Russian-olive (Elaeagnus angustifolia L.) was common as an ornamental plant in the southwestern United States early in the 20th century and was introduced in the 1930s in the U.S. Great Plains for soil conservation (Stannard et al. Reference Stannard, Ogle, Holzworth, Scianna and Sunleaf2002). Russian-olive produces thousands of oblong fruits per tree late in the fall (Katz and Shafroth Reference Katz and Shafroth2003). Fruits may be vertebrate or water dispersed, and seeds are long-lived (Scianna et al. Reference Scianna, Kilian and Muscha2012). Trees begin to produce fruit as early as 5 yr of age and do so reliably around 10 yr of age (Lesica and Miles Reference Lesica and Miles1999). Population maintenance includes stump and root sprouting after fire, mechanical injury, or other disturbance (Stannard et al. Reference Stannard, Ogle, Holzworth, Scianna and Sunleaf2002). Russian-olive is invasive in riparian areas throughout the western United States (Nagler et al. Reference Nagler, Glenn, Jarnevich and Shafroth2011) and has the potential for cascading effects, including changing beaver population dynamics, restructuring watershed food webs, and altering nitrogen cycling in both local terrestrial and aquatic habitats (Lesica and Miles Reference Lesica and Miles1999; Mineau et al. Reference Mineau, Baxter and Marcarelli2011; Pearce and Smith Reference Pearce and Smith2001).
Box 1 Management Implications
Russian-olive (Elaeagnus angustifolia L.) is invasive in riparian areas throughout the western United States. Trees can grow close together, creating a dense canopy that will shade out other trees and understory plants and eventually replace the native components of riparian ecosystems. We tested the effectiveness of a revegetation planting to increase native plant diversity and cover and reduce secondary invasion.
Cutting the Russian-olive trees using a tree shear to ground level, immediately treating the stumps with herbicide, and removing the trees and stacking into burn piles was very effective (96% kill rate). The limited number of stumps that resprouted did so for 2 yr postremoval, so yearly spraying of resprouts was necessary for complete kill. Russian-olive reinvasion via seedling emergence was substantial; therefore, reducing seedbanks may be critical for long-term control. Russian-olive trees do not produce seeds until they are 5 to 10 yr of age, so young Russian-olives do not need to be removed every year, but we recommend inspecting removal sites every other year to control reinvasion. Active revegetation successfully increased native plant diversity, but plantings may need to be in place longer than 5 yr before competitive exclusion of invasive species is apparent.
Riparian corridors contribute significantly to regional native biodiversity (Naiman et al. Reference Naiman, Decamps and Pollock1993) and provide many other ecosystem services such as groundwater recharge, regulation of streamflows, irrigation for agricultural uses, and streambank stabilization (Richardson et al. Reference Richardson, Holmes, Esler, Galatowitsch, Stromberg, Kirkman, Pysek and Hobbs2007). The diversity and abundance of alien plants have increased in riparian zones throughout the world (Richardson et al. Reference Richardson, Holmes, Esler, Galatowitsch, Stromberg, Kirkman, Pysek and Hobbs2007). Riparian populations of Russian-olive reduce recreational and agricultural use in addition to threatening native populations of cottonwood (Populus spp.) and willow (Salix spp.) trees (Lesica and Miles Reference Lesica and Miles2001).
Russian-olive tree removal can create a high level of disturbance, reducing existing plant biomass, removing canopy cover, and providing opportunities for other invasive species to colonize (i.e., secondary invasion) as well as creating a new set point for succession to begin (Blanchard and Holmes Reference Blanchard and Holmes2008; Corenblit et al. Reference Corenblit, Tabacchi, Steiger and Gurnell2007; Gabler and Siemann Reference Gabler and Siemann2012; Srivastava and Vellend Reference Srivastava and Vellend2005). After removal of invasive species, secondary invasions often follow (Blanchard and Holmes Reference Blanchard and Holmes2008; Denslow and D’Antonio Reference Denslow and D’Antonio2005; Galatowitsch and Richardson Reference Galatowitsch and Richardson2005; Rinella et al. Reference Rinella, Maxwell, Fay, Weaver and Sheley2009), and natural successional patterns may change (Blanchard and Holmes Reference Blanchard and Holmes2008; DeMeester and Richter Reference DeMeester and Richter2010). Legacies from invasive plants may inhibit native plant germination and growth (e.g., Corbin and D’Antonio Reference Corbin and D’Antonio2004; Perkins et al. Reference Perkins, Hatfield and Espeland2016; Zavaleta et al. Reference Zavaleta, Hobbs and Mooney2001), which may make postremoval tactics such as revegetation critical for increasing the likelihood of native plant colonization and establishment but may also make revegetation more likely to fail.
