Introduction
Smoking remains a worldwide health issue. Cigarette smoke contains about 4000 chemical substances, most of which have known toxic effects. These substances include polycyclic aromatic hydrocarbons (PAHs) such as benzoapyrene, nitrosamises, heavy metals (cadmium), alkaloids (nicotine end its major metabolite, cotinine) and aromatic amines. Possibly reflecting the multiple toxicological effects and targets of the chemicals contained in cigarettes, smoking has been causally related with a wide spectrum of deleterious effects on womens’ reproductive processes (Dechanet et al., Reference Dechanet, Anahory, Mathieu Daude, Quantin, Reyftmann, Hamamah, Hedon and Dechaud2011). These include early menopause, premature follicle loss, abnormal follicle growth and impairment of morphology and oocytes maturation (Neal et al., Reference Neal, Zhu, Holloway and Foster2007; Sadeu & Foster Reference Sadeu and Foster2011; Jennings et al., Reference Jennings, Merriman, Beckett, Hansbro and Jones2011; Paixao et al., Reference Paixao, Gaspar-Reis, Gonzalez, Santos, Santana, Santos, Spritzer and Nascimento-Saba2012). Different studies have evaluated the molecular mechanisms behind the ovotoxicity elicited by cigarette smoke (Nampoothiri et al., Reference Nampoothiri, Agarwal and Gupta2007; Tuttle et al., Reference Tuttle, Stämpfli and Foster2009; Sobinoff et al., Reference Sobinoff, Pye, Nixon, Roman and McLaughlin2012, Reference Sobinoff, Beckett, Jarnicki, Sutherland, McCluskey, Hansbro and McLaughlin2013; Gannon et al., Reference Gannon, Stampfli and Foster2012, Reference Gannon, Stampfli and Foster2013, Ganesan et al., Reference Ganesan, Bhattacharya and Keating2013, Mai et al., Reference Mai, Lei, Yu, Du and Liu2014), with redox balance alteration regarded as a main molecular determinant implicated (Nampoothiri et al., Reference Nampoothiri, Agarwal and Gupta2007; Lim & Luderer., Reference Lim and Luderer2011; Sobinoff et al., Reference Sobinoff, Pye, Nixon, Roman and McLaughlin2012, Reference Ganesan, Bhattacharya and Keating2013; Mai et al., Reference Mai, Lei, Yu, Du and Liu2014 Siddique et al., Reference Siddique, Sadeau, Foster, Feng and Zhu2014; Opuwari & Henkel, Reference Opuwari and Henkel2016).
The term redox balance indicates the physiological stability between free radicals and antioxidant defense. Cells are protected by defense systems in order to prevent oxidative damage and remove free radicals. An alteration of this balance towards an increase in pro-oxidants other than anti-oxidants establishes a condition called oxidative stress (Valko et al., Reference Valko, Leibfritz, Moncola, Cronin, Mazura and Telser2007). Among the free radicals, the main reactive oxygen species include superoxide anion (O2 –) and hydrogen peroxide (H2O2) that are physiologically stabilized by cytosolic copper–zinc superoxide dismutase (SOD1) and mitochondrial manganese-SOD (SOD2) and catalase respectively. In particular, SOD1 and SOD2 reduce superoxide anion to hydrogen peroxide and molecular oxygen (Valko et al., Reference Valko, Leibfritz, Moncola, Cronin, Mazura and Telser2007):
Catalase reduces hydrogen peroxide to water (Valko et al., Reference Valko, Leibfritz, Moncola, Cronin, Mazura and Telser2007):
There is evidence that cigarette smoke exposure is associated with an increase in ROS formation in oocytes (Sobinoff et al., Reference Sobinoff, Pye, Nixon, Roman and McLaughlin2012, Reference Sobinoff, Beckett, Jarnicki, Sutherland, McCluskey, Hansbro and McLaughlin2013), in follicles cultured in vitro (Siddique et al., Reference Siddique, Sadeau, Foster, Feng and Zhu2014), and in mice ovaries exposed to this stress (Mai et al., Reference Mai, Lei, Yu, Du and Liu2014). Little information is known about the effects of cigarette smoke exposure on granulosa cell redox status. Granulosa cells (GCs) are steroidogenic cells that surround the oocyte and have an important role in follicular development, oocyte maturation and atresia (Karuputhula et al., Reference Karuputhula, Chattopadhyay, Chakravarty and Chaudhury2013). Antioxidant enzymes play an important role in protection from oxidative stress damages. At very low concentration, ROS are second messengers that are important to modulate the expression of genes enrolled in physiological process of gametes and embryos, such as oocyte maturation, ovarian steroidogenesis, luteolisis and corpus luteal functions (Agarwal et al., Reference Agarwal, Gupta and Sharma2005). An increase in reactive oxygen species concentration induces pathological processes that involve the female reproductive tract.
