Annual bluegrass is a common weed of warm-season turfgrass during winter dormancy. Turfgrass managers rely on PRE or POST herbicide applications to selectively control annual bluegrass in warm-season turfgrass given that plants can produce as many as 185,000 viable seeds in the top 2.5 cm of soil, which can germinate at day and night soil temperatures ranging from 19 and 10 C to 39 and 29 C (McElroy et al. Reference McElroy, Walker, Wehtje and van Santen2004; Watschke et al. Reference Watschke, Long and Duich1979). High fecundity and adaptability to different growing conditions, along with robust genetic diversity (Mao and Huff Reference Mao and Huff2012), likely couples with herbicide selection pressure to create herbicide-resistant annual bluegrass populations. To date, herbicide resistance has been documented in annual bluegrass more than it has in any other turfgrass weed (Heap Reference Heap2016). Annual bluegrass populations evolving resistance to herbicidal inhibitors of mitosis (e.g., prodiamine), photosystem II (e.g., simazine), acetolactate synthase (e.g., trifloxysulfuron), or enolpyruvylshikimate-3-phosphate synthase (EPSPS) inhibitors (e.g., glyphosate) have all been documented in managed turfgrass systems (Binkholder et al. Reference Binkholder, Fresenburg, Teuton, Xiong and Smeda2011; Isgrigg et al. Reference Isgrigg, Yelverton, Brownie and Warren2002; Kelly et al. Reference Kelly, Coats and Luthe1999; McElroy et al. Reference McElroy, Flessner, Wang, Dane, Walker and Wehtje2013).
Multiple resistance results when weeds have more than one resistance mechanism that confers resistance to multiple herbicides within the same or different modes of action (Vencill et al. Reference Vencill, Nichols, Webster, Soteres, Mallory-Smith, Burgos, Johnson and McClelland2012). To date, there has only been a single reported case of annual bluegrass evolving multiple resistance in managed turfgrass: a phenotype resistant to simazine and trifloxysulfuron (Brosnan et al. Reference Brosnan, Breeden, Vargas and Grier2015). Genetic sequencing of these annual bluegrass plants revealed a target site mutation (Ser-264-Gly) on the D1 protein that is known to confer resistance to photosystem II–inhibiting herbicides, as well as a new mutation of acetolactate synthase (Ala-205-Phe) that conferred broad spectrum resistance to imidazolinone, sulfonylurea, triazolopyrimidines, sulfonylamino-carbonyl-triazolinones, and pyrimidinyl (thio) benzoate herbicides (Brosnan et al. Reference Brosnan, Vargas, Breeden, Grier, Aponte, Tresch and LaForest2016).
Recent research has found mixing herbicides with different modes of action to be more effective in delaying herbicide resistance than rotating between herbicides with different modes of action over time. However, herbicide mixture components must have efficacy against the target weed individually in order to decrease survival probabilities of the plants and reduce the frequency of resistance alleles in the overall weed population (Evans et al. Reference Evans, Tranel, Hager, Schutte, Wu, Chatham and Davis2016).
In the spring of 2012, poor annual bluegrass control (<50%) was reported in golf course roughs in Alcoa, Tennessee (35.75°N, 83.88°W) following treatment with a tank mixture of prodiamine (1,120 gha−1) and glyphosate (840 gaeha−1) during hybrid bermudagrass dormancy. This application had been made at this location for over ten consecutive years without rotation (JD Murr, personal communication). We hypothesized that annual bluegrass at this location had evolved resistance to both prodiamine and glyphosate. Therefore, our objectives were 1) to determine if this annual bluegrass phenotype was resistant to both prodiamine and glyphosate, and 2) to determine the efficacy of herbicide mixtures for controlling this phenotype in the field.
