Anaemia is a major public health concern among women in several low-income countries(Reference Yasutake, He and Decker1). In 2011, the WHO estimated that nearly 528·7 million women globally were anaemic, mainly in South East Asian (42 %) and African regions (39 %)(2). Similarly, about 20·2 million women were severely anaemic, including 0·8 million pregnant women and 19·4 million non-pregnant women(2). The prevalence of moderate–severe anaemia is high in African countries(Reference Yasutake, He and Decker1).
Evidence suggests that specifying anaemia severity levels is important for proper intervention, particularly in settings that require strengthened control efforts to change the burden of anaemia to lower severity levels(Reference Kassebaum, Jasrasaria and Naghavi3,Reference Balarajan, Ramakrishnan and Özaltin4) . Ethiopia is one of the countries with a high burden of maternal undernutrition, which exceeds 20 %(5). A recent study indicates that undernutrition is even more worse in pregnant women and 38 % of them were undernourished(Reference Workicho, Belachew and Ghosh6). Findings from the 2016 Ethiopian Demographic Health Survey (EDHS) indicate that anaemia is a moderate public health problem, and all anaemia levels have increased from 2011 to 2016(7). Anaemia is a key indicator of poor health among women(Reference Bates, McKew and Sarkinfada8), and several health problems among women have been linked to anaemia, including the risk of maternal morbidity and mortality(Reference Lumbiganon, Laopaiboon and Intarut9), preterm birth(Reference Levy, Fraser and Katz10), low birth weight(Reference Levy, Fraser and Katz10) and perinatal death(Reference Little, Brocard and Elliott11). Moderate–severe anaemia could lead to high blood loss during delivery and the postpartum period(Reference Kavle, Stoltzfus and Khalfan12), which can be prevented by targeting the main factors of moderate–severe anaemia before and during pregnancy. Thus, preventing anaemia in women of reproductive age can improve maternal and perinatal health conditions(Reference Balarajan, Ramakrishnan and Özaltin4) and subsequent pregnancy and birth outcomes.
Worldwide, the main contributing factors for anaemia are dietary deficiencies, high parity and infections (e.g. geohelminths)(Reference Balarajan, Ramakrishnan and Özaltin4,Reference Lover, Hartman and Chia13) . Moreover, the risk factors of anaemia among women in low-income countries are varied, contextual and multifaceted. In sub-Saharan Africa, Fe deficiency, malaria, low economic status, illiteracy, multiparity and having an intestinal parasitic infection were the main predictors of anaemia in women of reproductive age(Reference Asres, Yemane and Gedefaw14). In Ethiopia, the analysis of previous EDHS data has indicated that wealth index, use of family planning, ANC use and breast-feeding for 2 years were factors associated with lower odds of having anaemia in lactating mothers(Reference Lakew, Biadgilign and Haile15) and most other studies have focused on pregnant women(Reference Kefiyalew, Zemene and Asres16–Reference Gebre and Mulugeta19). However, there is limited evidence assessing predictors for anaemia severity levels (mild, moderate or severe) among women of reproductive age in Ethiopia(Reference Asres, Yemane and Gedefaw14,Reference Haidar20) .
Additionally, the impact of different factors of anaemia among women of reproductive age has not been evaluated using population attributable fraction (PAF). PAF is an important tool to measure the impact of factors in the population. The PAF offer estimates of the proportion of anaemia cases that could be prevented if a particular factor was eliminated or at least reduced in the population. PAF takes into consideration the strength of the association between factors, the outcome of interest and the prevalence of the factors in the population(Reference Gefeller21). Thus, a high association between a disease and a factor might have a low population impact if the factor is rare. Conversely, a low association between a disease and a factor may have a high impact on public health if the factor is common(Reference Rose22). In this sense, the PAF is important for understanding the public health impact of the factors in the population and can assist in prioritising public health intervention strategies(Reference Rezende and Eluf-Neto23). PAF are useful for indicating where preventive efforts should be focused to achieve the greatest potential reductions in the outcome of interest(Reference Gerstman24).
The aim of this study was to identify independent factors for different severity levels of anaemia among Ethiopian women through a multivariable multinomial logistic regression model. This study also aimed to quantify the PAF to understand the relative contribution of different factors to the occurrence of anaemia using a large-scale, population-based cross-sectional study.
Methods
Study design and setting
This study used data collected in the 2016 EDHS, which was a population-based cross-sectional study(7) conducted to provide the latest estimates of key demographic and health.
Indicators
The 2016 EDHS data sets are publicly available from MEASURE DHS (measuredhs.com).
