > 5 mIU/l was 2·9 % (Table 2).
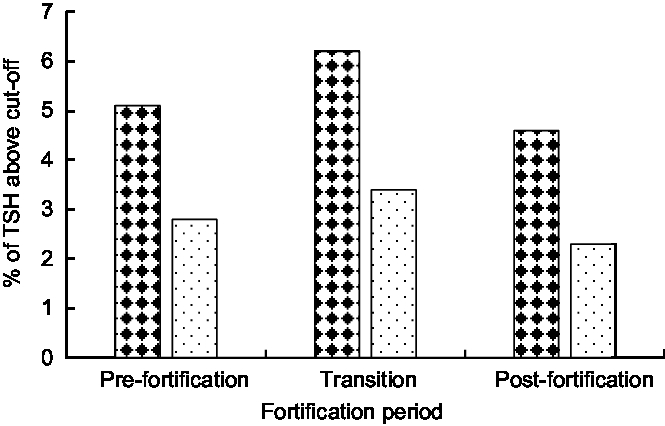
Fig. 3 Percentage of newborns with thyroid-stimulating hormone (TSH) concentration exceeding different cut-offs (![]() , TSH > 5 mIU/l;
, TSH > 5 mIU/l; ![]() , TSH > 6 mIU/l) by fortification period, 2005–2016, South Australia
, TSH > 6 mIU/l) by fortification period, 2005–2016, South Australia
Effect of mandatory iodine fortification of bread on iodine status of the population
In the primary unadjusted segmented regression analysis, newborns in the post-fortification period had a 10 % lower risk of having TSH > 5 mIU/l than newborns in the pre-fortification group (IRR = 0·90; 95 % CI 0·87, 0·94). Newborns in the transitional period had a 22 % higher risk of having TSH > 5 mIU/l compared with newborns in the pre-fortification period (IRR = 1·22; 95 % CI 1·13, 1·31; Table 3).
Table 3 The effect of fortification on the proportion of newborns with thyroid-stimulating hormone (TSH) concentration > 5 mIU/l using segmented regression analysis, 2005–2016, South Australia (n 211 033)
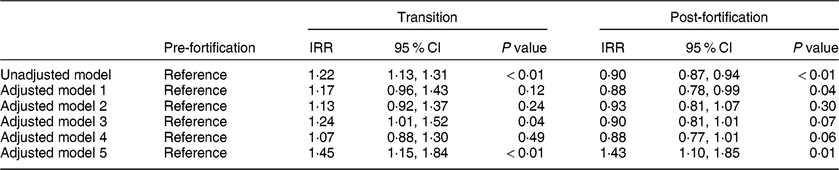
IRR, incidence rate ratio.
Model 1: adjusted for only the linear and quadratic effects for the percentage of infants with low birth weight.
Model 2: adjusted for only the linear and quadratic effects for the percentage of infants’ blood sampled in each 6 h period from birth.
Model 3: adjusted for only season at birth.
Model 4: adjusted for season, linear and quadratic effects for the percentage of infants’ blood sampled in each 6 h period from birth and linear and quadratic effects for the percentage of infants with low birth weight.
Model 5: adjusted model that includes time in months and also adjusted for season, linear and quadratic effects for the percentage of infants’ blood sampled in each 6 h period from birth and linear and quadratic effects for the percentage of infants with low birth weight.
Effect estimates (IRR) were similar in the adjusted exploratory models (models 1–4) except for model 5 when time in months was included in the adjustment, which changed the direction of the effect in the post-fortification period and substantially increased the CI (Table 3). There was no evidence of an interaction between the fortification periods and time in months (P interaction = 0·35) and hence the interaction term (fortification groups and time) was not included in the final adjusted models.
Based on the predicted values of the exploratory segmented regression including fortification group and time in months in the model, there was a jump in the proportion of TSH > 5 mIU/l at the commencement of the iodine fortification, followed by a decreasing trend throughout the post-fortification period which was similar to the trend seen in the pre-fortification period (Fig. 4). Based on the counterfactual model, the proportion of TSH > 5 mIU/l would also decrease in the post-fortification period even if the mandatory iodine fortification of bread was not introduced in October 2009 (Fig. 4).