Materials and Methods
Plant Collection
One hundred annual bluegrass plants were mechanically harvested from golf course roughs (Alcoa, TN; 35.75°N, 83.88°W) using a Hound Dog weeder (Weed Hound, The Ames Companies Inc., Camp Hill, PA) on March 18, 2014, and transplanted in a glasshouse in Knoxville, Tennessee (35.56°N, 83.56°W). Considering that annual bluegrass is a self-pollinated species (Ellis Reference Ellis1973), harvested plants were given a unique identifier and clonally propagated into a minimum of four single-tiller samples per harvested plant. Tillers were established in 164-cm3 cone-tainers (SC10 Super Cell Cone-tainer, Steuwe & Sons, Tangent, OR) filled with peat moss growing medium (Growing Mix #2, Conrad Fafard, Inc., Agawam, MA). The unique identifier allowed for screening experiments to be conducted using tillers directly associated with a specific plant harvested from the field site. This process produced 890 individual tillers from 100 whole plants. After transplanting, all plant material remained in a glasshouse with day and night temperatures averaging 29 and 19 C, respectively. Plants received irrigation three times a day from an overhead misting system in order to maintain adequate moisture and prevent drought stress. Plants were maintained at an approximate height of 4 cm by cutting with scissors weekly. Nutrients were supplied at a rate of 24 kg N ha−1 every 14 d using a complete fertilizer (20-20-20 Soluble Fertilizer with Minor Elements, Southern Agricultural Insecticides Inc., Hendersonville, NC).
Screening for Resistance Within the Sampled Population
Screening experiments were conducted in the aforementioned glasshouse in 2014 to determine the percentage of annual bluegrass plants in the sampled population that were resistant to prodiamine, glyphosate, or both.
Glyphosate Resistance
On April 29, 2014, clonal tillers of all 100 plants harvested from the field site were treated with glyphosate at 840 gha−1 using a single flat-fan nozzle (8004EVS TeeJet®, Spraying Systems Co., Wheaton, IL) in an enclosed spray chamber (Generation III Research Sprayer, DeVries Manufacturing, Hollandale, MN) calibrated to deliver 215 L ha−1. Plants were at least a single tiller in size when herbicide was applied, and the plants were maintained under the previously described glasshouse conditions. Annual bluegrass response to glyphosate was visually assessed on a 0% (no injury) to 100% (complete necrosis) scale relative to a non-treated check 3 wk after treatment. Plants injured ≤30% were placed in a glyphosate-resistant group, while those injured ≥70% were classified as susceptible.
Prodiamine Resistance
Tillers of all 100 plants harvested from the field site were screened for prodiamine resistance using hydroponic methods of Brosnan et al. (Reference Brosnan, Reasor, Vargas, Breeden, Kopsell, Cutulle and Mueller2014) in the previously described glasshouse. Tillers were transplanted into polyethylene containers (Roughneck®, Rubbermaid Commercial Products LLC, Winchester, VA) filled with 10 L of full-strength Hoagland solution (Hoagland and Arnon Reference Hoagland and Arnon1950) aerated with a blower (VB-007S, Sweetwater, Ft. Collins, CO) and air stones (HAGEN Elite 1” Cube Air Stone, Rolf C. Hagen Corp., Mansfield, MA). Ten holes (0.4 cm diameter) were drilled into the lid of each container at a 5.3 cm spacing. One hundred individual annual bluegrass tillers were transplanted into these holes on April 29, 2014. Root length of all tillers was trimmed to 5 cm before transplanting, and all 100 tillers were placed into containers with roots submerged into the nutrient solution containing 0.04 mM prodiamine. In a previous study, a prodiamine concentration of 0.04 mM was required to reduce the rooting of annual bluegrass from this field location by 50%, compared to a concentration of 2.8×10−6 mM for a known susceptible phenotype of annual bluegrass (Brosnan et al. Reference Brosnan, Reasor, Vargas, Breeden, Kopsell, Cutulle and Mueller2014). Therefore, all 100 plants in this experiment were exposed to 0.04 mM prodiamine in hydroponic culture to identify prodiamine-resistant and -susceptible individuals within the population sampled from the field site. At 10 days after treatment (DAT), all tillers were harvested and root length was measured. Root growth beyond the 5 cm benchmark was used to classify plants as likely resistant to prodiamine, while root length of 5 cm or less resulted in plants being classified as likely susceptible to prodiamine.
