Fe is a nutritionally essential trace element that functions through incorporation into proteins and enzymes, including Hb, myoglobin, cytochrome c and aconitase(Reference Dallman1). The negative effects of Fe-deficiency anaemia on work capacity and physical performance have been well characterised in humans(Reference Gardner, Edgerton and Senewiratne2–Reference Celsing and Ekblom4) and are attributed to insufficient O2 transport by Hb to peripheral tissues(Reference Hinton, Giordano and Brownlie5). The effects of moderate Fe deficiency without anaemia on performance are not as well described, although recent studies indicate that Fe supplementation may improve exercise endurance(Reference Hinton, Giordano and Brownlie5) and maintain ventilatory threshold in Fe-deficient non-anaemic athletes(Reference Hinton and Sinclair6). Furthermore, Fe deficiency without anaemia results in impaired aerobic adaptation and endurance capacity in untrained women(Reference Brownlie, Utermohlen and Hinton7, Reference Brownlie, Utermohlen and Hinton8).
Fe deficiency and Fe-deficiency anaemia affect billions of individuals worldwide(Reference DeMaeyer and Adiels-Tegman9, Reference Stoltzfus10). In the developed world, the greatest prevalence of poor Fe status is observed in premenopausal women, mainly due to suboptimal Fe intake and the regular loss of Fe through the menstrual cycle(Reference Harvey, Armah and Dainty11). Recent data from the USA and the UK suggest that Fe deficiency affects between 16 and 21 % of premenopausal women(Reference Looker, Cogswell and Gunter12, Reference Heath and Fairweather-Tait13). Female soldiers represent a population that may be at risk for poor Fe status, as these soldiers are of premenopausal age, may not consume adequate Fe(Reference King, Fridlund and Askew14) and often enter military service with low Fe stores(Reference Dubnov, Foldes and Mann15). Poor Fe status could adversely affect female soldiers, as maintaining optimal physical performance is critical for operational deployment and the completion of required training courses, including basic combat training (BCT).
A number of studies indicate that physical activity has a negative effect on Fe status in women(Reference Blum and Sherman16, Reference Ashenden, Martin and Dobson17). Furthermore, cross-sectional studies indicate that military training results in an increased prevalence of Fe deficiency and Fe-deficiency anaemia in female soldiers(Reference McClung, Marchitelli and Friedl18). As such, the primary objective of the present longitudinal study was to confirm findings from previous cross-sectional studies indicating an effect of military training on Fe status. A secondary objective was to determine whether changes in Fe status indicators during training were predictive of physical performance at the end of the training period.
Experimental methods
Volunteers and experimental design
The present study was approved by the Human Use Review Committee at the US Army Research Institute of Environmental Medicine. Human subjects participated in these studies after giving their free and informed voluntary consent. Investigators adhered to Army Regulation 70-25 and US Army Medical Research and Materiel Command Regulation 70-25 on the use of volunteers in research.
A total of ninety-four female soldiers completed this longitudinal study. The first timepoint was within 1 week of arrival at BCT (pre); the second timepoint was within 1 week of completion of BCT (post). All volunteers were not pregnant and had not exercised in the 8 h before fasted blood collection. Height and body weight were assessed at both timepoints. BCT consists of 9 weeks' military training, including both aerobic and muscle strength training(Reference Knapik, Canham-Chervak and Hauret19). Formalised daily physical training sessions occurred 4–6 d/week, and included 1–1.5 h cardiorespiratory and muscle strength training(Reference Knapik, Darakjy and Hauret20). Cardiorespiratory training included activities such as road marching, distance running and sprinting. Muscle strength training included callisthenic exercises, sit-ups and push-ups. In addition to formalised physical training, military training included rappelling, bayonet training, live fire exercises, obstacle courses and prolonged standing in formation(Reference McClung, Marchitelli and Friedl18). Recent studies using pedometers to assess ambulatory activity during BCT indicate that trainees perform an average of over 16 000 steps/d, the equivalent of nearly 12 km(Reference Knapik, Darakjy and Hauret20). In comparison, data from civilian studies estimate average ambulatory activity to be between 6000 and 8000 steps/d(Reference Knapik, Darakjy and Hauret20, Reference Tudor-Locke, Ainsworth and Whitt21).
