The fish farming industry is constrained by the limited availability and high price of fish oil and fishmeal (FM) for feed, whereas the higher availability and lower price of vegetable oils and plant proteins (PP) make the latter the most viable alternatives(Reference Montero, Benitez-Dorta and Caballero1,Reference Hardy, Tacon, Stickney and MacVey2) . Such PP/vegetable oil-based feeds are more prone to be nutritionally unbalanced and can even incorporate some antinutritional factors that might negatively impact fish physiological processes, ultimately impacting fish growth, health and welfare(Reference Yaqoob and Calder3–Reference Francis, Makkar and Becker6). PP sources tend to be low in some essential amino acids (AA)(Reference Médale and Kaushik7), often lysine and methionine(Reference Jobling8), and their use as replacements for FM requires a careful combination of plant proteins and/or supplementation with specific crystalline AA, to satisfy the nutritional requirements of the species(Reference Espe, Lemme and Petri9). Moreover, fish requirement of some AA appears to increase when fish are fed a PP diet, since their feeding intake, growth and protein utilisation may be reduced(Reference Kaushik, Cravedi and Lalles10). Therefore, establishment of optimal dietary requirements and characterisation of the AA profile of the alternative protein sources are imperative in fish nutrition research(Reference Li, Mai and Trushenski11). Nonetheless, AA are not only characterised as the building blocks for protein synthesis, key for growth, but also regulate key metabolic pathways in other biological processes such as reproduction and immune defences(Reference Li, Mai and Trushenski11), acting as precursors for the synthesis of hormones and metabolites as polyamines, serotonin, nitric oxide and glutathione(Reference Li, Meininger and Hawker12). The immune system is in fact highly dependent on AA availability since their metabolism is found altered in stress and inflammatory situations(Reference Li, Mai and Trushenski11,Reference Conceicao, Aragao and Dias13–Reference Costas, Aragão and Mancera17) . A growing interest on the role of several AA in the immune functions of fish, as in higher vertebrates, has shown that specific AA can specially modulate the innate immune responses(Reference Azeredo, Machado and Afonso18–Reference Costas, Conceicao and Dias22). Additionally, the AA requirement levels often established by optimal growth overlook the metabolic needs associated with immune responses, health, reproduction and cell signalling(Reference Li, Mai and Trushenski11). Hence, underestimation of the true AA requirement level may occur.
The interaction between nutrition and immune system is well recognised and raised the discussion about the so-called functional AA(Reference Wu23). Methionine is an example of the relationship between nutrition and immunity. It is often the first limiting AA in fish diets, particularly in those containing high levels of PP sources (e.g. soyabean)(Reference Mai, Wan and Ai24). Likewise, methionine has a key role in the immune system. As a precursor of S-adenosylmethionine, a universal methyl donor group, methionine participates in the regulation of many cellular events involved in polyamine synthesis, formation of signalling molecules essential for cellular function, hormones, bioactive amines, enzymes, neurotransmitters, nitric oxide, DNA methylation and the control of inflammation(Reference Igarashi and Kashiwagi25–Reference Williams and Schalinske28). In fact, increased levels of dietary methionine, above the required optimal growth, led to an improved immune response in poultry(Reference Wu, Cui and Peng29,Reference Bunchasak30) , while in fish, recent results show that methionine dietary supplementation improved seabass cellular immune status without evidences of activation of pro-inflammatory mechanisms(Reference Machado, Azeredo and Diaz-Rosales20,Reference Machado, Azeredo and Fontinha21) . Moreover, increased methionine level improved disease resistance against Photobacterium damselae subsp. piscicida in European seabass(Reference Machado, Azeredo and Fontinha21). On the other hand, Wu et al. (Reference Wu, Cui and Peng29) discussed that also methionine dietary deficiency could impair cellular immune function in broilers. Therefore, an accurate estimation of the dietary methionine requirement in fish needs to be intensively studied, in particular in the present feed formulation scenarios. In addition, methionine also presents key roles in fish health management.
This work intended to compare the effects on the immune mechanisms of an extreme feed formulation (0 % FM, low methionine) compared with a fishmeal-based diet (FM), and to graded levels of methionine, after a short and prolonged feeding period.
Materials and methods
Experimental diets
Four PP-based diets (Table 1) were formulated and manufactured by SPAROS Lda. The M0·65 diet was formulated to meet the estimated AA requirements for European seabass(Reference Kaushik31), except for a deficiency in methionine. Three other diets were identical to the M0·65 diet but supplemented with graded levels of crystalline methionine at 0·22, 0·63 and 0·88 % of feed: 0·85 % methionine in feed (CTRL, at requirement(Reference Kaushik31)), 1·25 % methionine in feed (MET1·25, above requirement) and 1·5 % methionine in feed (MET1·5, above requirement). Moreover, a high fishmeal diet with 1·18 % methionine in feed (FM, above requirement) was formulated as a positive control (FM).