Confirming Resistance in Progeny
Research was conducted in the aforementioned glasshouse in 2015 to confirm resistance to prodiamine and glyphosate in the progeny of plants that survived treatment with both of these herbicides during the screening experiments. In all confirmation experiments, irrigation was supplied via an overhead misting system three times a day. Once non-treated plants reached 2.5 cm in height, nutrients were supplied at a rate of 24 kg N ha−1 every 14 d using a complete fertilizer. Maximum and minimum air temperature conditions in the glasshouse during confirmation experiments averaged 30 and 20 C, respectively. Chlorantraniliprole (5-bromo-N-[4-chloro-2-methyl-6-(methylcarbamoyl)phenyl]-2-(3-chloropyridin-2-yl)pyrazole-3-carboxamide; Acelepryn® Turf Insecticide, Syngenta Professional Products, Greensboro, NC) was applied at 0.23 kgha−1 on an as-needed basis to control insect pests. Annual bluegrass control in all experiments was visually assessed on a 0% (no control) to 100% (complete necrosis) scale relative to a non-treated check 35 DAT in prodiamine experiments and 21 DAT in experiments evaluating responses to glyphosate. Once all visual control data were collected, aboveground biomass was measured by harvesting all tissue above the soil line. Plant shoots were then placed in a drying oven at 35 C for 5 d and then weighed. Data are expressed as a percentage of the non-treated control.
Prodiamine Resistance
Seeds from plants exhibiting resistance to prodiamine in previously described screening experiments were collected and stored at −20 C during the spring of 2014. After a 4-mo vernalization period, greenhouse pots containing 1,049 cm3 of Sequatchie silt loam soil (fine-loamy, siliceous, semiactive, thermic Humic Hapludult) were surface seeded with prodiamine-resistant germplasm on February 5, 2015. Companion pots were surface seeded with prodiamine-susceptible germplasm (Penn State University, University Park, PA) on the same date. Immediately after seeding, pots were treated with prodiamine at 0; 105; 210; 420; 840; 1,680; 3,360; and 6,720 gha−1 using a previously described spray chamber. Treatments were arranged in a completely randomized design with four replications, and were repeated in time. Applications for the second experiment made on March 13, 2015.
Glyphosate Resistance
Seeds from plants exhibiting resistance to glyphosate in screening experiments were collected and stored as previously described for prodiamine. Cone-tainers filled with peat moss growing medium were seeded with glyphosate-resistant germplasm on October 30, 2014. An annual bluegrass phenotype known to be susceptible to glyphosate (Penn State University) was seeded in a similar manner on the same date. Cone-tainers were incubated under previously described conditions until plants developed a minimum of three tillers. Resistant and susceptible plants were treated with glyphosate at 0; 105; 210; 420; 840; 1,680; 3,360; and 6,720 gha−1 on February 19, 2015 using the previously described spray chamber calibrated to deliver 215 L ha−1. Treatments were arranged in a completely randomized design with four replications, and were repeated in time. Applications for the second experiment made on March 13, 2015.
A separate experiment was conducted to quantify shikimic acid accumulation after exposing resistant and susceptible tillers to glyphosate, similar to that performed by Mueller et al. (Reference Mueller, Massey, Hayes, Main and Stewart2003). Plants were treated with glyphosate at 420 gha−1 using the previously described spray chamber. Annual bluegrass aboveground tissue was harvested using scissors from each cone-tainer at 0, 1, 2, 3, and 6 DAT. Treatments were arranged in a completely randomized design with twenty-sprayed replications of each phenotype on each harvest date. In order to provide sufficient biomass for shikimic acid analysis, these twenty replicates were combined to produce four composite replicates of each phenotype on each harvest date. Aboveground plant tissue harvested 0, 1, 2, 3, and 6 DAT was stored in a freezer (−20 C) within an hour of harvest and remained there until shikimic acid was extracted and analyzed. All samples were extracted and analyzed within 34 d of harvest. Shikimic acid accumulation studies were initiated on February 10, 2015 and repeated on March 3, 2015. Maximum and minimum air temperature conditions in the glasshouse during the time the data were collected averaged 30 and 19 C, respectively.