Active restoration such as revegetation can increase species diversity (Srivastava and Vellend Reference Srivastava and Vellend2005), preempt niche space, prevent establishment of invasive plant species (Blanchard and Holmes Reference Blanchard and Holmes2008), and assist in recolonization by native plants (Ehrenfeld Reference Ehrenfeld2000). However, ruderal species, nonnative species, and/or invasive species are often a problem in revegetation projects (Mulhouse and Galatowitsch Reference Mulhouse and Galatowitsch2003; Zedler and Callaway Reference Zedler and Callaway1999; but see Rinella et al. Reference Rinella, Mangold, Espeland, Sheley and Jacobs2012). Other research on tree invasions has shown that understory species that survive invasions are a drastically reduced subset of understory species in uninvaded areas (de Abreu and Durigan Reference de Abreu and Durigan2011; Galatowitsch and Richardson Reference Galatowitsch and Richardson2005; Tererai et al. Reference Tererai, Gaertner, Jacobs and Richardson2015; Zavaleta et al. Reference Zavaleta, Hobbs and Mooney2001); therefore, revegetation may be required to return the full complement of native understory species to a site after tree removal. Revegetating a site with active restoration can be costly (Kimball et al. Reference Kimball, Kulow, Sorenson, Balazs, Fang, Davis, O’Connell and Huxman2015) with variable success rates. Allowing a site to revegetate passively via colonization from neighboring plant populations or implementing non-revegetation restoration practices (such as grazing removal) can be less costly but may not be as effective (Gornish et al. Reference Gornish, Lennox, Lewis, Tate and Jackson2017; Ruwanza et al. Reference Ruwanza, Gaertner and Richardson2013). Because the establishment of a native plant community passively brings both aboveground and belowground fauna to the site (Hobbs and Cramer Reference Hobbs and Cramer2008), revegetation may speed the return of ecosystem functions other than plant species diversity (Palmer et al. Reference Palmer, Ambrose and Poff1997).
In previous research (Espeland et al. Reference Espeland, Petersen, Muscha, Scianna and Kilian2014a), we found that after Russian-olive removal, revegetation increased the diversity of desirable native species compared with unplanted controls after 2 yr. Because of the disturbance involved in revegetation (as in Robichaud et al. Reference Robichaud, Beyers and Neary2000), the slow-growing, late seral species often planted in revegetation projects (Suding Reference Suding2011) are unlikely to exclude ruderal weeds (e.g., Espeland and Perkins Reference Espeland and Perkins2017) and fast-growing invasive species (Galatowitsch and Richardson Reference Galatowitsch and Richardson2005). Very early in the restoration process, plantings may have low vegetative cover. At this stage, reinvasion of Russian-olive and secondary invasions of other species may be limited by propagule pressure, not competitive exclusion. After establishment, the growth of native species may reach a stage at which native species are able to competitively exclude secondary invasions and reinvasion of Russian-olive (e.g., Levine et al. Reference Levine, Adler and Yelenik2004). The prior publication on this research (Espeland et al. Reference Espeland, Petersen, Muscha, Scianna and Kilian2014a) focused on the establishment stage of revegetation; 3 yr hence we expect competitive exclusion to begin to drive plant community succession (Richardson et al. Reference Richardson, Holmes, Esler, Galatowitsch, Stromberg, Kirkman, Pysek and Hobbs2007) toward native species dominance.
Preventing Russian-olive reinvasion after removal means minimizing stump resprouting (Stannard et al. Reference Stannard, Ogle, Holzworth, Scianna and Sunleaf2002) and establishment from seed. We expect stump resprout rates to be primarily determined by the effectiveness of the control technique, not by revegetation success, because resprouting is not likely to be limited by competition. Reinvasion of Russian-olive removal sites via seed germination would depend on propagule pressure (i.e., seedbanks and dispersal, as in Blanchard and Holmes Reference Blanchard and Holmes2008) until competitive exclusion comes into play.