Antioxidant enzymes have been localized in the granulosa and thecal cells of the growing follicle (Agarwal et al., Reference Agarwal, Gupta and Sharma2005). In particular, in granulosa cells, antioxidant enzymes play an important role in scavenging reactive oxygen species generated during the synthesis of steroid hormones, and catalase activity is stimulated by both FSH and an increase in E2 levels (Tatone et al., Reference Tatone, Carbone, Falone, Aimola, Giardinelli, Caserta, Marci, Pandolfi, Ragnelli and Amicarelli2006).
The aim of this study was to evaluate, for the first time, the consequences of the habit of smoking on the levels of antioxidant enzymes (SOD1, SOD2 and CAT) in granulosa cells of women smokers undergoing IVF treatments.
Materials and methods
Patient selection
In total, 40 women, attending an IVF programme in the unit for assisted reproductive technology of the Ortona General Hospital, Italy, were studied. The study group consisted of 20 smokers [13 ± 6 cigarettes/day; mean ± standard deviation (SD)] and 20 non-smoking patients who were undergoing IVF techniques. Patients taking micronutrient supplements were excluded from the study. Patients’ smoking habits were assessed by a questionnaire. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinky Declaration of 1975, as revised in 2008.
Ovarian stimulation was induced in all patients using a combination of GnRH agonist/antagonist and recombinant FSH (Puregon; Merck Shape and Dome, Italy; Gonal F, Merck Serono, Italy) at the starting daily doses ranging from 150 IU to 225 IU. The stimulation was controlled using serum estradiol measurement and serial ultrasound amounts of follicle numbers and diameters. Oocyte retrieval was performed by transvaginal aspiration 36 h after recombinant hCG (Ovitrelle, Merck Serono, Italy) administration. The luteal phase was supported with intramuscular injections of 100 mg progesterone (Prontogest, IBSA, Italy). A pregnancy test was performed by quantifying serum beta-hCG 14 days after embryo transfer. Clinical pregnancy was defined as the presence of a gestational sac with fetal heart activity visible on an ultrasound scan at 7 weeks’ gestation.
GCs isolation and in vitro fertilization
For each patient enrolled in this study, follicular aspirates, free of blood contamination, after removal of the oocyte, were collected in a tube that was left standing for 5 min to allow mural GCs to sediment. The cell pellet was gently re-suspended in 5 ml of Ham's F-10 medium (Sigma Aldrich). After centrifugation on a density gradient (Ficoll-Hypaque; Sigma Aldrich) at 3000 rpm for 10 min, GCs were purified collecting the top layer of the suspension and taking care to avoid the layers containing lymphocytes and red blood cells. GCs were washed with 5 ml of Ham's F-10 medium and to prevent cell aggregation, were treated with hyaluronidase solution (0.1% vol/wt in Ham's F-10) for 15 min. The entire procedure was completed within 1 h after follicle aspiration to prevent cell death.
After 2 h from ovum pick-up, eggs were denuded and their stage of maturation was recorded. Oocytes in metaphase II stage were fertilized by intracytoplasmic sperm injection (ICSI). Italian legislation requires that embryo production must not exceed what is strictly necessary based on individual patient characteristics. Embryos were classified into types A, B, and C according to the following morphological characteristics (Tiboni et al., 2012): type A or excellent embryos composed with regular blastomeres with equal or non-equal size without fragmentation; type B or good embryos with blastomeres of equal or non-equal size with < of 20% of the embryo volume with enucleate fragments; and type C or fair embryos with anucleate fragments in 20–50% of the volume of the embryo.