Annual bluegrass tissue was ground with a mortar and pestle in liquid nitrogen and then weighed in centrifuge tubes. Five milliliters of 1M HCl was added per gram of tissue. The tubes were placed on a reciprocating shaker (Fisher Scientific, Hampton, NH) at 80 rpm for 16 h. HCl extractions were filtered through a 0.45-mm syringe filter into a 4-mL vial for liquid chromatography analysis. All samples were analyzed 1 d after extraction using a liquid chromatograph (Mueller et al. Reference Mueller, Barnett, Brosnan and Steckel2011) equipped with an ultraviolet detector (215 nm wavelength). Shikimic acid accumulation in plant tissue (mg shikimic acid per kg annual bluegrass fresh weight) was quantified and plotted over harvest intervals (DAT), with error bars representing standard error of the mean for each phenotype at each harvest interval.
Statistical Analysis of Confirmation Experiments
For all confirmation experiments, analysis of variance was performed using SAS® (SAS Institute, Cary, NC) to determine if data from both experimental runs could be combined. No significant interactions with experimental run were detected, allowing data to be combined and subjected to non-linear regression analysis in Prism (Prism 6.0 for Mac OS X, GraphPad Software, La Jolla, CA). Responses for resistant and susceptible annual bluegrass in all experiments were compared using a global sums-of-squares F-test at α=0.05.
Annual bluegrass control data were analyzed using the following non-linear regression equation:
In this equation, Rate50 represents the herbicide rate (X) at which 50% annual bluegrass control was reached. Bottom and Top represent asymptotes that were constrained to 0% and 100%, respectively, as annual bluegrass control could only range from 0% to 100%. K represents the slope of the best-fit line to model the response of resistant and susceptible phenotypes to increasing rates of herbicide.
All dry biomass data were analyzed using the following exponential decay non-linear regression equation:
In this equation, Y0 and Plateau were constrained to 100 and 0, respectively, as dry biomass only ranged from 0% to 100% of the non-treated check regardless of herbicide rate. K represented the slope of the best-fit line to model the response of each phenotype to increasing rates of herbicide (X). The herbicide rate required to reduce dry biomass to 50% of the non-treated (GR50) was calculated in Prism using the formula GR50=ln (2)/K.
Shikimic acid accumulation data were analyzed using the following one-phase exponential association non-linear regression equation:
The parameters of Equation 3 were similar to those of Equation 2, except that Y0 and Plateau were not constrained, and X represented d after glyphosate treatment at 420 gha−1. This approach allowed the maximum shikimic acid concentration accumulated in each phenotype to be calculated (i.e., Plateau). All data and regression analysis files are available at https://dx.doi.org/10.6084/m9.figshare.2066319.v5.
Efficacy of Herbicide Mixtures
Field research was conducted in golf course roughs located in Alcoa, TN (35.75°N, 83.88°W) with the objective of evaluating several herbicide mixtures for controlling an annual bluegrass phenotype with putative resistance to mitotic-inhibiting herbicides (e.g., prodiamine) and glyphosate. Soil at this location was a Dewey silty clay loam (fine, kaolinitic, thermic typic Paleudult), while the predominant turf species was hybrid bermudagrass mowed once weekly at 4.5 cm. The site received no supplemental irrigation, and no supplemental nutrition was applied during the experiment.
In order to effectively manage resistance, herbicide mixtures must contain active ingredients that can control the target weed when applied individually (Evans et al. Reference Evans, Tranel, Hager, Schutte, Wu, Chatham and Davis2016). Given that resistance to mitotic-inhibiting herbicides and glyphosate were both suspected, herbicide mixture treatments in the current study were designed using alternative modes of action. All mixture treatments evaluated in the current study contained herbicides with labeling for annual bluegrass control when applied individually. A complete list of herbicide mixture treatments and application rates is presented in Table 1. All treatments were mixed in water and applied using a CO2-pressurized boom sprayer equipped with 8002 flat-fan spray nozzles (Teejet®, Wheaton, IL) positioned to create a 1.2-m spray swath. In 2014, treatments were applied on October 20 to 1.5 by 2.4 m plots at a carrier volume of 281 L ha−1. In 2015, treatments were applied on October 21 to 1.5 by 2.1 m plots at a carrier volume of 374 L ha−1. Each year, annual bluegrass had emerged in the plots on the date of herbicide treatment, but the plants had not begun to tiller. In March and April of 2014 and 2015, annual bluegrass control was visually assessed on a 0% (no control) to 100% (complete necrosis) scale relative to a non-treated check. Assessment dates corresponded to 21 and 25 wk after treatment each year.