In this publication, we report on Russian-olive stand regeneration 5 yr postremoval, and we hypothesize that active revegetation after removal is necessary to reduce secondary invasion and to increase native plant diversity and cover. Our hypotheses were: (1) revegetation increasingly excludes invasive species as the planting matures, compared with non-revegetated controls; (2) revegetation increases native understory cover and diversity compared with controls; (3) transplanted tree and shrub species vary in survivorship, with some species unable to establish; and (4) resprouting dominates Russian-olive stand regeneration after removal, and the contribution of seeds to reinvasion decreases as seedbanks become depleted. Hypotheses 3 and 4 are evaluated descriptively, whereas experimental controls are available to assess hypotheses 1 and 2 statistically.
Materials and Methods
Study Location
This study was conducted within the mixed-grass prairie of the Northern Great Plains at the 22,500-ha USDA–Agricultural Research Service (USDA-ARS) Fort Keogh Livestock and Range Research Laboratory located near Miles City, MT 46°24.431 N, 105°52.618 W. Native vegetation includes grama–needlegrass–wheatgrass (Bouteloua–Stipa–Agropyron) mix (Barker and Whitman Reference Barker and Whitman1988) with shrubs including silver sagebrush (Artemisia cana Pursh ssp. cana), big sagebrush (Artemisia tridentata Nutt.), winterfat [Krascheninnikovia lanata (Pursh) A. Meeuse & Smit, formerly Ceratoides lanata (Pursh) J. T. Howell], and small trees such as common juniper (Juniperus communis L.). Average annual precipitation is 316 mm, of which 85% is received April to October.
Experimental Design
Four replicate 0.5-ha blocks were established adjacent to the Yellowstone River on a Glendive fine sandy loam soil. Blocks were located in closed-canopy Russian-olive forest, equidistant from the edge of the river. Russian-olive trees were completely removed from these blocks in April and May of 2011 using a John Deere 326D skid steer with tree shear and herbicide-spray attachments (Grace Manufacturing, 11200 Turkey Ridge Drive, Plato, MO 65552). Triclopyr (Element® 4, Dow Agrosciences, 9330 Zionsville Road, Indianapolis, IN 46268) was applied at 479 g ai L−1 with an oil carrier (Blue Basal Bark oil, Dow Agrosciences) immediately following a cut-stump treatment. This was the equivalent of a 25% v/v treatment on a product basis. The cost per hectare of removing these 2,500 trees (excluding equipment costs) was 17.7 person-hours, 39.5 L of fuel, and US$427 in herbicide costs (7.9 L of triclopyr ha−1). Resprouts and new Russian-olive seedlings were spot sprayed with a backpack using a mixture consisting of 2.59 g ai L−1 Element® 4, 1.29 g ai L−1 Milestone® (aminopyralid, Dow Agrosciences, Canada, Suite 2100, 450 1st Street SW, Calgary, AB T2P 1M4, Canada), and 2.6 g surfactant mixed in water in fall of 2011 and 2012. Spot-treatment spray application was also conducted in summer of 2013 to 2016 and included saltcedar (Tamarix ramosissima Ledeb.). Some Russian-olive seedlings were hand pulled in 2014 and 2015; in other years, herbicide use ranged from 1.2 to 8.4 L of the spot-spray treatment, mixed as mentioned above, per hectare. We recorded the number of treated saplings per block.
Each block was divided equally into five revegetation treatments. For logistic reasons, non-revegetated controls were always in the center and other treatments were randomized within blocks (Figure 1). We seeded all revegetation treatments with a herbaceous mix, and trees and shrubs were transplanted in a factorial design. Revegetation occurred in April of 2012, because a 50-yr flood in May 2011 placed most plots underwater until the beginning of July. Local sources supplied 1-yr-old conservation-grade woody stock for the tree and shrub plantings. All plots except controls were sprayed with glyphosate (Roundup PowerMax®, Monsanto, 800 N Lindbergh Boulevard, St Louis, MO 63167) in the fall of 2011 and before revegetation in spring of 2012. Glyphosate use was 0.54 kg ai ha−1 and spraying took 1.6 person-hours ha−1. Herbaceous seed was applied to all plots (described below). One treatment (“H”) received only this seeding; “T” plots received approximately 38 trees plot−1; “S” plots received approximately 150 shrubs; and “TS” plots received 75 shrubs and 20 trees, with equal numbers of each species planted in each experimental unit. Each tree and shrub transplant received approximately 3.75 L of water upon planting and none thereafter. Weed barrier fabric (0.91 m by 0.91 m) surrounded half the transplanted individuals. Transplanted woody species were narrowleaf cottonwood (Populus angustifolia James), plains cottonwood [Populus deltoides W. Bartram ex Marshall ssp. monilifera (Aiton) Eckenwalder], boxelder (Acer negundo L.), green ash (Fraxinus pennsylvanica Marshall), golden currant (Ribes aureum Pursh), common chokecherry (Prunus virginiana L.), silver buffaloberry [Shepherdia argentea (Pursh) Nutt.], and Woods’ rose (Rosa woodsii Lindl.). Excluding weed fabric, transplanting costs were 10 person-hours and US$111 in materials per hectare. The tree and shrub treatments were established to examine the potential for facilitation among plant life history groups. Since trees and shrubs were still too small to interact with each other in 2016, we use T, S, and TS plots only to report on survival of transplanted species in this paper. We did not use these plots for the analysis of understory vegetation because of the potential for hidden treatments (as in Huston Reference Huston1997).