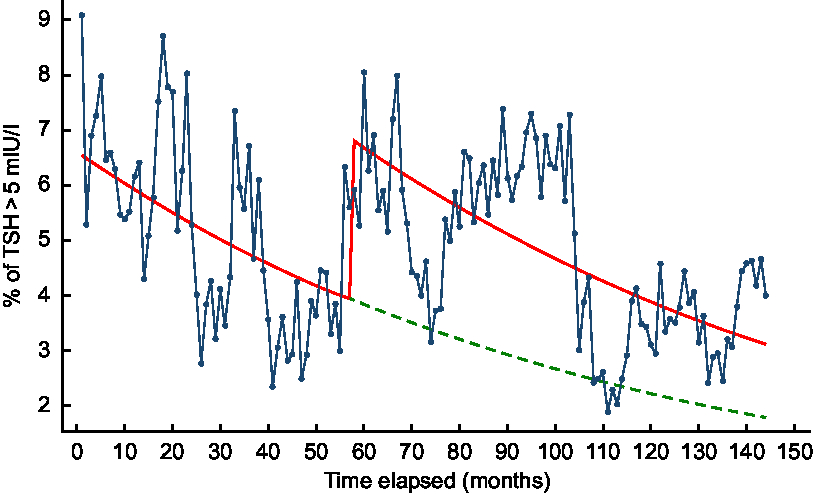
Fig. 4 Percentage of newborn thyroid-stimulating hormone (TSH) concentration >5 mIU/l across time in months based on a segmented regression model, 2005–2016, South Australia: ![]() , observed data;
, observed data; ![]() , predicted values based on an unadjusted regression model including fortification group and time;
, predicted values based on an unadjusted regression model including fortification group and time;![]() , predicted trend without introduction of fortification based on an unadjusted regression model including fortification group and time
, predicted trend without introduction of fortification based on an unadjusted regression model including fortification group and time
Discussion
The present study assessed the effect of the mandatory iodine fortification programme in the South Australian population. Using one of the recommended markers of population iodine status, the percentage of newborns with TSH concentration >5 mIU/l, South Australia would be classified as mild iodine deficiency post-fortification. Our finding of mild iodine deficiency in South Australia using newborn TSH as a marker is in contrast to iodine sufficiency defined by median UIC among school-aged children(16), pregnant women(Reference Condo, Huyhn and Anderson17) and breast-milk iodine concentration(Reference Huynh, Condo and Gibson18) in South Australia post-fortification. The majority of newborn blood samples were collected between 48 and 72 h after birth, and this may increase the percentage of newborns with TSH > 5 mIU/l and contribute to the discrepancy in the classification of iodine status between the two markers of population iodine status of UIC and newborn TSH. The WHO criteria specify using newborn TSH assessed between 48 and 96 h after birth to classify iodine status of populations. Our data showed that TSH concentrations in samples collected 48–72 h after birth were higher than in samples collected 72–96 h after birth. The population became iodine sufficient if samples collected between 72 and 96 h after birth were used. A similar finding has also been reported in Western Australia, where the population was iodine sufficient based on the median UIC(16) but mildly iodine deficient using TSH as a marker in the post-fortification period(Reference Clapin, Lewis and Greed19). In the Western Australian study, the percentage of newborns with TSH > 5 mIU/l was higher in samples collected 48–72 h after birth(Reference Clapin, Lewis and Greed19) similar to our study. Discrepancies in classifying population iodine status using newborn TSH concentration v. median UIC have also been reported elsewhere(Reference Vandevijvere, Coucke and Vanderpas20,Reference Evans, Barry Nix and Hillier25,Reference Vanderpump26) . Evans et al.(Reference Evans, Barry Nix and Hillier25) and Vandevijvere et al.(Reference Vandevijvere, Coucke and Vanderpas20) reported iodine deficiency by median UIC(Reference Vandevijvere, Coucke and Vanderpas20,Reference Vanderpump26) although a population study using newborn TSH showed iodine sufficiency. However, TSH was measured from blood sampled between 72 and 120 h of age, outside the WHO recommendation, which may partly explain the difference. Furthermore, the acute perinatal stress during the early neonatal period may increase the TSH concentration in healthy newborns with a blood sample taken prior to 72 h compared with newborns sampled after 72 h of birth(Reference Lee24,Reference Rashmi and Sekhri28) .
Many countries have shifted to earlier blood sampling for neonatal screening due to earlier discharge from hospitals. This change in practice may affect the newborn TSH values and the percentage with TSH > 5 mIU/l at a population level even though blood samples are collected within the WHO’s recommended time frame of 48–96 h. In countries with earlier discharge, applying the 3 % TSH cut-off suggested for samples collected 48–96 h after birth may not be appropriate since early samples have a higher TSH concentration and this may lead to incorrect classification of iodine status. When a higher cut-off of 6 mIU/l for TSH was used, we found that South Australia was iodine sufficient post-fortification, consistent with the finding from the 2011–2012 National Health Survey which indicated iodine sufficiency in South Australia(16). In contrast, Evans et al.(Reference Evans, Barry Nix and Hillier25) called for WHO to modify its criteria and showed that using cut-offs of 2 and 3 mIU/l for samples collected from 72 h after birth was consistent with UIC. Together, these data suggest that in countries with earlier blood sampling for newborn TSH, increasing either the cut-off TSH value of 5 mIU/l, or the percentage of newborns with TSH > 5 mIU/l used to define iodine deficiency, or applying specific cut-offs for TSH depending on when the newborn blood is sampled, may improve the consistency between TSH and median UIC in the classification of iodine status. In such countries like Australia, the newborn TSH concentration >5 mIU/l at its current cut-off of >3 % may not be an appropriate marker for classifying the iodine status of the population.