Table 1 Herbicide mixture treatments evaluated for control of annual bluegrass with suspected multiple resistance to glyphosate and prodiamine in hybrid bermudagrass golf course roughs in Alcoa, TN (35.75°N, 83.88°W) during 2014 and 2015.

a All herbicides were manufactured by Syngenta Professional Products (Greensboro, NC) and applied at maximum label rates for annual bluegrass control in managed turf.
Experimental design in both years of the study was a randomized complete block with three replications. All annual bluegrass control data were subjected to statistical analysis using R software (version 3.2.3). Expected means squares of McIntosh (Reference McIntosh1983) determined that data could be combined over years prior to being subjected to analysis of variance using the ‘ExpDes’ package in R. Means were separated using Fisher’s least significant difference test at α=0.05 when appropriate. All data and analysis files are available at https://dx.doi.org/10.6084/m9.figshare.2066319.v5.
Results and Discussion
Screening for Resistance Within the Sampled Population
Eighty-one of the 100 annual bluegrass plants harvested from the field site screened positive for glyphosate and prodiamine resistance, whereas only a single plant was susceptible to both herbicides. A total of 96 of the 100 annual bluegrass plants sampled screened positive for glyphosate resistance, 15 of which were prodiamine susceptible. Conversely, 84 of the 100 plants sampled were classified as likely prodiamine resistant, only three of which were deemed glyphosate susceptible. These responses suggest that the majority of the sampled annual bluegrass population was likely resistant to both prodiamine and glyphosate; however, this response needed to be confirmed from seed in progeny to ensure that these traits were heritable (Burgos et al. Reference Burgos, Tranel, Streibig, Davis, Shaner, Norsworthy and Ritz2013).
Confirming Resistance in Progeny
Prodiamine Resistance
Control of resistant and susceptible annual bluegrass phenotypes varied in response to increasing rates of prodiamine (Figure 1, Table 2). Non-linear regression curves fit control data collected on the resistant and susceptible phenotypes, and were significantly different from each other (P<0.0001). Rates of prodiamine required to control the resistant and susceptible phenotypes 50% (GR50 values) were 4,117 and 132gha−1, respectively. Dry biomass data were similar to control assessments in that non-linear regression curves modeling reductions in biomass for the resistant and susceptible phenotypes were significantly different from one another (P<0.0001) (Figure 2, Table 2). Prodiamine rates needed to reduce dry biomass of the resistant and susceptible phenotypes by 50% were 1,006 and 45 gha−1, respectively, equating to a resistance factor of 22 based on dry biomass reductions. These results are similar to research conducted by Cutulle et al. (Reference Cutulle, McElroy, Millwood, Sorochan and Stewart2009), who identified a phenotype of annual bluegrass 26 times less sensitive to prodiamine than a known susceptible phenotype.

Figure 1 Control of prodiamine-resistant and -susceptible annual bluegrass 35 days after treatment with prodiamine at 0; 105; 210; 420; 840; 1,680; 3,360; and 6,720 gha−1 during the spring of 2015 in a glasshouse (Knoxville, TN; 35.56°N, 83.56°W). Error bars represent standard error of the mean.

Figure 2 Dry biomass reduction of prodiamine-resistant and -susceptible annual bluegrass 35 days after treatment with prodiamine at 0; 105; 210; 420; 840; 1,680; 3,360; and 6,720 gha−1 during the spring of 2015 in a glasshouse (Knoxville, TN; 35.56°N, 83.56°W). Error bars represent standard error of the mean.