SOD1, SOD2 and catalase mRNA quantification by qRT-PCR
Total RNA extraction
Total RNA was isolated and subjected to DNase I treatment, using the RNAqueous-4 PCR Kit (ThermoFisher Scientific) according to the manufacturer's instructions. RNA concentrations were quantified by determination of optical density at 260 nm and 280 nm on a UV spectrophotometer. All RNA used in this study had a 260:280 ratio between 1.9 and 2.1. The integrity of the RNA sample was confirmed by agarose gel electrophoresis, which showed the presence of intact 18S and 28S ribosomal RNA bands.
Reverse transcription
Synthesis of cDNA was performed using the High-Capacity Reverse Transcription Kit (ThermoFisher Scientific) with the reaction conditions recommended by the manufacturer. Briefly, a mixture containing 1 µg of RNA, 2 µl 10× RT Buffer, 0.8 µl of 25× dNTP 100 mM mixture, 2 µl 10× Random Primers, 1 µl MultiScribe Reverse Transcriptase (50 U/µl), 1 µl RNase Inhibitor (20 U/µl) and RNAse-free water to reach a final volume of 20 µl. The reaction was incubated at 25°C for 10 min, 37°C for 120 min, 85°C for 5 min and then at 4°C. cDNAs were stored at −20°C until use.
Quantitative real-time PCR
Quantitative real-time PCR assays (ABI PRISM 7700 Sequence Detection System User Bulletin#2 P7N 4303859, Applied Biosystems, USA) for the Relative Quantitation of human superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2) and catalase (CAT) gene expression versus the endogenous control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were performed using TaqMan™ technology (PE Applied Biosystems, USA ThermoFisher Scientific) on the ABI Prism 7900 HT Sequence Detection System Instrument (PE Applied Biosystems, USA), connected to a Sequence Detector Software (SDS version 2.0, PE Applied Biosystems, USA) for collection and analysis of data. We previously validated GAPDH as housekeeping gene in this cell type.
According to manufacturer recommendation the reaction mixture (25μl) for each gene and endogenous control gene (GAPDH) was prepared as follows: 0.5 µl of cDNA obtained from 1 µg of transcripted RNA, 12.5 µl TaqMan Universal PCR Master Mix 2×, 1.25 µl TaqMan Gene Expression Assay 20×, containing a FAM dye-labeled TaqMan MGB probe and 2 unlabeled PCR primers, and RNAse free-water to reach a final reaction volume of 25 µl. Reactions were performed in a MicroAmp Optical 96-well reaction plate (PE Applied Biosystems, USA). Amplification conditions were 95°C 10 min (hot start), followed by 40 cycles of 95°C 15 s, 60°C 1 min. All reactions were performed in triplicate and repeated twice. The relative quantification of SOD1, SOD2 and CAT gene expression was evaluated with data from SDS Software. Within each experiment, the fold change between treatment and control was determined by transforming logarithmic data to linear data using the arithmetical formula: RQ = 2−ΔΔCT, according to the comparative Ct method, where ΔΔCT = (Avg. CT gene × in smoker − Avg. CT GAPDH in smoker) − (Avg. CT gene × in non-smoker − Avg. CT GADPH in non-smoker), where Avg. CT is the average of CT numbers of three replicates in the smoking or not smoking group. The values represent the fold change of the gene in the smoker group relative to non-smoker one. For the ΔΔCT calculation to be valid, relative efficiency plots were performed. The efficiency of the target amplification and the efficiency of the reference amplification must be approximately equal (ABI PRISM 7700 Sequence Detection System User Bulletin #2 P7N 4303859, Applied Biosystems, USA; not shown). A sensitive method for assessing if two amplicons have the same efficiency is to look at how ΔCT varies with template dilution. We found that GAPDH responded to these parameters as assumed by ABI PRISM 7700 Sequence Detection System User (Bulletin #2 P7N 4303859, Applied Biosystems, USA).
Outcome measures and statistical analysis
Statistical analysis was performed using the chi-squared test for proportions, and Student's t-test for continuous variables. A P-value < 0.05 was considered as significant.