Upon entry to BCT, 1-mile (1·6 km) run time was collected as an indicator of initial fitness level. The mean 1-mile run time of this population was 9·9 (sd 1·1) min (n 71), which may be converted to a mean VO2 peak of 39·4 (sd 3·4) ml/kg per min using a published equation for the estimation of VO2 peak from running time(Reference Cureton, Sloniger and O'Bannon22). Hence, the average aerobic fitness levels of the volunteers that participated in the present study corresponds to approximately the 60th percentile for women aged 20–29 years(Reference Whatley23). Other demographic characteristics of the volunteers appear in Table 1.
Table 1 Volunteer demographics
(Mean values and standard deviations for ninety-four subjects)
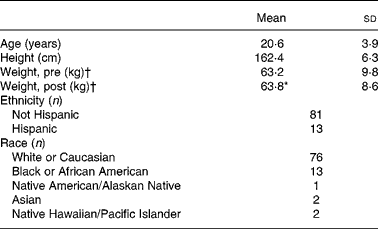
* Mean value was significantly different from that for ‘Weight, pre’ (P ≤ 0·05; paired t test).
† ‘Pre’ measures were collected during week 1 of basic combat training; ‘post’ measures were collected during the final week.
Sample collection and analysis
Blood was collected by antecubital venepuncture into tubes (Vacutainer; Becton-Dickinson, Franklin Lakes, NJ, USA) containing the appropriate anticoagulant. Erythrocyte distribution width (RDW) and Hb were determined in whole blood on-site using a haematology analyser (Ac·T; Beckman Coulter, Fullerton, CA, USA; Sysmex XE-2100; Kobe, Japan). Serum samples were processed on-site, sampled, frozen and shipped to the Pennington Biomedical Research Center (Baton Rouge, LA, USA) for Fe status indicator assays. Total Fe-binding capacity and serum Fe were determined using a Beckman Coulter Synchron CX7 (Beckman Coulter). Serum ferritin was determined by microparticle enzyme immunoassay (IMx; Abbott Laboratories, Abbott Park, IL, USA). Transferrin saturation (TS) was determined by dividing serum Fe by total Fe-binding capacity(Reference Looker, Gunter and Johnson24). Soluble transferrin receptor (sTfR) was determined in serum using an immunoassay according to the manufacturer's instructions (Quantikine IVD; R&D Systems, Inc., Minneapolis, MN, USA). In the case of this immunoassay for sTfR, Fe deficiency is indicated where values exceed 28·1 nmol/l.
Statistical analysis
Statistical analysis was performed using commercially available statistical software (SPSS 15.0; SPSS, Inc., Chicago, IL, USA). Descriptive statistics are presented as mean values and standard deviations. Before means comparisons, normal distribution of variables was determined using the Kolmogorov–Smirnov test. For longitudinal comparisons of Fe status indicators, normally distributed means were compared using paired t tests. For Fe status indicators that were not normally distributed, medians were compared using the Wilcoxon signed ranks test. Correlations between Fe status indicators and 2-mile (3·2 km) run time were evaluated using Pearson's correlation and Spearman's rank correlation analyses as appropriate. Partial correlations between longitudinal changes in each Fe status indicator and 2-mile (3·2 km) run time were conducted while controlling for the pre-BCT concentration of each Fe status indicator. For all statistical analyses a maximum P value of 0·05 was the necessary condition for statistical significance.
Results
Longitudinal changes in iron status following basic combat training
Nearly all Fe status indicators suggest a decline in Fe status at the end of the training period (Table 2). Markers of Fe transport and storage were diminished, as TS and serum ferritin were reduced (P ≤ 0·01) post-BCT. Mean TS was 42·7 % lower at the end of BCT; median serum ferritin values decreased by 20·1 %. Further, median RDW and sTfR were increased (P ≤ 0·01) at the end of BCT, also indicating reduced Fe status. Post-BCT RDW median levels were 107·1 % of the pre-BCT levels, whereas post-BCT sTfR median levels were 131·3 % of the starting levels. Seven individuals would have been considered Fe deficient at the start of BCT using the sTfR cut-off value of 28·1 nmol/l, whereas seventeen individuals would have been considered Fe deficient post-BCT. Hb values were significantly (P ≤ 0·01) higher post-BCT compared with pre-BCT.