Table 2 Regression parameters for control and biomass data, presented in figures, representing annual bluegrass response to increasing rates of prodiamine and glyphosate in glasshouse experiments (Knoxville, TN; 35.56°N, 83.56°W) in 2015.
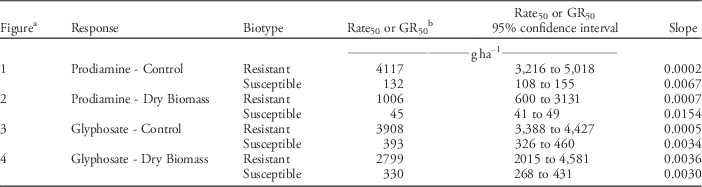
a Control data in Figures 1 and 3 modeled using the equation Control (%)=Bottom+(Top – Bottom)/(1+10((Rate50–X) ×K)), where Rate50 represents the herbicide rate (X) at which 50% annual bluegrass control was reached. Bottom and Top represent asymptotes that are constrained to 0 and 100, and K represents the slope of the best-fit line to model the response of resistant and susceptible biotypes to increasing rates of herbicide. Dry biomass data in Figures 2 and 4 modeled using the equation dry biomass (% non-treated)=(Y0 − Plateau) × exp (−KX)+Plateau, where Y0 and Plateau are constrained to 100 and 0, K represents the slope of the best-fit line to model the response of each biotype to increasing rates of herbicide (X). The herbicide rate required to reduce dry biomass to 50% of the non-treated (GR50) was calculated using the formula: GR50=ln (2)/K.
b Prodiamine rate (gha−1) required to control annual bluegrass by 50% (Rate50) or reduce dry biomass to 50% of the non-treated (GR50).
Glyphosate Resistance
Significant differences in annual bluegrass control were observed 21 d after treating progeny of resistant and susceptible phenotypes with increasing rates of glyphosate (Figure 3, Table 2). Non-linear regression curves fit control data collected on the resistant and susceptible phenotypes and were significantly different from one another (P<0.0001). Glyphosate rates that resulted in 50% control of the resistant and susceptible phenotypes were 3,908 and 393 gha−1, respectively. Dry biomass data supported assessments of annual bluegrass control, as approximately 8.5 times more glyphosate (1,940 gha−1) was required to reduce dry biomass of the resistant phenotype by 50% compared to a known glyphosate-susceptible phenotype (229 gha−1; Figure 4, Table 2). This response is within the range of reduced glyphosate sensitivity (4 to 12 times lower sensitivity) reported by other researchers studying glyphosate-resistant annual bluegrass (Binkholder et al. Reference Binkholder, Fresenburg, Teuton, Xiong and Smeda2011; Brosnan et al. Reference Brosnan, Breeden and Mueller2012; Cross et al. Reference Cross, McCarty, Tharayil, McElroy, Chen, McCullough, Powell and Bridges2015).

Figure 3 Control of glyphosate-resistant and -susceptible annual bluegrass 35 days after treatment with glyphosate at 0; 105; 210; 420; 840; 1,680; 3,360; and 6,720 gha−1 during the spring of 2015 in a glasshouse (Knoxville, TN; 35.56°N, 83.56°W). Error bars represent standard error of the mean.

Figure 4 Dry biomass reduction of glyphosate-resistant and -susceptible annual bluegrass 35 days after treatment with glyphosate at 0; 105; 210; 420; 840; 1,680; 3,360; and 6,720 gha−1 during the spring of 2015 in a glasshouse (Knoxville, TN; 35.56°N, 83.56°W). Error bars represent standard error of the mean.