Sampling and sample size
The 2016 EDHS used a two-stage cluster sampling technique. The first stage involved selecting 645 clusters (primary sampling units) with probability proportional to the size (the number of households in the cluster). The second stage involved the systematic sampling of households from the selected clusters. A sample of 18 008 households was then selected from the clusters. Of this, 16 650 households were successfully interviewed, with 16 583 eligible women identified for individual interviews. A total of 15 683 women aged 15–49 years were interviewed, and haemoglobin (Hgb) levels were measured for 14 923 of them(7). Data collection took place from 18 January to 27 June 2016 (~5 months).
Measurements
HemoCue was used to measure Hgb levels of women, and all Hgb values were adjusted for altitude and smoking status. Different anaemia levels were defined using WHO cut-off points(25). Pregnant women with a Hgb value < 11 g/dl and non-pregnant women with a Hgb level < 12 g/dl were considered to have anaemia. Similarly, anaemia was classified according to its severity levels as severe (Hgb value < 7 g/dl) and moderate (7·0–9·9 g/dl) in all women. Mild anaemia was classified as a Hgb level of 10·0–10·9 g/dl in pregnant women and a Hgb level of 10·0–11·9 g/dl in non-pregnant women based on WHO recommendations(25). For this study, anaemia was grouped into three categories: (1) non-anaemic; (2) mild anaemia and (3) moderate–severe anaemia. Due to the small number of severe anaemia cases, moderate and severe anaemia cases were merged into a single category for analysis purpose.
The determinant (exposure) variables included for analysis were age (15–24, 25–34 and 35–49 years), educational status (no formal education, primary, secondary and tertiary), marital status (single, married/living together and divorced/widowed), place of residence (urban and rural), wealth index (poorest, poorer, middle, richer and richest), the number of children ever born (0, 1–3 and ≥4), births in the last 5 years (0 or 1–2), currently pregnant (at the time of the survey: pregnant and not sure/non-pregnant), currently breastfeeding (yes and no), current contraceptive use (yes and no), toilet facility (improved and unimproved) and water source (improved and unimproved).
Statistical analysis of the data
All analyses were conducted using Stata version 14 (Stata Corp, stata.com). Complex sample analysis methods were used(Reference Lee and Forthofer26,27) , which took into consideration the DHS sampling design by incorporating the sampling frame information (primary sampling units and strata) and weights in all analyses, presented as percentages with 95 % CI. Using Stata, the survey analysis module commands [SVY] were used to account for the complex sampling design, including the sampling weight. The prevalence rate of anaemia (any, mild and moderate–severe) was estimated in terms of different factors such as residence, education status, age, wealth index and gravidity.
The independent predictors of mild and moderate–severe anaemia were identified using a multinomial logistic regression model, with non-anaemia used as the reference. A multinomial regression model was selected for the analysis when it was determined that the assumption of proportional odds was not satisfied for an ordinal logistical model, as recommended by Hosmer and Lemeshow(Reference Hosmer and Lemeshow28). Moreover, we wanted to estimate a separate coefficient for each category (mild and moderate–severe) of the outcome, using one category (non-anaemia) as the reference. In this regard, the multinomial model was suitable for estimating the coefficient for each category of the outcome variable. Thus, multinomial logistic regression could enable the extraction of more information from the data and prevent the loss of information due to the collapsing of categories. In addition, the multinomial logistic regression had further advantages: (1) it does not assume a linear relationship between the dependent variable and independent variables, and (2) normally distributed error terms are not assumed(Reference Hosmer and Lemeshow28).
Given the hierarchical structure of the sample, a multivariable multilevel logistic model was initially applied to assess the associations between different factors and any anaemia (yes and no) among women in Ethiopia. Subsequently, multilevel multinomial logistic regression models were used to identify independent predictors for different levels of anaemia (mild and moderate–severe compared with no anaemia). The AOR with 95 % CI was computed using generalised structural equation models (Stata command GSEM)(29,Reference Rabe-Hesketh and Skrondal30) , which allows for the fitting of complex models and takes into consideration the hierarchical structure of the data(Reference Garson31). A P < 0·05 was used as a measure of statistical significance in the final model. The random effects (variation of effects) were measured by a variance partition coefficient(Reference Merlo, Chaix and Ohlsson32), which measures the cluster variability in the multi-level models.
PAF were estimated to assess the contribution of each factor to the occurrence of anaemia (any, mild, moderate–severe) among women. PAF were estimated by using AOR from the multivariable logistic regression model for each variable that was significantly associated with anaemia (any, mild, moderate–severe). Estimating attributable fractions using logistic regression analysis was initiated by Bruzzi et al.(Reference Bruzzi, Green and Byar33), developed by Eide and Gefeller(Reference Eide and Gefeller34), and was operationalised by Rückinger and colleagues(Reference Rückinger, Kries and Toschke35). Ideally, risk ratios would be used to estimate PAF. However, the OR calculated from cross-sectional and case-control studies can also be used to compute PAF(Reference Gerstman24) when risk ratios are not appropriate or available. Thus, in this study, OR was used to estimate the PAF because EDHS was a cross-sectional study and OR was calculated through logistic regression.