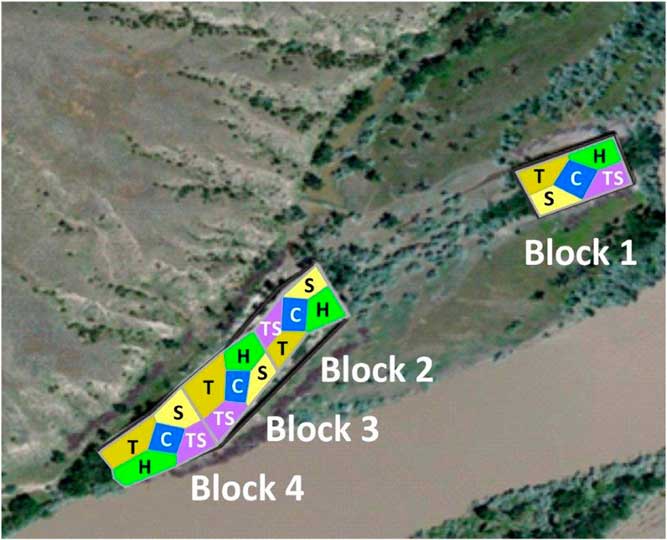
Figure 1 Aerial view of removal plots along the Yellowstone River near Miles City, MT, including block and treatment placement. Treatments were: C, control plots that did not receive any active restoration; H, herbaceous seeds were broadcast seeded in plots; S, herbaceous seeding with shrub transplants; T, herbaceous seeding with tree transplants; and TS, herbaceous seeding with shrub and tree transplants.
Herbaceous species seeded were: slender wheatgrass [Elymus trachycaulus (Link) Gould ex Shinners], western wheatgrass [Pascopyrum smithii (Rydb.) Á. Löve], Prairie cordgrass (Spartina pectinata Link), switchgrass (Panicum virgatum L.), common yarrow (Achillea millefolium L.), upright prairie coneflower [Ratibida columnifera (Nutt.) Wood & Standl.], American vetch (Vicia americana Muhl. ex Willd.), Canada milkvetch (Astragalus canadensis L.), white prairie clover (Dalea candida Michx. ex Willd.), purple prairie clover (Dalea purpurea Vent.), Maximilian sunflower (Helianthus maximiliani Schrad.), blue flax (Linum perenne L.), Rocky Mountain beeplant (Cleome serrulata Pursh.), and Rocky Mountain penstemon (Penstemon strictus Benth.). Herbaceous seed was broadcast, and harrows and hand rakes ensured seed–soil contact. The total application of all seed was 1.2 kg live seed ha−1 (see Supplementary Material for seed mix fractions) at a cost of 0.7 person-hours and US$105 in materials per hectare. All plots were fenced to USDA–Natural Resource Conservation Service (USDA-NRCS) wildlife fence (NRCS 2006, 2008) specifications to protect the woody plants from wildlife and cattle browsing. Riparian tree plantings are unlikely to survive without protection from herbivory (Sweeney and Czapka Reference Sweeney and Czapka2004).
Data Collection
In all treatment plots and non-revegetated controls, understory plant cover by functional group (native perennial grass, invasive perennial grass, nonnative forb, forb, invasive annual grass, seeded species) was collected using the line-point intercept method (Jonasson Reference Jonasson1988) along two 25-m transects per experimental unit, with 50 evenly spaced data-collection points on each transect. We also recorded the herbaceous species present throughout each growing season in the entire plot. We tracked secondary invasion by recording dynamics of invasive species: black henbane (Hyoscyamus niger L.), smooth brome (Bromus inermis Leyss.), clasping pepperweed (Lepidium perfoliatum L.), halogeton [Halogeton glomeratus (Stephen ex Bieb.) C. A. Mey.], houndstongue (Cynoglossum officinale L.), nonnative thistles [Cirsium arvense (L.) Scop.; Cirsium vulgare (Savi) Ten.], spotted knapweed (Centaurea stoebe L.), absinth wormwood (Artemisia absinthium L.), Kentucky bluegrass (Poa pratensis L.), crested wheatgrass [Agropyron cristatum (L.) Gaertn.], annual brome grasses (Bromus arvensis L.; Bromus tectorum L.), Siberian elm (Ulmus pumila L.), and saltcedar. We also documented native species that naturally colonized the plots and were not seeded species. We did not have any invasive native species at our site.