Table 2 Longitudinal changes in iron status during military training
(Mean values and standard deviations or medians and ranges)
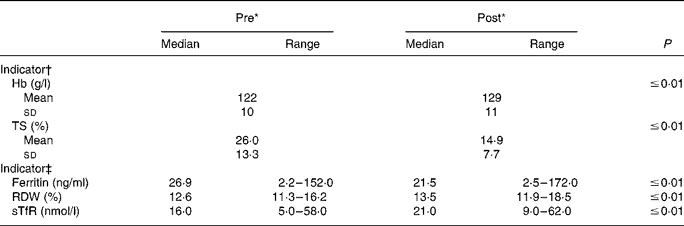
TS, transferrin saturation; RDW, erythrocyte distribution width; sTfR, soluble transferrin receptor.
* ‘Pre’ measures were collected during week 1 of basic combat training; ‘post’ measures were collected during the final week.
† P values were determined using paired t tests (n 94).
‡ P values were determined using the Wilcoxon signed ranks test (n 93–94).
Correlations between iron status indicators and physical performance
Correlation analyses between Fe status indicators and 2-mile (3·2 km) running performance at the end of BCT were performed (Table 3). TS, RDW and Hb at the start of BCT were associated (P ≤ 0·05) with 2-mile (3·2 km) running performance at the end of BCT. Furthermore, both Hb and RDW at the end of BCT were associated (P ≤ 0·05) with running time. When longitudinal change scores were calculated and utilised in correlation analyses, only the change in sTfR was associated (P ≤ 0·05) with running performance. The r value was positive, indicating that the longitudinal decrement in Fe status reflected by increased sTfR was associated with diminished running performance at the end of BCT.
Table 3 Correlations between iron status indicators and running performance during military training†
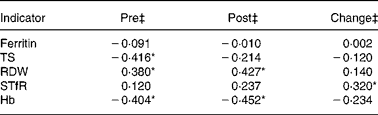
TS, transferrin saturation; RDW, erythrocyte distribution width; sTfR, soluble transferrin receptor.
* Significant (P ≤ 0·05) correlation between Fe status indicators and 2-mile (3·2 km) run times.
† Pearson's correlation and Spearman's rank correlation coefficients (r values) were determined using Fe status indicator values and time to completion of a 2-mile (3·2 km) run at the end of basic combat training (n 59).
‡ ‘Pre’ samples were collected during week 1 of basic combat training; ‘post’ measures were collected during the final week. ‘Change’ values represent correlation between 2-mile (3·2 km) run times and the difference between pre- and post-values.
Discussion
Inadequate Fe intake(Reference King, Fridlund and Askew14) and diminished Fe status(Reference Dubnov, Foldes and Mann15) have been reported previously in female military personnel. Furthermore, cross-sectional studies indicate that Fe status declines in female soldiers during BCT(Reference McClung, Marchitelli and Friedl18). As such, the primary objective of the present study was to determine the longitudinal effect of BCT on Fe status. Our major finding was that all markers of Fe nutriture, with the exception of Hb, indicate diminished Fe status following the training period. Diminished Fe status may have negative consequences for female soldiers, as the period following BCT includes advanced individualised training and possibly operational deployment; activities which require optimal immune, cognitive and physical capabilities.