The PAF were calculated using the following formula(Reference Gerstman24), which has been used in different studies(Reference Agho, Ezeh and Issaka36–Reference Issaka, Agho and Ezeh38); all of which estimated PAF using AOR from a cross-sectional or case-control study.
PAF = P (AOR−1)/AOR
where P is the proportion of women with factors among cases (ratio of exposed cases relative to a total number of cases), and AOR is the adjusted OR (the association between factors and anaemia).
Results
Sociodemographic characteristics and prevalence of anaemia
The proportion of mild and moderate–severe anaemia among women was estimated to be 17·8 % (95 % CI 16·7, 19·0 %) and 5·8 % (95 % CI 4·9, 6·8 %), respectively (Table 1). A higher prevalence rate of any anaemia was observed among women who had higher gravidity (≥4) (28·8 % (95 % CI 26·7, 30·9 %)) compared with women who had no previous births (18·2 % (95 % CI 16·4, 20·1 %)). The prevalence of any anaemia was significantly greater in pregnant women (29·1 % (95 % CI 24·9, 33·7 %)) than non-pregnant women (23·2 % (95 % CI 21·6, 24·9 %)). Similarly, a higher prevalence of moderate–severe anaemia was observed in pregnant women (12·6 % (95 % CI 10·0, 15·8 %)) compared with non-pregnant women (5·3 % (95 % CI 4·4, 6·3 %)) (Table 1)
Table 1 Characteristics of the study population and prevalence of various levels of anaemia among Ethiopian women aged 15–49 years, 2016
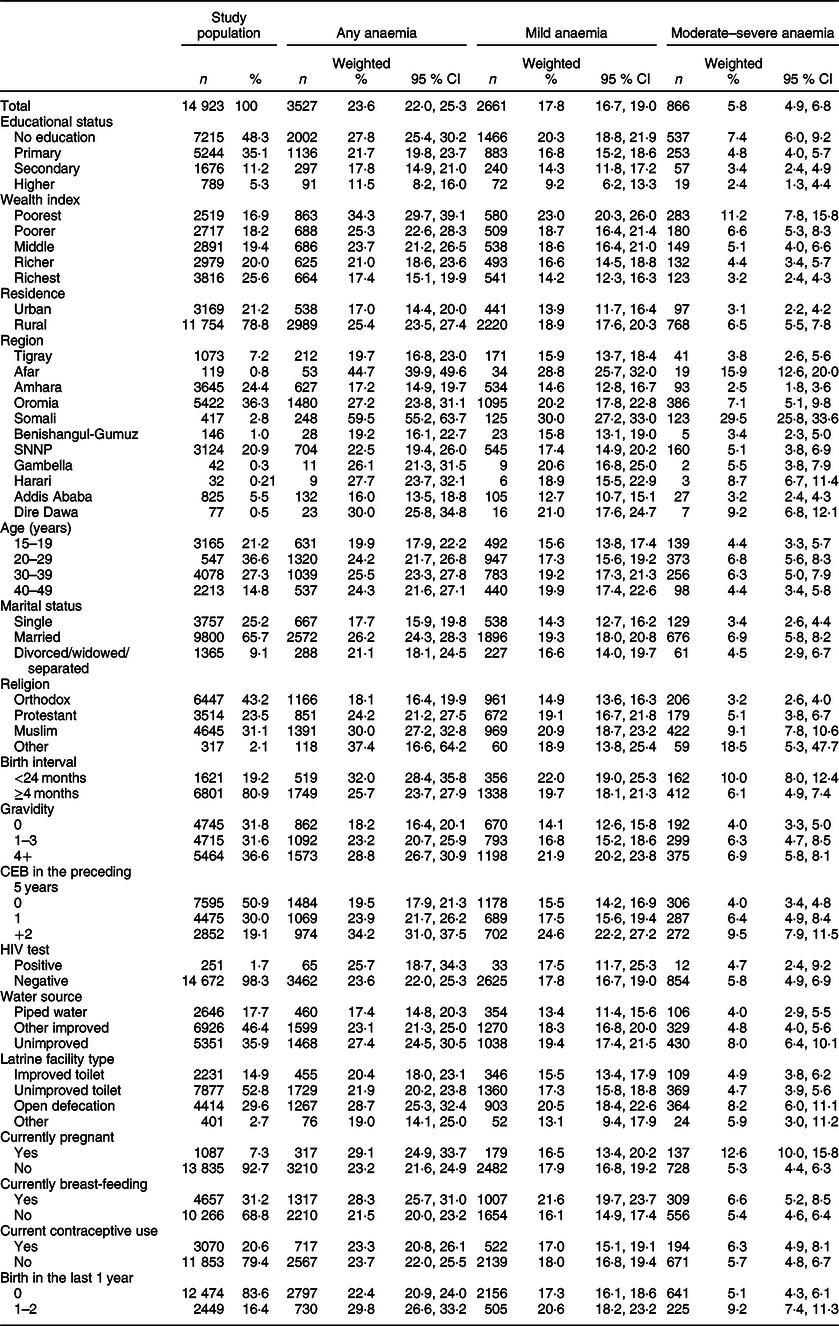
n, number; SNNP, Southern Nations Nationalities and People; CEB, Children Ever Born.