Different cut-offs have been applied to newborn TSH for estimating population iodine status in the literature. Most studies use the 5 mIU/l TSH cut-off(Reference Rahman, Savige and Deacon12,Reference Vandevijvere, Coucke and Vanderpas20,Reference Evans, Barry Nix and Hillier25) while others use values rounded to one decimal place (5·0 mIU/l)(Reference Clapin, Lewis and Greed19,Reference Burns, Mayne and O’Herlihy36) . The WHO recommends using TSH > 5 mIU/l(6,7) , however recent sensitive TSH assays make reporting of TSH levels to one decimal place possible. It has been shown that using TSH results rounded to the nearest whole number and applying a 5 mIU/l cut-off decreased the percentage of newborns with elevated TSH concentration compared with using TSH results rounded to one decimal place and applying a 5·0 mIU/l cut-off(Reference Clapin, Lewis and Greed19). This discrepancy is due to rounding TSH results between 5·0 and 5·5 mIU/l to a whole number (5 mIU/l) and applying a 5 mIU/l cut-off. As the recent TSH assay methods are more sensitive and able to measure TSH concentration to more than one decimal place(Reference Clapin, Lewis and Greed19,Reference Spencer, De Groot, Chrousos and Dungan37) , an update to the TSH cut-off value with one decimal place is suggested.
There have been debates over whether the current WHO criterion of newborn TSH concentration >5 mIU/l above 3 % is appropriate to define iodine deficiency(Reference Li and Eastman8,Reference Clapin, Lewis and Greed19,Reference Vandevijvere, Coucke and Vanderpas20,Reference Evans, Barry Nix and Hillier25,Reference Burns, Mayne and O’Herlihy36) . Due to the discrepancy between TSH and UIC to classify population iodine status, Burns et al.(Reference Burns, Mayne and O’Herlihy36) and Vandevijvere et al.(Reference Vandevijvere, Coucke and Vanderpas20) suggested to use the trend of percentage of TSH > 5 mIU/l over time to see the change in the iodine status of populations. Although trends are important to observe changes in iodine status over time, a cut-off on the percentage of newborn TSH > 5 mIU/l is required to classify iodine status in populations using this marker.
The post-fortification group had a 10 % lower risk of newborn TSH > 5 mIU/l compared with the pre-fortification group. This indicates the success of both the mandatory iodine fortification of bread and the recommendation for routine iodine supplementation in pregnancy in Australia to lower the risk of iodine deficiency. This finding is consistent with studies in Ireland(Reference Burns, Mayne and O’Herlihy36), Armenia(Reference Hutchings, Tovmasyan and Hovsepyan38) and Switzerland(Reference Zimmermann, Aeberli and Torresani39) that showed a declining trend in the percentage of newborn TSH > 5 mlU/l after the introduction of iodine fortification or salt iodisation. However, an exploratory analysis controlling for timing of blood sample collection, birth weight, season at birth and the underlying time trend resulted in a reversing of the effect estimate and a loss of precision. These seemingly contradictory results are due in part to the underlying time trend, which suggests that the percentage of newborns with TSH concentration >5 mlU/l was decreasing over time even before fortification was introduced. Although the actual percentage of TSH above 5 mIU/l in the post-fortification period is lower than in the pre-fortification period, it is higher than the predicted percentage when taking into consideration the decreasing time trend. There is no clear explanation for the decreasing time trend in the pre-fortification period. Increased awareness of the re-emergence of iodine deficiency in Australia from the 2006 national iodine nutrition survey may have led to increasing iodised salt use in place of non-iodised salt in the population, which may partly explain the decreasing time trend observed pre-fortification(Reference Li, Chapman and Agho40).