All transplanted trees and shrubs were mapped at planting in spring 2012. Each living tree and shrub transplant was individually tagged in spring of 2013. Maps were compared with locations of live plants to determine survivorship over the first winter. Tagged plants were recensused in 2016, and their survival was recorded.
Each summer of 2011 to 2016, Russian-olive saplings were counted in each block and sprayed with herbicide or hand pulled. In 2011 and 2012, resprouts were counted separately from seedlings. No reprouts were observed in 2014 to 2016 western wheatgrass [Pascopyrum smithii (Rydb.) A. Love].
Data Analysis
We tested the influence of year, treatment, year by treatment interaction, and block (random factor) on three invasive cover variables (invasive perennial grass, nonnative forbs, and annual bromes) and three native cover variables (native perennial grass, native forbs, and seeded species) in a multivariate analysis of variance (MANOVA; JMP v. 12.1.0, SAS Institute, Cary, NC). When the MANOVA was significant [year F(4,70)=14.486, P<0.001; treatment F(1,70)=5.153; P<0.027, year*treatment F(4,70)=3.405, P<0.014], we used protected generalized linear mixed models to analyze the influence of year, treatment, year by treatment interaction, and block (random factor) on each cover group separately. We used the same model statement in a MANOVA for four diversity measures: total, native, invasive, and seeded species diversity. When the MANOVA was significant [year F(4, 30)=7.3151, P=0.0003; treatment and treatment*year interaction P>0.3], we used protected generalized linear mixed models to analyze the influence of year on each diversity group separately. All cover and diversity data were normally distributed (Shapiro-Wilk test W>0.84), and cover data were square-root transformed for analysis to improve the distribution of residuals. All averages are presented ±1 SD.
We calculated conditional survival probabilities for individuals from each transplanted tree and shrub species. The probability that an individual of age x survives to age x+1, P(x), is calculated as:
where S(x) is number of individuals survived to time x, and S(0) is the original number of individuals planted. Probabilities were generated from data pooled across all plots combined.
Results and Discussion
Our removal technique of cutting Russian-olive trees with a tree shear to ground level and immediately spraying the stump with herbicide resulted in a 4% resprout rate the following year (Table 1). No resprouts were detected in the third year after removal and all following years (Table 1). We observed no seedlings emerging in early summer in 2011, the year we conducted tree removal.
Table 1 Number of Russian-olive saplings sprayed or hand pulled after removal in 2011, separated by establishment type (resprout or seedling), and average number of saplings treated per block (±1 SD) and percent stand regeneration (out of the 2,500 trees removed).

a Approximately 200 saltcedar saplings were sprayed, and counts of resprouts and seedlings were combined in 2013.
b Approximately 100 saltcedar saplings were also sprayed in 2014.
c Only Block 1 was treated in 2015.
d The 2016 data include saplings emerged in 2015 and 2016 for Blocks 2–4.
Block 1 consistently had the highest Russian-olive sapling numbers establishing from seed, averaging 1,474 trees year−1 (433, 206, 941, 5,383, and 408 for years 2012 to 2016, respectively); Block 3 had the lowest numbers, with an average of 18.5 saplings year−1 (13, 17, 7, 36, and 37 for years 2012 to 2016, respectively). The stand-regeneration rate was lowest in 2011 (the year of removal), which was the only year when resprouts contributed more to stand regeneration than seedling establishment (Table 1). Stand-regeneration rates varied considerably among years, with estimates ranging from 4% to 215%.
Half or more of cottonwood and boxelder transplants were lost to transplant shock and/or winterkill in the first year (Figure 2). In following years, cottonwood survival remained poor, and compared with other species, cottonwood essentially failed to establish from our plantings. Other species survival remained relatively high and trended upward between 2014 and 2016. Woods’ rose was especially susceptible to herbicide-spray drift from follow-up Russian-olive control: 40% of the mortality in 2014 was due to herbicide damage. However, remaining Woods’ rose transplants maintained relatively high survival into 2016.