Diminished Fe status has been observed in a number of populations following physical training. Early studies documented diminished Hb in athletes following periods of intense training(Reference Yoshimura25, Reference Radomski, Sabiston and Isoard26). The diminished Hb observed in these populations was often described as ‘sports anaemia’ and was attributed to the plasma volume expansion that occurs with physical training. More recently, the effect of physical training on Fe status has been demonstrated using multivariable models of Fe status(Reference McClung, Marchitelli and Friedl18) and newly developed indicators of Fe status, suggesting that physical training may result in true degradations in overall Fe status, as opposed to an acute decrement in Hb. For example, Di Santolo et al. (Reference Di Santolo, Stel and Banfi27) recently reported that athletes who performed regular physical exercise (about 11 h/week) had diminished serum Fe and TS as well as increased sTfR as compared with sedentary controls. Furthermore, reduced serum ferritin levels(Reference Ashenden, Martin and Dobson17) and diminished serum Fe(Reference Magazanik, Weinstein and Dlin28) have been reported in female athletes during physical training. In the present study, we found that Fe status was reduced in female soldiers following a standardised military training regimen as demonstrated using a series of Fe status indicators, including serum ferritin, TS and sTfR. One weakness of the present study was the inability to control for potential changes in plasma volume that may have been experienced during military training. However, the use of multiple Fe status indictors diminishes the potential confounding effect of changes in plasma volume, as these indicators provide a dynamic response to diminished Fe status. For example, serum ferritin was reduced following BCT, which could indicate diminished Fe status, or dilution due to increased plasma volume. However, sTfR was elevated, demonstrating that Fe status was truly diminished, as sTfR may not have been significantly elevated had plasma volume been confounding our data.
The reduced levels of early Fe status indicators observed in female soldiers following BCT in the present longitudinal study confirm previous cross-sectional studies. However, the earlier cross-sectional studies found that the prevalence of anaemia was increased following military training(Reference McClung, Marchitelli and Friedl18), whereas our data indicate that despite decreases in all other Fe status indicators, Hb was marginally elevated ( < 10 %) following training. This effect of BCT on Hb levels may indicate that the degree of Fe deficiency following BCT observed in the present study is less than reported in earlier studies, as Fe deficiency is known to occur in stages(Reference Dallman1). Alternatively, increased Hb levels coupled with diminished serum ferritin levels could indicate a shift in Fe metabolism away from storage proteins to proteins that function in O2 delivery. Elevated Hb levels coupled with diminished serum ferritin levels following exercise have been observed in previous studies. Blum et al. (Reference Blum, Sherman and Boileau29) describe elevated Hb levels in premenopausal women following 6 weeks' participation in an aerobic exercise programme. This increase in Hb was coupled with reduced serum ferritin. Interestingly, Hb levels returned to baseline levels following 13 weeks' participation in the exercise programme, whereas serum ferritin levels remained diminished. The authors concluded that the stress of exercise may have stimulated an increase in the production of erythrocytes, resulting in increased Hb, which caused an increased mobilisation of Fe from ferritin. A second potential explanation for the increased Hb levels observed in the present study could be reduced plasma volume due to dehydration. However, reduced plasma volume would have resulted in not only elevation in Hb levels, but other Fe status indicators as well. Regardless, our data indicate that early indicators of Fe storage and transport were negatively affected by BCT, even if Hb was increased.
Although the definitive mechanism explaining diminished Fe status following periods of physical training remains to be elucidated, a number of hypotheses have been proposed. Classical hypotheses include diminished dietary Fe intake(Reference King, Fridlund and Askew14), gastrointestinal bleeding(Reference Stewart, Ahlquist and McGill30), Fe loss through sweat(Reference Brune, Magnusson and Persson31), increased whole-body Fe turnover(Reference Ehn, Carlmark and Hoglund32) and haematuria due to erythrocyte rupture in the foot during running(Reference Siegel, Hennekens and Solomon33). Dietary Fe intake was not assessed in the present study, although recent studies with female recruits in the Israeli Defence Forces report average Fe intakes of approximately 15 mg/d, which would account for 83 % of the current RDA (18 mg/d) for Fe in the corresponding demographic group(Reference Israeli, Merkel and Constantini34). In that study, mean dietary Fe intake was not different between volunteers with normal Fe status as compared with those with Fe deficiency or Fe-deficiency anaemia, suggesting that the development of poor Fe status in female soldiers may involve a multifaceted mechanism. Studies with athletic populations suggest that diminished Fe status may occur as a result of the production of pro-inflammatory cytokines, such as IL-6, in response to acute bouts of exercise, which may subsequently result in the release of hepcidin(Reference Ganz and Nemeth35, Reference Peeling, Dawson and Goodman36). Hepcidin, a recently discovered protein that is synthesised and exported by the liver, confers a major role in Fe homeostasis, as it affects both Fe absorption at the enterocyte and cellular Fe export through the binding and degradation of ferroportin(Reference Ganz and Nemeth35). This hypothesis is supported by the findings of Roecker et al. (Reference Roecker, Meier-Buttermilch and Brechtel37), who reported increased urinary hepcidin excretion in female athletes following a marathon. Future studies should attempt to determine whether military training results in pro-inflammatory cytokine and subsequent hepcidin release.