Multivariable analysis of factors
Factors for any anaemia among women
The multilevel logistic regression results show that having no formal education (AOR = 1·37; 95 % CI 1·10, 1·72), having only a primary education (AOR = 1·24; 95 % CI 1·00, 1·53), being of rural residence (AOR = 1·29; 95 % CI 1·02, 1·63), being in the poorest wealth quantile (AOR =1·29; 95 % CI 95 % CI 1·01, 1·60), having higher gravidity (≥4 births; AOR = 1·39; 95 % CI 1·13, 1·69), being HIV-positive (AOR = 2·11; 95 % CI 1·59, 2·79), currently breast-feeding (AOR = 1·09; 95 % CI 1·03, 1·28), having menstruated in the last 6 weeks (AOR = 1·1; 95 % CI 1·01, 1·23) and open defecation (AOR = 1·18 ; 95 % CI 1·00, 1·39) were all significantly associated with the occurrence of any anaemia among women of reproductive age after adjusting for all other variables in the model. Current contraceptive use (AOR = 1·01; 95 % CI 0·91, 1·11) and unimproved water source (AOR = 1·03; 95 % CI 0·83, 1·27) were not significantly associated with any anaemia (Table 2).
Table 2 Adjusted OR from multilevel logistics regression and population attributable fractions for factors associated with any anaemia among women in Ethiopia, 2016
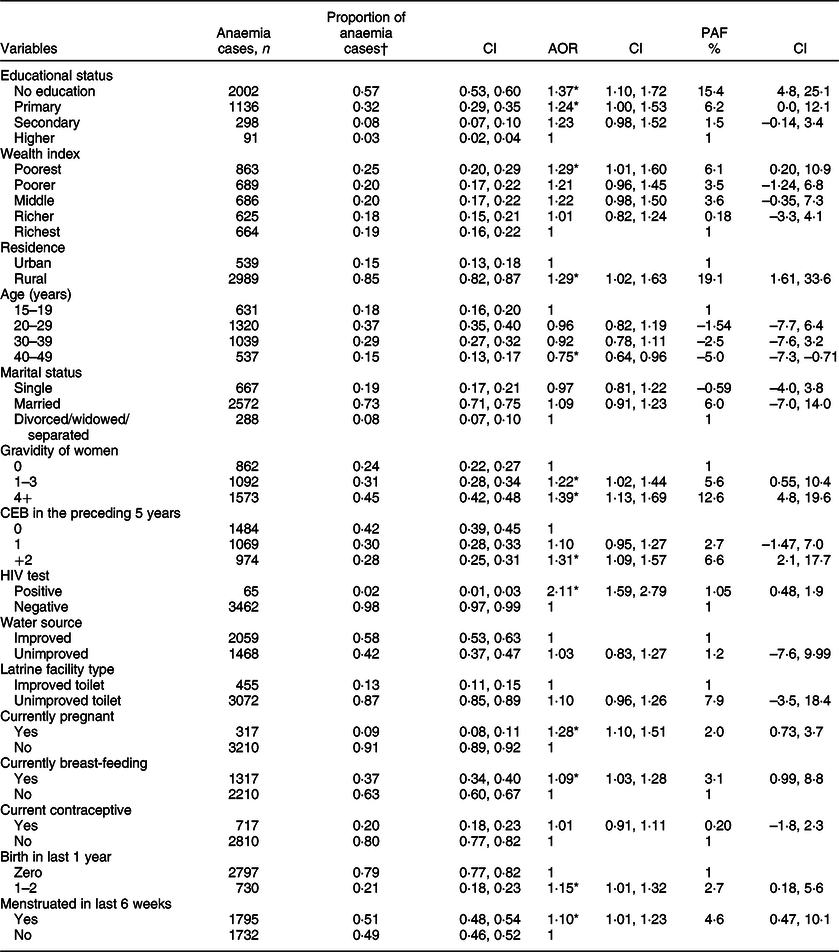
PAF, population attributable fractions.