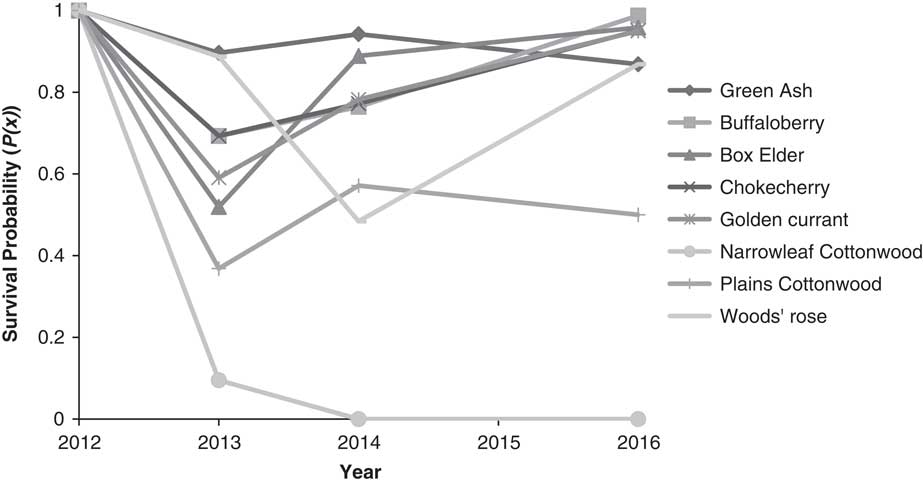
Figure 2 Conditional survival probability to time x+1, given survival to time x from initial planting (2012) to 2016. Several species had substantial mortality the first year after planting. However, survival has largely increased over time for the remaining transplants, with the exception of narrowleaf cottonwood.
All planted understory species established in the plots, with the exception of American vetch, which was seeded at a very low rate (see Supplementary Material). While the interaction term for revegetation over time (treatment*year) was significant in the MANOVA, it was not significant for any single cover group, which indicates that the community as a whole had a nuanced response to revegetation. All plant cover groups responded to the main effect of year. Native perennial grass [F(1,67)=9.8885, P<0.0001], seeded species [F(1, 67)=8.6743, P<0.0001], and annual bromes [F(1,67)=9.4877, P<0.0001] increased over time (Figure 3A), while nonnative forbs (despite an initial increase in 2013) [F(1,67)=13.7476, P<0.0001] and native forbs [F(1,67)=4.0611, P<0.006] decreased over time (Figure 3B). Invasive perennial grass cover decreased initially in 2013 and continued to increase over time [Figure 3C, F(1,67)=3.884, P<0.007]. Only invasive perennial grass cover responded to the revegetation treatment [F(1, 67)=12.230, P<0.001] with cover much higher in controls compared with revegetated plots (25.7±21.2% vs. 7.7±13.4%). All categories of species diversity except invasive species diversity (P>0.06) increased over time (P<0.004, Figure 3D). Contrary to our expectations, revegetation did not appear to competitively exclude secondary invasions, even after five growing seasons. The establishment phase of secondary invasion appeared to be unaffected by revegetation: the number of invasive species did not differ between planted plots and unplanted controls. However, in terms of growth (Figure 3C), the cover of invasive grass species was lower in revegetated plots compared with non-revegetated controls. Because native cover was not significantly higher in revegetated plots, we cannot say with certainty that revegetation provided competitive exclusion of invasive grasses. The herbicide treatment as part of revegetation may be responsible for the reduction in invasive grass cover, although the strength of the treatment effect could be a combination of herbicide application, seeded species establishment (i.e., revegetation), and subsequent competitive pressure. Few restoration experiments extend monitoring to the stage at which competitive interactions can be observed (Blanchard and Holmes Reference Blanchard and Holmes2008; Rinella et al. Reference Rinella, Mangold, Espeland, Sheley and Jacobs2012), and we have not observed competitive exclusion of secondary invasive species in this study. Revisiting revegetation plantings many years after their installation shows that successful revegetation establishment does not always confer the function of competitive exclusion of weeds (Rinella et al. Reference Rinella, Mangold, Espeland, Sheley and Jacobs2012). Because invasive grasses in the Northern Great Plains reduce overall native plant species diversity (Toledo et al. Reference Toledo, Sanderson, Spaeth, Hendrickson and Printz2014), reduce wildlife habitat (Ellis-Felege et al. Reference Ellis-Felege, Dixon and Wilson2013), and have the potential to prohibit shrub establishment (Rinella et al. Reference Rinella, Hammond, Bryant and Kozar2015), the secondary invasion we observed may represent as significant a threat to native riparian ecosystems as Russian-olive.