Results
SOD1, SOD2 and CAT gene expression in GCs of smokers and non-smokers
We carried out qRT-PCR analysis on GCs isolated from a pool of follicles of each patient in order to evaluate mRNA levels of SOD1, SOD2 and catalase in smokers and non-smokers. Figure 1 shows the relative mRNA levels between the two groups. These data revealed an overexpression of SOD1, SOD2 and catalase mRNA levels in smokers. In particular, the relative level of SOD2 mRNA in smokers was 1.7 ± 1.2, whereas for non-smokers it was 1.0 ± 0.3 (Fig. 1B). The difference was statistically significant (P = 0.03). In the same way, catalase expression was upregulated in GCs retrieved from smokers. The relative level of catalase mRNA in this group was 1.6 ± 0.7 whereas for non-smokers it was 1.1 ± 0.4 (Fig. 1C). This difference was statistically significant (P = 0.01).
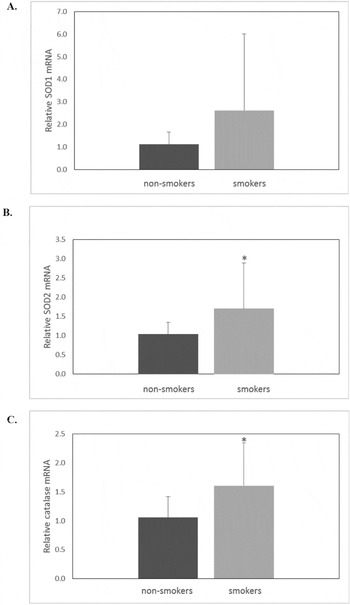
Figure 1 SOD1 (A), SOD2 (B) and catalase (C) mRNA expression levels in granulosa cells from non-smoker and smoker patients. Values are means ± SD from comparative real time results obtained from non-smoker (n = 20) and smoker (n = 20) patients enrolled in the study. Student's t-test was used for statistical analysis for smoker versus non-smoker patients: *P < 0.05.
IVF outcome analysis
Table 1 displays demographic and baseline characteristics of the two study groups. There were no statistically significant differences between the mean ages, causes of infertility, basal FSH and body mass index (BMI). Table 2 shows IVF outcomes of the two study groups. Comparable results were observed in terms of number of retrieved oocytes, number of metaphase II oocytes, quality of transferred embryos, live birth rate, twin pregnancy and pregnancy loss. Conversely, fertilization rate, implantation rate and clinical pregnancy rate were significantly different between smokers and non-smokers patients. In particular, smokers had a lower fertilization rate compared with non-smokers (73% in non-smokers versus 56% in smokers). Regarding the implantation rate, it was 22.8% in non-smokers and 5.3% in smokers (P = 0.008). Our study revealed also a 55% rate of clinical pregnancies in non-smokers, and 10% rate of clinical pregnancies in smokers (P = 0.002).
Table 1 Main demographic and baseline characteristics of non-smoker and smoker patients
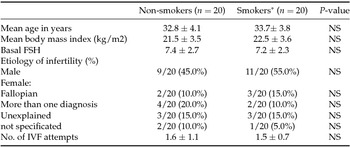
*13 ± 6 cigarettes/day.
Values are expressed as means ± SD or n (%).
Table 2 IVF cycle outcomes in non-smoker and smoker patients
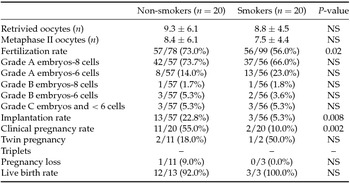
Values are expressed as means ± standard deviation (SD) or n (%).
Discussion
This study analyzed the relationship between cigarette smoking and antioxidants levels in human granulosa cells and a correlation between the habit of smoking in women and clinical outcomes of assisted reproductive techniques.
Experimental studies on animal models focusing on the effects of cigarette smoke on ovaries showed interesting results. Nampoothiri et al. (Reference Nampoothiri, Agarwal and Gupta2007) and Sobinoff et al. (Reference Sobinoff, Pye, Nixon, Roman and McLaughlin2012) demonstrated that cigarette smoking is correlated with an alteration in the redox balance in oocytes and granulosa cells exposed in vitro to compounds in cigarettes. In particular, Nampoothiri et al. (Reference Nampoothiri, Agarwal and Gupta2007) established a significant decrease in SOD activity and GSH content, and a significant increase in catalase activity after lead and cadmium administration to rat granulosa cells. This altered balance between free radicals and antioxidants in granulosa cells was associated with an increased lipid peroxidation in GCs membrane structures. Sobinoff et al. (Reference Sobinoff, Pye, Nixon, Roman and McLaughlin2012) tested the effects of BaP on mouse ovarian transcriptome, demonstrating that BaP exposure is associated with increased levels of mitochondrial ROS in oolemma membrane resulting in a considerable plasma membrane lipid peroxidation. Siddique et al., (Reference Siddique, Sadeau, Foster, Feng and Zhu2014) and Mai et al., (Reference Mai, Lei, Yu, Du and Liu2014) found an impact of cigarette smoke on antioxidant enzymes in follicles cultured in vitro and in mouse ovaries exposed to this stress, respectively.