*P < 0·05.
† Proportion of anaemic women exposed to a factor (ratio of exposed cases to total cases).
Population attributable fractions/proportions
Around 15·4 % (95 % CI 4·8, 25·1 %) of anaemia in women from the study population is attributable to having no formal education. About 19 % (95 % CI 1·61, 33·6) of anaemia cases among women of reproductive age might be attributable to being of rural residence. Around 6 % of anaemia cases could be attributable to being in the poorest wealth quantile. Furthermore, the proportion of anaemic cases in the study population that could be attributed to having access to an unimproved latrine facility was estimated to be 8 % (95 % CI −3·5, 18·4 %). Similarly, an estimated 3 % (95 % CI 0·99, 9·9 %) of cases of anaemia among women could be attributed to being currently breast-feeding. Around 13 % (95 % CI 4·8, 19·6 %) of anaemia cases were attributable to having higher gravidity (≥4) (Table 2).
Factors associated with mild anaemia and moderate–severe anaemia
The results of the multilevel multinomial logistic regression analyses of factors for mild anaemia and moderate–severe anaemia are presented in Table 3.
Table 3 Adjusted OR (AOR) from multilevel multinomial logistics regression, and population attributable fraction for factors associated with mild and moderate–severe anaemia among women in Ethiopia, 2016
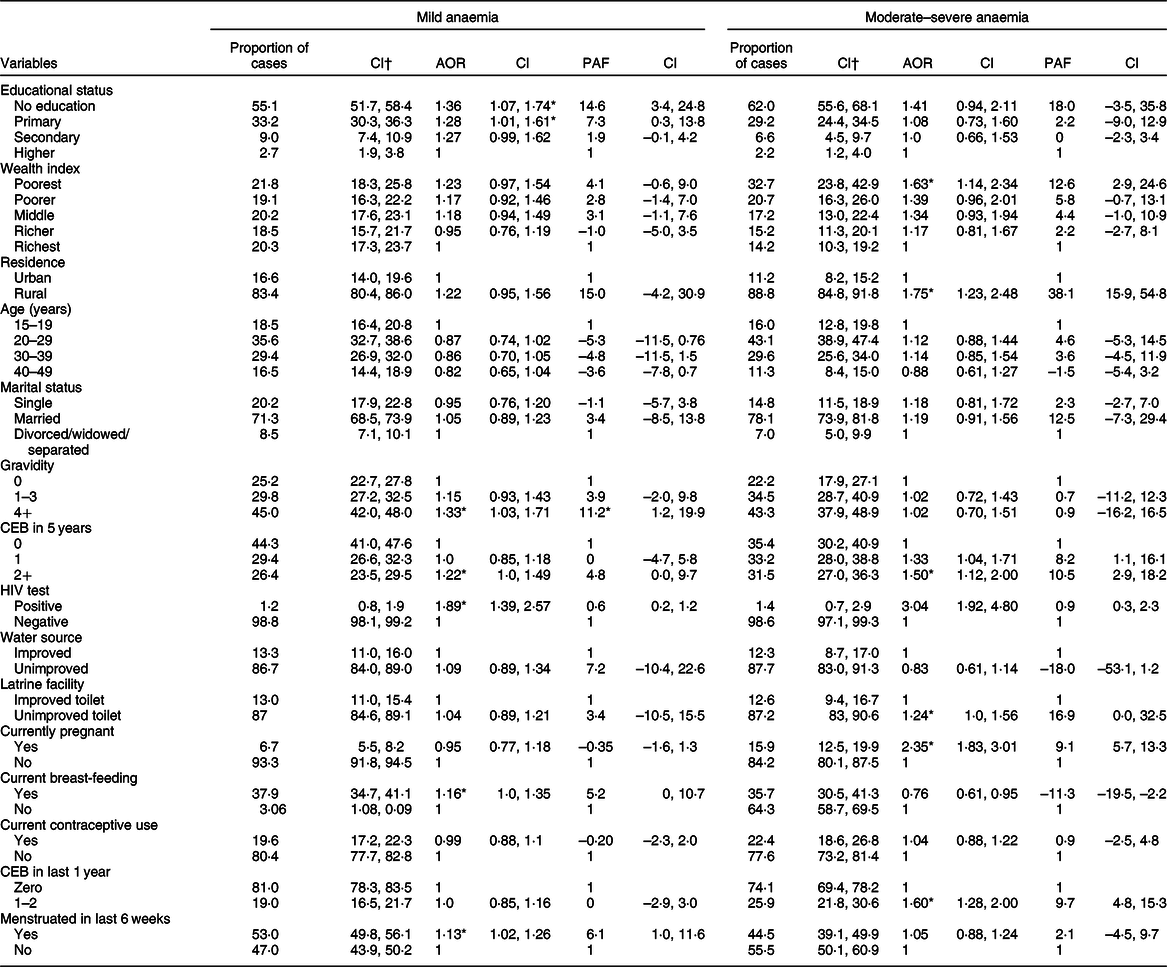
PAF, Population Attributable Fractions; CEB, Children Ever Born.