Figure 3 Cover and diversity response to time: (A) native perennial grass, annual bromes, and seeded species increased over time (P<0.05); (B) nonnative forbs and native forbs decreased over time; (C) invasive perennial grass cover was greater in non-revegetated controls, and increased over time in both revegetated and control plants (P<0.05); (D) total, seeded, and native species diversity increased over time (P<0.05), while nonnative species diversity did not change (P<0.06). Note that D is on a logarithmic scale.
Russian-olive removal is a long-term commitment: stands are capable of complete regeneration from seed, and resprouts must be controlled for at least two consecutive years postremoval with the method we used. Removal with a skid steer resulted in soil disturbance that was combined with a 50-yr flood just after removal; these events may have altered both Russian-olive resprout rates and the composition of the understory vegetation compared with less disturbance (Richardson et al. Reference Richardson, Holmes, Esler, Galatowitsch, Stromberg, Kirkman, Pysek and Hobbs2007). The first year after removal, a 4% resprout rate included resprouts from tree roots that were exposed by floodwater action. This percentage decreases to 0.4% if we report only stump resprouts, which likely would have been the case had the flood not occurred. Smaller trees are more likely to be killed by herbicide, and spraying larger trees has a greater likelihood of damaging adjacent, desirable vegetation due to spray drift. Nonfoliar treatment techniques such as basal bark herbicide application may avoid these problems but were not tested. Biological control of this tree (potential agents listed in Bean et al. Reference Bean, Norton, Jashenko and Schaffner2008) will likely lead to different limitations to revegetation and reinvasion, just as would be the case if control was achieved from methods other than those used in this experiment. No matter the technique, effective control of reinvasion can have costs to desirable vegetation if appropriate care is not exercised (Blanchard and Holmes Reference Blanchard and Holmes2008).
Our plot design had important effects on our results. Because it is surrounded by untreated land, Block 1 has more opportunities for gravity-based seed inputs from surrounding Russian-olive trees compared with the other experimental blocks. Also, water-dispersed fruits were more likely to settle at this block due to geomorphological factors: this block experienced a river backflow due to an ice jam in March of 2014. Stand regeneration was consistently highest in Block 1. The average 18.4% per year regeneration rate we observed in Block 3 is more likely to reflect reinvasion dynamics when Russian-olive control is successfully undertaken at the stand level and when water and wildlife dispersal are low. While Russian-olive seed is very long-lived in laboratory storage (Scianna et al. Reference Scianna, Kilian and Muscha2012), seed buried deeper than 5 cm rarely emerges and can experience 100% mortality (Hybner and Espeland Reference Hybner and Espeland2014), which is promising for Russian-olive control in depositional environments such as on the banks of the undammed Yellowstone River (e.g., Graumlich et al. Reference Graumlich, Pisaric, Waggoner, Littell and King2002). Because Russian-olive trees do not consistently set fruit every year until they reach about 10 yr of age (Lesica and Miles Reference Lesica and Miles1999), it is possible to control reinvasions by conducting reentry kills less frequently than once per year. High stand-regeneration rates throughout the study period illustrate the importance of repeated site inspection and periodic control of this invasive species.
Transplanted tree and shrub survivorship was high, even though southeastern Montana experienced in 2012 one of the four driest years on record since 1878 (National Climatic Data Center, Asheville, NC; Table 2). Low cottonwood survival from our transplants does not indicate the inability of cottonwood to grow in soils with a Russian-olive legacy: natural establishment after the flood led to considerable numbers of plains cottonwood seedlings in the experiment area, particularly in the control plots that did not receive herbicide or the additional disturbance of seeding and transplanting in 2012. Cottonwood typically has low seedling survivorship alongside unregulated rivers (Andersen Reference Andersen2005; Bradley and Smith Reference Bradley and Smith1986; Horton and Clark Reference Horton and Clark2001; Li Kui and Stella Reference Li Kui and Stella2016), but due to prolific germination and emergence, this species is able to establish new populations even with very low survival. Because establishment of native cottonwood and willow trees is limited by shade, Russian-olive prevents their recruitment (Lesica and Miles Reference Lesica and Miles2001); therefore, these native trees are unlikely to maintain their populations without intervention in the face of continuing Russian-olive invasion.
Table 2 Percent understory plant cover and growing-season rainfall amounts per year of the study.