Shikimic acid accumulation studies also suggest glyphosate resistance in this annual bluegrass phenotype. Glyphosate-susceptible plants accumulated 50% more shikimic acid 6 DAT than those resistant to glyphosate (Figure 5, Table 3). Glyphosate-resistant annual bluegrass accumulated a maximum of 394 mg kg−1 shikimic acid 6 DAT, while 898 mg kg−1 accumulated in annual bluegrass with susceptibility to glyphosate. Similarly, Brosnan et al. (Reference Brosnan, Breeden and Mueller2012) and Cross et al. (Reference Cross, McCarty, Tharayil, McElroy, Chen, McCullough, Powell and Bridges2015) reported shikimate concentrations to be 50% lower in a glyphosate-resistant annual bluegrass compared to susceptible plants at 3 and 6 DAT.

Figure 5 Shikimic acid concentrations (mg kg−1) in glyphosate-resistant and -susceptible annual bluegrass following treatment with glyphosate at 420 gha−1. Error bars represent standard error of the mean for each biotype at each timing.
Table 3 Regression parameters modeling shikimic acid concentrations (mg kg−1) in glyphosate-resistant and -susceptible annual bluegrass following treatment with glyphosate at 420 gha−1 in glasshouse experiments (Knoxville, TN; 35.56°N, 83.56°W) in 2015.

a Responses modeled using a one-phase exponential association non-linear regression equation, shikimic acid=Y0+(Plateau − Y0)(1 − exp(−KX)), where Y0 and Plateau were the minimum and maximum shikimic acid accumulation, K represented the slope of the best-fit line, and X represented days after treatment with glyphosate at 420 gha−1.
Efficacy of Herbicide Mixtures
All POST-applied herbicide mixtures evaluated in the current study effectively controlled annual bluegrass with resistance to prodiamine and glyphosate (Table 4). October applications of herbicide mixtures containing trifloxysulfuron, simazine, S-metolachlor, or mesotrione controlled this resistant annual bluegrass biotype 86% to 100% in March and 84% to 98% in April, with no significant differences detected among treatments (Table 4).
Table 4 Control of annual bluegrass with multiple resistance to glyphosate and prodiamine with herbicide mixtures applied to hybrid bermudagrass golf course roughs in Alcoa, TN (35.75°N, 83.88°W) following applications made in October 2014 and 2015. Means represent combined responses of four replications over two years.

a Annual bluegrass control assessed on a 0% (no control) to 100% (complete necrosis) scale relative to a non-treated check plot. Assessments made in March and April represent 21 and 25 weeks after treatment, respectively. b Herbicide mixtures were applied on October 20, 2014 and October 21, 2015, and included non-ionic surfactant (Activator-90, Loveland Products, Greeley, CO) at 0.25% v/v.
Implications for Turf Managers
Our findings documented the second instance of annual bluegrass evolving multiple resistance in a managed turfgrass system, and the first case of annual bluegrass with resistance to both PRE and POST herbicides. However, we demonstrated that POST-applied herbicide mixtures can effectively control this annual bluegrass biotype with resistance to prodiamine and glyphosate. However, herbicides included in these mixtures were all active on annual bluegrass and applied at maximum label rate. Our results present a baseline outlining the relative percentage of annual bluegrass plants in the sampled population that were resistant to both prodiamine and glyphosate, either herbicide individually, or neither herbicide. Future research should explore if use of herbicide mixtures evaluated in the current study change the percentage of resistant plants over time.
Our research is limited in that we did not fully determine the mechanisms conferring resistance in this annual bluegrass population. Genetic sequencing work did identify a Pro-106-Ala substitution on the EPSPS gene within this population, a target site alteration that has been shown to confer glyphosate resistance in annual bluegrass (Cross et al. Reference Cross, McCarty, Tharayil, McElroy, Chen, McCullough, Powell and Bridges2015). Complete sequences for EPSPS in the annual bluegrass biotype studied herein are available in GenBank (KU382756 – KU382773).
Acknowledgements
The authors would like to thank Robert Trigiano, Sarah Boggess, Phil Wadl, Brian Scheffler, Meg Staton, Javier Vargas, and Daniel Farnsworth for their efforts in this research, as well as Doug Karcher for his assistance with statistical analysis. This work was supported by the Tennessee Agricultural Experiment Station and Syngenta Crop Protection. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the University of Tennessee Institute of Agriculture.