* P < 0·05.
† Proportion of anaemic women exposed to factors (ratio exposed cases to total cases).
Multivariable multinomial analysis shows that having no formal education (AOR = 1·36; 95 % CI 1·07, 1·74), breast-feeding (AOR = 1·16; 95 % CI 1·0, 1·35), higher gravidity of women (≥4 births; AOR = 1·33; 95 % CI 1·03, 1·71), HIV infection (AOR = 1·89; 95 % CI 1·39, 2·57) and menstruation in the last 6 weeks (AOR = 1·13; 95 % CI 1·02, 1·26) were factors independently associated with mild anaemia. Similarly, rural residence (AOR = 1·75; 95 % CI 1·23, 2·48), birth in the last year (AOR = 1·60; 95 % CI 1·28, 2·0), birth in the last 5 years (AOR = 1·50; 95 % CI 1·12, 2·0), currently pregnant (AOR = 2·35; 95 % CI 1·83, 3·01), unimproved latrine facility (AOR = 1·24; 95 % CI 1·0, 1·56) and poorest wealth index (AOR = 1·63; 95 % CI 1·14, 2·34) were independently associated with moderate–severe anaemia (Table 3).
Population attributable fractions
The proportions of mild and moderate–severe anaemia attributable to having no formal education were estimated to be 14·6 and 18 % among women, respectively. Proportions of moderate–severe anaemia attributable to current pregnancy were 9·1 % (Table 3). Being a rural resident was attributable to 38·1 % of moderate–severe anaemia cases among women. In addition, 12·6 % of moderate–severe anaemia cases might be attributable to household wealth being rated in the poorest wealth quantile. The proportion of mild anaemia cases attributable to high gravidity of women (≥4) and currently breast-feeding was 11·2 % and 5·2 %, respectively. Similarly, 16·9 and 10·5 % of moderate–severe anaemia cases among women were due to unimproved latrine facilities, birth in the last 5 years preceding the survey, respectively (Table 3).
Random effects analysis
The results of random effect analysis showed that there was variation in log odds of different types of anaemia across communities (τ 2 = 0·907). According to the variance partition coefficient, about 21·6 % of the variance in the odds of different anaemia types in women could be attributed to community-level factors. The variation in odds of different anaemia types across communities remained statistically significant even after adjusting for individual- and community-level factors. About 9 % of the odds of anaemia variation across communities was observed in the full model (model 2) (Table 4).
Table 4 Measure of variation for different anaemia types at the cluster level (effect of variation from the random intercept model)
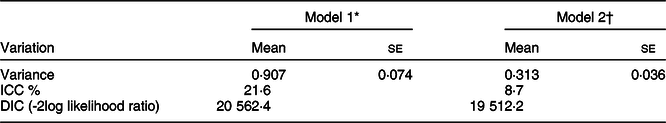
ICC, Intra-cluster Correlation Coefficient; DIC, Deviance Information Criterion.
* Empty model (without the predictors).
† Adjusted for predictor factors.
Discussion
This study assessed the impact of predictors on all levels of anaemia among women using large-scale population data. The findings indicate that having no formal education, currently breast-feeding, having higher (≥4) gravidity, having HIV infection and having menstruation in the past 6 weeks preceding the survey were factors independently associated with higher odds of mild anaemia. Similarly, living in a rural area, having an unimproved latrine facility, giving birth in the past year, being currently pregnant, being of the poorest wealth status and having a HIV infection were independently associated with moderate–severe anaemia. Moreover, having rural residency, no formal education, high gravidity, being in the poorest wealth quantile and having unimproved latrine facilities were positively associated with the occurrence of any anaemia among women. These results are consistent with other study findings from low-income countries, which reported associations between socio-economic status and all anaemia levels(Reference Bentley and Griffiths39–Reference Perumal41).