Indicators of Fe status in the volunteers that participated in the present study were associated with physical performance. Levels of Hb and RDW were correlated with 2-mile (3·2 km) run times at the end of the study. The correlation between Hb levels and running performance was not unexpected, as the negative effects of reduced O2 delivery to tissue on physical performance have been well described(Reference Beard and Tobin38). However, our finding that the change in sTfR over the course of the training period was correlated with 2-mile (3·2 km) run time is novel and indicates that the decrement in Fe status observed in female soldiers had an effect on the ability of these soldiers to perform aerobically demanding tasks. Others have demonstrated the important relationship between sTfR and physical performance. Brownlie et al. (Reference Brownlie, Utermohlen and Hinton7, Reference Brownlie, Utermohlen and Hinton8) reported improved work rate, maximal O2 uptake and performance on time trials using a cycle ergometer following Fe supplementation in individuals with elevated sTfR levels as compared with individuals with normal levels. Similar to our findings, changes in sTfR levels through these studies were correlated with improvements in performance at the end of the training period, indicating that sTfR may be an accurate measure of Fe status that is predictive of physical performance during training periods. It should be noted, however, that most studies indicate that the relationship between sTfR and performance is typically strongest in individuals with levels of sTfR that approach Fe deficiency. In the present study, sTfR-derived cut-off values for Fe deficiency indicate that seven volunteers were Fe deficient pre-BCT; seventeen volunteers were Fe deficient post-BCT.
In summary, the major finding of the present longitudinal study was that indicators of Fe status were diminished following BCT in female soldiers. Fe status indicators were predictive of physical performance, and the decrement in sTfR observed during the training period correlated with 2-mile (3·2 km) run time. These findings indicate that Fe status is affected by training in female soldiers and that degraded Fe status during training affects aerobic performance. Future studies should aim to determine the cause of degraded Fe status during training periods in athletes and soldiers, to include the relative contribution of changes in plasma volume, dietary Fe intake and the effects of inflammation. Preventing the decrement in Fe status that occurs during training periods may be essential to optimise physical performance in female soldiers, athletes and workers regularly engaged in physically demanding tasks.
Acknowledgements
First and foremost, the authors wish to acknowledge the soldier volunteers that participated in the present study as well as the command staff at Fort Jackson, South Carolina for allowing access to soldiers. The authors also wish to acknowledge Dr Jennifer Rood and her staff at the Pennington Biomedical Research Center for their contributions to data collection and performance of Fe status indicator assays. Nancy Andersen, Louis Marchitelli, Nelson Morales-Martinez, Michael Stanger and Sgt Tyson Tarr all made significant technical contributions to the experiments detailed in this paper. J. P. M. contributed to study design, data collection and manuscript preparation. J. P. K., S. J. C. and K. W. W. participated in data collection, statistical analysis and study management. H. R. L. and A. J. Y. contributed to study design and manuscript preparation. The present study was supported by the US Army Medical Research and Materiel Command.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defense. Any citations of commercial organisations and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organisations.
Portions of this paper were presented in abstract form at Experimental Biology 2007, 28 April–2 May and NATO HFM-158, 13–15 October 2008.
The authors have no conflicts of interest to report.