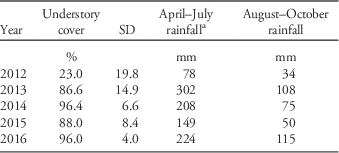
a April–July rainfall is shown to indicate rainfall that occurred before the cover measurement taken that year.
Placing non-revegetated plots at the center of each block maximized the potential for seeded species to colonize this neighboring unseeded area, which may be partly responsible for the lack of effect our revegetation treatment had on native species’ cover and diversity. The disturbance of tree removal that created substantial bare ground, the flood, and subsequent drought were likely the largest drivers of changes in understory plant cover in the short term (as in Richardson et al. Reference Richardson, Holmes, Esler, Galatowitsch, Stromberg, Kirkman, Pysek and Hobbs2007). Additionally, at the time of study completion, seeded species had moved beyond the boundaries of the plots. Data analyses conducted in 2013 showed a main effect of revegetation on native species diversity (Espeland et al. Reference Espeland, Petersen, Muscha, Scianna and Kilian2014a), but the longer time frame of the study we report on here has allowed plot boundaries to blur. Thus, our study may unintentionally support the use of “seed islands”—seeding small areas to provide a source for native species to recolonize degraded lands—in restoration to improve landscapes (as in Reever Morghan et al. Reference Reever Morghan, Sheley, Denny and Pokorny2005).
Removing Russian-olive in dense, closed-canopy populations with a skid steer resulted in considerable bare ground. This disturbance may be responsible for the “year” main effect we observed in plant cover. Both native and introduced forbs appeared to capitalize on the disturbance with high cover early in the experiment; perennial grasses increased their cover over time following disturbance. While many studies show that restoration can introduce high levels of problem species (summarized in Robichaud et al. Reference Robichaud, Beyers and Neary2000), all our experimental plots experienced very high levels of disturbance, equalizing that particular invasion vector among controls and treatments. Conducting Russian-olive removal using a technique resulting in less soil disturbance (e.g., with a chainsaw instead of a skid steer) would probably result in very different restoration and reinvasion outcomes.
Ecological research in habitats with high levels of historical and contemporary disturbance means natural variation can occlude the effects of experimental treatments (Richardson et al. Reference Richardson, Holmes, Esler, Galatowitsch, Stromberg, Kirkman, Pysek and Hobbs2007; Sweeney and Czapka Reference Sweeney and Czapka2004). We were fortunate that none of our plots were destroyed via natural fluvial processes, and even though large blocks of ice were found inside the wildlife fence in 1 yr, we did not lose any plots to scouring (as in Espeland et al. Reference Espeland, Rand and Delaney2014b). Because stand-regeneration (or reinvasion) numbers varied exponentially among blocks, future ecological research on Russian-olive removal should include not only controlling for tree density and distance from the river (as we did in this study) but also seedbank (Blanchard and Holmes Reference Blanchard and Holmes2008) and dispersal factors that include fluvial geomorphology (Nagler et al. Reference Nagler, Glenn, Jarnevich and Shafroth2011) and wildlife corridors (Stannard et al. Reference Stannard, Ogle, Holzworth, Scianna and Sunleaf2002). Because seeds are so plentiful under closed-canopy populations, a seedbank-reduction technique such as scraping (e.g., Morgan Reference Morgan1999) or seed-eating biological control (Bean et al. Reference Bean, Norton, Jashenko and Schaffner2008) may be beneficial, or other techniques could be developed to exploit the low survival of buried seed (Hybner and Espeland Reference Hybner and Espeland2014). This research shows the potential to regain native plant diversity and structure (i.e., trees and shrubs) in riparian areas after the removal of invasive trees.
Acknowledgments
The authors would like to thank the Fort Keogh Summer 2010 range crew, Roger Hybner, Maureen O’Mara, Sue Bellows, Susie Reil, Stacie Kageyama, Brooke Shipp, Dustin Strong, Mark Henning, Holger Jensen, Phil Smith, Kenny Strobel, Jenny Woodward-Paddock, Darren Zentner, Ross Oyler, Patrick Hagemeister, Martin Ellenburg, Kristie Nile, Jerry Cline, Patrick Rolling, Valerie Riter, Scott Brady, Bernie Garber, and Ming-Yu Stephens for their labor in project installation and monitoring. The Bureau of Land Management and the National Wild Turkey Foundation provided additional project funding. Thanks to John Gaskin and two anonymous reviewers for comments on the paper. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2017.36