A relevant question to ask is how much of the burden of anaemia could be avoided if these factors were addressed, or reduced, in the population. PAF is a measure of the overall effect of a factor on the problem/outcome of interest at the population level(Reference Rezende and Eluf-Neto23). The PAF estimates of this study showed that the proportion of mild anaemia cases attributable to high gravidity (≥4), currently breast-feeding and menstruation in the last 6 weeks was 11·2 %, 5·2 and 6·1 %, respectively, and could potentially be reduced if these factors are addressed in the population. Similarly, the proportion of moderate–severe anaemia attributable to living in a rural area, having no formal education, being in the poorest wealth quantile and giving birth in the last 5 years was 38·1, 18, 12·6 and 10·5 %, respectively. The higher proportion of any anaemia cases could be attributed to rural residency (19 %), having no formal education (15 %), high gravidity (13 %), poorest wealth quantile (6 %) and having unimproved latrine facilities (8 %). These are theoretical calculations, but they illustrate the important role these factors play in determining the occurrence of anaemia among women in this population. Thus, rural residence, illiteracy and being in a low wealth quantile would be predicted to have a substantial effect on the occurrence of anaemia among women in Ethiopia. Similar findings have been reported in other studies from developing countries, in that high proportions of anaemia cases were observed in women of rural residence, with low education and being in a low wealth quantile(Reference Ganapathi and Kumar42,Reference Balarajan, Fawzi and Subramanian43) .
Research has recognised low socio-economic status as a predictor of higher odds of anaemia among reproductive-age women(Reference Asres, Yemane and Gedefaw14,Reference Kamruzzaman, Rabbani and Saw44,Reference Siddiqui, Goli and Reja45) . A higher prevalence of anaemia was observed in women with low socio-economic status compared with women with high socio-economic status(Reference Sadeghian, Fatourechi and Lesanpezeshki46,Reference Wirth, Woodruff and Engle-Stone47) . Our results are in line with these findings and indicated that women from households in the poorest wealth quantile were at higher risk of having moderate–severe anaemia. About 13 % of moderate–severe anaemia cases among women could be attributed to being in the poorest wealth quantile; this is consistent with a previous finding that showed that higher odds of moderate–severe anaemia cases were associated with the poorest wealth quantile(Reference Wilunda, Massawe and Jackson48). Moreover, the current study revealed that the odds of any anaemia among women was higher among the poorest compared with the richest women, which aligns with study findings in Bangladesh(Reference Kamruzzaman, Rabbani and Saw44,Reference Ghose, Tang and Yaya49) and Ethiopia(Reference Asres, Yemane and Gedefaw14). A possible explanation is that, as the income level of women is lower, the women may not be able to purchase adequate or varied foodstuffs and subsequently do not consume a diversified nutritious diet(Reference Lenz, Olinto and Dias-da-Costa50). As a result, women are unable to obtain adequate nutrients and are subsequently exposed to anaemia. Furthermore, women with no formal education are at higher odds of anaemia compared with women with a tertiary education. The proportion of any anaemia attributable to having no education was nearly 15 %. This result is consistent with previous study results conducted in Ethiopia(Reference Asres, Yemane and Gedefaw14), Timor-Leste(Reference Lover, Hartman and Chia13), India(Reference Ganapathi and Kumar42,Reference Balarajan, Fawzi and Subramanian43) , Bangladesh(Reference Kamruzzaman, Rabbani and Saw44,Reference Ghose, Tang and Yaya49) and Senegal(Reference Diégane, Faye Adama and Khadim51), which reported that low educational status was associated with an increased odds of anaemia among women. Evidence has also shown that low educational status is associated with higher odds of moderate–severe anaemia among women(Reference Ngnie-Teta, Kuate-Defo and Receveur40,Reference Wilunda, Massawe and Jackson48) . The effect of low education on the odds of anaemia could be due to the low capacity to pursue health care and limited awareness about diversified food intake or health risks.
The odds of any anaemia and moderate–severe anaemia were higher in rural compared with urban women, but the odds of mild anaemia were not statistically different between urban and rural women. The odds of any anaemia and moderate–severe anaemia attributable to rural residence are 19 and 38 %, respectively, which is consistent with evidence, suggesting that higher moderate–severe anaemia prevalence was more noticeable in individuals of rural residence(Reference Perumal41,Reference Ghose, Tang and Yaya49) . A higher prevalence of moderate–severe anaemia and any anaemia in rural areas could be attributed to differences in availability and utilisation of health care, diversified foods, infection risk and fertility preferences(Reference Balarajan, Ramakrishnan and Özaltin4,Reference Bentley and Griffiths39) . Moreover, having unimproved latrine facilities was associated with an increased odds of any anaemia and moderate–severe anaemia among women. This result is in line with other study findings from low-income settings(Reference Ganapathi and Kumar42,Reference Wilunda, Massawe and Jackson48,Reference Diégane, Faye Adama and Khadim51) . This may be partially explained by the fact that unimproved toilet facilities could decrease sanitation standards and increase the risk of intestinal infections(Reference Freeman, Garn and Sclar52), which are risk factors for anaemia(Reference Shaw and Friedman53).