Previous studies conducted on women undergoing IVF treatments, demonstrated that age influences antioxidant enzyme expression in follicular fluid (Carbone et al., Reference Carbone, Tatone, Delle Monache, Marci, Caserta, Colonna and Amicarelli2003) and in granulosa cells (Tatone et al., Reference Tatone, Carbone, Falone, Aimola, Giardinelli, Caserta, Marci, Pandolfi, Ragnelli and Amicarelli2006). Carbone et al. (Reference Carbone, Tatone, Delle Monache, Marci, Caserta, Colonna and Amicarelli2003) found that follicular fluid from older women exhibited a reduced level of glutathione transferase and catalase activities and a higher level of SOD activity. Tatone et al. (Reference Tatone, Carbone, Falone, Aimola, Giardinelli, Caserta, Marci, Pandolfi, Ragnelli and Amicarelli2006) found a reduced expression of SOD1, SOD2 and catalase in granulosa cells of women ≥ 38 years. Recent findings (Ávila et al., Reference Ávila, González-Fernández, Rotoli, Hernández and Palumbo2016) revealed that women with endometriosis and PCOS have lower antioxidant production capacity in their granulosa cells that may contribute to abnormal follicular development and infertility. A reactive oxygen species accumulation in the granulosa cells correlates with diminished expression of follicle-stimulating hormone receptor (FSHR) and a dysregulation of the FSHR signaling pathway and may be implicated in altered steroidogenic function and poor response to FSH in women (Ávila et al., Reference Ávila, González-Fernández, Rotoli, Hernández and Palumbo2016).
To the best of our knowledge, this is the first study that evaluated the association between the habit of smoking and the antioxidant enzymes gene expression (SOD1, SOD2 and CAT) in granulosa cells of smoker women undergoing IVF treatments. We used mural granulosa cells for our experiments, which are somatic cells that constitute the oocyte microenvironment together with cumulus cells (Aydos et al., Reference Aydos, Gurel, Oztemur Islakoglu, Noyan, Gokce, Ecemis, Kaya, Aksu and Gur Dedeoglu2016). Mural granulosa cells have an important role for correct oocyte development; one of the main functions is arresting the oocyte in the meiotic phase until ovulation by secreting oocyte maturation inhibitor (Kawamura et al., Reference Kawamura, Cheng, Kawamura, Takae, Okada and Kawagoe2011). When the menstrual cycle begins, these cells receive external hormone signals to resume oocyte maturation. This activation signal is then transmitted to the cumulus cells, which are in direct contact with the oocyte with gap junction interaction (Aydos et al., Reference Aydos, Gurel, Oztemur Islakoglu, Noyan, Gokce, Ecemis, Kaya, Aksu and Gur Dedeoglu2016).
We found a statistically significant increase in SOD2 and catalase mRNA levels in smokers and an increase, not statistically significant, in SOD1. It is well known for granulosa cells that the antioxidant enzymes evaluated in our study play an important role in scavenging superoxide anions and hydrogen peroxide generated during steroidogenesis (Tatone et al., Reference Tatone, Carbone, Falone, Aimola, Giardinelli, Caserta, Marci, Pandolfi, Ragnelli and Amicarelli2006).
The overexpression of these genes found in this study is probably due to an accumulation of reactive oxygen species cigarette smoke-induced. These increased levels of antioxidant enzymes may represent a necessity of granulosa cells to act against cigarette smoke-induced reactive oxygen species in order to neutralize them and to prevent their diffusion into the oocyte (Carbone et al., Reference Carbone, Tatone, Delle Monache, Marci, Caserta, Colonna and Amicarelli2003). It is known that oxidative stress plays a role in the etiopathogenesis of endometriosis, tubal factor infertility, and unexplained infertility (Ngo et al., Reference Ngo, Chéreau, Nicco, Weill, Chapron and Batteux2009; Kumar et al., Reference Kumar, Pathak, Kriplani, Ammini, Talwar and Dada2010; Menezo et al., Reference Menezo, Silvestris, Dale and Elder2016). The fact that patients suffering from these infertility factors were included in the study may have introduced confounding effects in the analysis of the results.