Surprisingly, the percentage of TSH > 5 mIU/l and the median TSH concentration increased during the transitional period despite that TSH was measured using the same method over the entire study period. Our finding of a transient increase in TSH concentration in the transitional period could be explained by the acute Wolf Chaikoff effect(Reference Leung and Braverman41), which refers to a transient inhibition of thyroid function when iodine-deficient populations exposed to a sudden increase in iodine intake, like in Australia from a combination of iodine supplementation in pregnancy and mandatory iodine fortification of bread during the transition period. A recent study reported that the mean iodine intake of pregnant women in South Australia who took iodine supplements at the recommended dose of 150 µg/d or above was 377 µg/d, which was well above the Recommended Dietary Intake for iodine in pregnancy(Reference Condo, Huyhn and Anderson17). In addition, based on the median UIC, 20 % of Australian children aged 2–3 years(42) and 24 % of South Australian infants aged 3 months(Reference Huynh, Condo and Gibson43) had iodine intake above the upper level of safe intake in the post-fortification period. A national bread consumption survey(42) conducted in 2010 also indicated that iodine content varied in the transitional period, with some as high as 270 µg/100 g bread in contrast to the target level of 46 µg/100 g bread(14). Implementation of both the mandatory iodine fortification of bread in October 2009(44) followed by the recommendation for routine iodine supplementation in pregnancy in January 2010(15) in Australia may have resulted in a transient inhibitory effect on thyroid function due to a sudden increase in iodine intake in pregnant women. The fetal thyroid system is more susceptible to both inadequate and excess iodine. Elevated newborn TSH concentration above 17 mIU/l was reported in an iodine-deficient population exposed to increased intake above requirements, suggesting a transient inhibitory effect on the synthesis of thyroid hormones(Reference Nishiyama, Mikeda and Okada45). A transient inhibitory effect from a sudden increase in iodine intake during the implementation of the fortification and supplementation programmes may contribute to a sharp increase in the percentage of TSH above 5 mIU/l in the transitional period(Reference Leung and Braverman41). In the post-fortification period, the population (including women of childbearing age) became iodine sufficient(16,Reference Condo, Huyhn and Anderson17) . The same level of iodine intake in the post-fortification period is unlikely to cause the acute Wolf Chaikoff effect as women entering pregnancy were no longer iodine deficient.
Our study reported seasonal variation in the percentage of newborns with TSH concentration >5 mIU/l in the study period and this was slightly higher in winter and spring compared with summer and autumn. A similar seasonal pattern was observed in Belgium(Reference Trumpff, Vandevijvere and Moreno-Reyes29). Seasonality in TSH concentration may due to variation in dietary iodine intake (mainly fish and iodine-fortified foods)(Reference Trumpff, Vandevijvere and Moreno-Reyes29) but there are no data on the seasonality of bread consumption in Australia. Cold weather conditions can increase thyroid hormone secretion and this may contribute to the higher TSH concentrations observed in winter and spring(Reference Ruppert, Sulyok and Varga46,Reference Fregly47) .
The current study has a number of limitations. First, iodine supplementation for pregnant women was recommended by National Health and Medical Research Council in January 2010, shortly after the implantation of the fortification programme. Therefore, it was not possible to examine the effect of the fortification separately from the supplementation programme. Second, gestational age was not controlled in the final model due to incomplete information but we controlled for birth weight as a proxy, which is highly correlated with gestational age(Reference Dobbins, Sullivan and Roberts30). Third, we did not adjust for the caesarean section rate because these data were not collected in the neonatal screening programme. However, the caesarean section rate was steady over the study period and is unlikely to affect the percentage of TSH > 5 mIU/l over the study period(34). Fourth, due to a move towards earlier discharge of mothers and newborns from hospital, a majority of newborns were sampled at 48 to 72 h after birth for newborn screening. As a result, there is a difference in the mean timing of blood sampling between the three fortification periods which may increase the percentage of newborns with TSH > 5 mIU/l in the post-fortification period. Although we attempted to control for this variation in the timing of blood sample collection in the analysis by considering the percentage of infants sampled in each 6 h period after birth, there may be a residual effect.
Conclusions
Using newborn TSH as a marker, South Australia would be classified as mild iodine deficiency post-fortification in contrast to iodine sufficiency based on the median UIC, the most widely used population marker of iodine status. Our study together with the literature suggests that newborn TSH concentration >5 mIU/l at its current cut-off of >3 % may not be a reliable marker for defining the iodine status of populations. There has been a change in clinical practice worldwide towards early discharge from hospital and newborn bloods are collected early for the newborn screening. Re-evaluation of the current WHO criteria on TSH as a marker of population iodine status is warranted in this context.
Acknowledgements
Acknowledgements: The authors are grateful to the South Australia newborn screening centre for providing the newborn screening data for infants born between 2005 and 2016. Financial support: M.M.W. was supported by The University of Adelaide Research Training Program scholarship. L.N.Y. was supported by an Australian National Health and Medical Research Council Early Career Fellowship (ID 1052388). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: S.J.Z. was responsible for the conception of the study and the overall conduct of the study; M.M.W., L.N.Y. and S.J.Z. were responsible for the design of the study; M.M.W. and L.N.Y. analysed and interpreted the data; L.G.S. and E.R. contributed to the design of the study and interpretation of the results; M.M.W. drafted the manuscript; all authors critically reviewed the manuscript. All authors approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Human Research Ethics Committee (HREC) of the University of Adelaide (reference number 22274).