The results of this study also revealed that higher gravidity of women was associated with increased odds of any anaemia and mild anaemia. Similarly, a higher number of births in the previous 5 years, as well as in the past year, was associated with an increased odds of moderate–severe anaemia among women. A similar finding was reported that moderate–severe anaemia was higher in women who had more births in the last 5 years(Reference Perumal41) and who had given birth in the previous year(Reference Lover, Hartman and Chia13). Similarly, studies from Ethiopia(Reference Asres, Yemane and Gedefaw14), Myanmar(Reference Win and Ko54), Iran(Reference Sadeghian, Fatourechi and Lesanpezeshki46) and India(Reference Siddiqui, Goli and Reja45) have reported that higher gravidity was associated with a higher odds of anaemia among women of reproductive age. This could be the fact that repeated pregnancies/higher gravidity reduces Fe stores, which leads to anaemia(Reference Perumal41,Reference Sadeghian, Fatourechi and Lesanpezeshki46,Reference Win and Ko54) .
Menstruation in the 6 weeks preceding the survey was associated with increased odds of anaemia among women. Similar results have been documented in other studies, in which high menstruation blood loss was associated with an increased odds of anaemia among women of reproductive age(Reference Asres, Yemane and Gedefaw14,Reference Ganapathi and Kumar42) . This could be the fact that excessive blood loss is linked directly to a depletion of Fe stores and leads to anaemia(Reference Harvey, Armah and Dainty55). The infection by HIV might also increase the risk of anaemia due to its effects on the bone marrow and the reduction of Hgb levels in the blood(Reference Kokici, Harxhi and Como56). However, our results indicate that HIV prevalence in women of reproductive age in Ethiopia is estimated to be about 1·7 % and was not the major contributor to anaemia(Reference Asres, Yemane and Gedefaw14).
Strengths and limitations of the study
One of the main strengths of this study is the large representative population-based survey data. The other strength of this study is the identification of predictors for different levels of anaemia among women of reproductive age using multilevel multinomial models. Furthermore, in this study, the relative contribution of each factor for the occurrence of anaemia among women was quantified through PAF, which will help to prioritise any intervention programmes. The PAF were estimated using AOR to obtain unbiased estimates(Reference Benichou57).
This study sets a benchmark for assessing the impact of the predictors at the population level, using large population-based cross-sectional data. However, the analyses and estimates of PAF for assessing the impact of factors depend on assumptions that cannot be addressed, including the potential for unincorporated factors and remaining confounding or non-causal relations. Since this study is an observational study, temporal and cause–effect relationships cannot be established. Recall bias might also be an issue for this study, as the DHS data relied on the memory of study participants. This error could inflate/deflate some of the estimates reported in this study. Another limitation of this study was the lack of data on some important factors in anaemia, such as dietary intake and helminthic infections.
Conclusion
The current study indicated that having no formal education, currently breast-feeding and higher (≥4) gravidity were factors associated with increased odds of mild anaemia among women. Furthermore, higher odds of moderate–severe anaemia were observed among women who were rural residents, had an unimproved latrine facility, gave birth in the past year, were currently pregnant, were in the poorest wealth quantile or were infected with HIV.
The PAF suggest that rural residency, low education, low wealth status, pregnancy and high gravidity contribute substantially to the occurrence of anaemia among women. The burden of different levels of anaemia among women in the population could potentially be reduced by employing a large range of approaches targeting these maternal and sociodemographic factors. Therefore, pregnant women, women with high gravidity and those with recent births, women with low education, low wealth status and women living in rural areas should be prioritised in any intervention programme targeting anaemia. Different approaches would be needed when targeting mild and moderate–severe anaemia. Mild anaemia could be reduced by setting intervention strategies targeting multigravida and breast-feeding women, while preventing moderate–severe anaemia may require working on improving income, educating women and improving living conditions through the accessibility of hygienic latrines. Further research needs to be conducted to identify potential factors associated with mild anaemia by incorporating data on dietary intake and infections (malaria and helminths).
Acknowledgements
Acknowledgements: The authors would like to thank the DHS Program for allowing us to use the EDHS data set. The authors also grateful to Miss Natalia Soeters and Peta Forder for their substantial help in language editing and reviewing the manuscript, respectively. Financial support: None. Conflict of interest: The authors declare that they have no conflicting interests. Authorship: Conceptualisation designing the study; interpreting the results; and drafting, writing, reviewing and approving the final manuscript: K.T.K., C.C., D.L. and E.D. Analysing the data: K.T.K. Data sharing statement: All data relevant for this study are included in this article. Ethics of human subject participation: Publicly available EDHS 2016 data were used for this study. The study has also been approved by the University of Newcastle Human Research Ethics Committee (H-2018-0045).