Clinical effects
In our report we found a strong influence of cigarette smoke on fertilization, implantation and clinical pregnancy rates. As previously shown, we found a fertilization rate of 73% in non-smokers and 56% in smokers. These results are in accordance with our previous study (Tiboni et al., Reference Tiboni, Bucciarelli, Giampietro, Sulplizio and Di Ilio2004) demonstrating a higher percentage fertilization rate in non-smokers (71.5%) than smokers (55.9%) in a retrospective analysis of 70 female patients undergoing IVF treatments (17 smokers and 43 non-smokers).
Gruber et al., (Reference Gruber, Just, Birner and Lösch2008) found an 85.7% fertilization rate in non-smokers versus 78.2% in smokers in a retrospective study that included 130 patients undergoing ICSI procedure (72 smokers and 58 non-smokers).
Implantation rate
Our findings are in line with Neal et al. (Reference Neal, Hughes, Holloway and Foster2005) and Benedict et al. (Reference Benedict, Missmer, Vahratian, Berry, Vitonis, Cramer and Meeker2011) who showed a significant decrease in the percentage of implants in smokers compared with non-smokers. In particular, Neal et al. (Reference Neal, Hughes, Holloway and Foster2005) found a rate of 12% in smokers compared with a 25% implant rate in non-smokers in a retrospective study of 225 female patients undergoing IVF procedures. Benedict et al. (Reference Benedict, Missmer, Vahratian, Berry, Vitonis, Cramer and Meeker2011) in their retrospective analysis of a prospective cohort study, showed a significant increase in the risk of implantation failure among women exposed to sidestream smoke.
Clinical pregnancy rates
Different authors found, in accordance with our findings, a decrease in clinical pregnancies in smokers (see Neal et al., Reference Neal, Hughes, Holloway and Foster2005; Waylen et al., Reference Waylen, Metwally, Jones, Wilkinson and Ledger2009; Ben-Haroush et al., Reference Ben-Haroush, Ashekenazi, Sapir, Pinkas, Fisch and Farhi2011; Freour et al., Reference Freour, Masson, Dessolle, Allaoua, Dejoie, Mirallie, Jean and Barriere2012). In particular, Neal et al. (Reference Neal, Hughes, Holloway and Foster2005) found a 40% rate of pregnancies in non-smokers and an 11.1% rate in smokers. Freour et al. (Reference Freour, Masson, Dessolle, Allaoua, Dejoie, Mirallie, Jean and Barriere2012) showed a clinical pregnancy rate per transfer of 21.1% in smokers (35.3% in non-smokers) in a retrospective study involving 277 women while Ben-Haroush et al. (Reference Ben-Haroush, Ashekenazi, Sapir, Pinkas, Fisch and Farhi2011) found an overall rate of 35.7% of clinical pregnancies in smokers (55.4% in non-smokers) in a cohort study including 237 patients (42 smokers and 195 non-smokers). Waylen et al. (Reference Waylen, Metwally, Jones, Wilkinson and Ledger2009) in their meta-analysis with a computerized search demonstrated that patients who smoked had significantly lower odds of clinical pregnancy per cycle (OR 0.56, 95% CI 0.43–0.73) than non-smokers.
Our findings are in contrast with some other studies (Wright et al., 2006; Fuentes et al., 2010; Cinar et al., 2014). In a retrospective analysis of 389 patients, Wright et al. (2006) described that the cigarette-smoking habit does not affect IVF procedure outcomes. Fuentes et al. (2010) failed to note, in a cohort prospective study including 166 patients, a significant association between women who smoked and reduced pregnancy rate. They also found a lower implantation rate, which was non-statistically significant, among women exposed to cigarette smoke compared with those who were not. Cinar et al. (2014) did not determine a significant correlation between the adverse effects of cigarette smoking and IVF outcomes.
Conflict of interest
There are no conflicts of interest.
Acknowledgements
This study was supported by internal funds.