Table 1. Formulation of the experimental diets
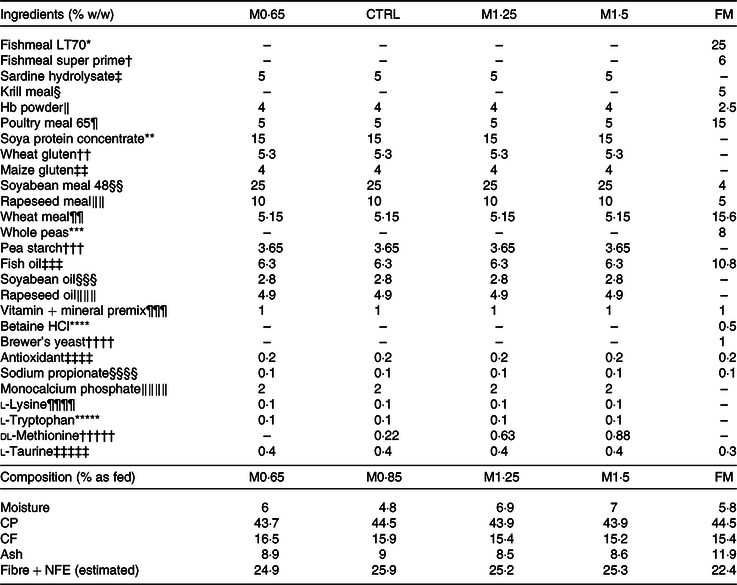
MET0·65, 0·65 % methionine in feed; CTRL, control; MET1·25, 1·25 % methionine in feed; MET1·5, 1·5 % methionine in feed; FM, fishmeal; MET0·85, 0·85 % methionine in feed; CP, crude protein; CF, crude fat; NFE, nitrogen-free extract.
* NORVIK LT70:70·7 % CP, 8·1 % CF; Pesquera Diamante.
† Diamante: 66·3 % CP, 11·5 % CF; Pesquera Diamante.
‡ TRIPLE S: 79·5 % CP, 0·2 % CF; Sopropêche.
§ Krill meal: 61·1 % CP, 17·4 % CF; Aker BioMarine.
‖ Porcine Hb: 91·6 % CP, 1·2 % CF; SONAC BV.
¶ Poultry meal: 62·4 % CP, 14·5 % CF; SAVINOR UTS.
** Soycomil P: 63 % CP, 0·8 % CF; ADM.
†† VITAL: 83·7 % CP, 1·6 % CF; ROQUETTE Frères.
‡‡ Maize gluten meal: 61 % CP, 6 % CF; COPAM.
§§ Dehulled solvent extracted soyabean meal: 47 % CP, 2·6 % CF; CARGILL.
‖‖ Defatted rapeseed meal: 34 % CP, 2 % CF; Premix Lda.
¶¶ Wheat meal: 10·2 % CP; 1·2 % CF; Casa Lanchinha.
*** Yellow peas: 19·6 % CP, 2·2 % CF; Ribeiro e Sousa Lda.
††† NASTAR: 90 % starch; Cosucra.
‡‡‡ Sopropêche.
§§§ Henry Lamotte Oils GmbH.
‖‖‖ J.C. Coimbra Lda.
¶¶¶ PREMIX Lda: vitamins (IU or mg/kg diet): dl-alpha tocopherol acetate, 100 mg; sodium menadione bisulphate, 25 mg; retinyl acetate, 20 000 IU; dl-cholecalciferol, 2000 IU; thiamin, 30 mg; riboflavin, 30 mg; pyridoxine, 20 mg; cyanocobalamin, 0·1 mg; nicotinic acid, 200 mg; folic acid, 15 mg; ascorbic acid, 500 mg; inositol, 500 mg; biotin, 3 mg; calcium pantothenate, 100 mg; choline chloride, 1000 mg and betaine, 500 mg. Minerals (g or mg/kg diet): copper sulphate, 9 mg; ferric sulphate, 6 mg; potassium iodide, 0·5 mg; manganese oxide, 9·6 mg; sodium selenite, 0·01 mg; zinc sulphate,7·5 mg; sodium chloride, 400 mg and excipient wheat middlings.
**** Beta-Key 95 %; ORFFA.
†††† PREMIX Lda.
‡‡‡‡ Paramega PX; Kemin Europe NV.
§§§§ Disporquímica.
‖‖‖‖ MCP: 22 % P, 16 % Ca; Fosfitalia.
¶¶¶¶ Lysine HCl 99 %; Ajinomoto Eurolysine SAS.
***** l-Tryptophan 98 %; Ajinomoto Eurolysine SAS.
††††† dl-Methionine for Aquaculture: 99 % methionine; Evonik Nutrition & Care GmbH.
‡‡‡‡‡ ORFFA.
Fish were fed by hand ad libitum three times/d, and the feeding trial lasted for 12 weeks. After total AA analysis in feed (Table 2), methionine content in MET0·65 was 20 % below CTRL, while methionine supplementation led to 39 and 70 % increase above CTRL (MET1·25 and MET1·5, respectively). Methionine supplementation levels were chosen according to previous works(Reference Azeredo, Machado and Afonso18,Reference Machado, Azeredo and Diaz-Rosales20,Reference Machado, Azeredo and Fontinha21) and with the aim to assess the effects of methionine deficiency and its graded supplementation levels in an alternative feed formulation (i.e. 0 % FM) context. Formulation and proximate composition of the experimental diets are presented in Table 1.
Table 2. Analysed amino acid composition of the experimental diets*

MET0·65, 0·65 % methionine in feed; CTRL, control; MET1·25, 1·25 % methionine in feed; MET1·5, 1·5 % methionine in feed; FM, fishmeal.
* Tryptophan was not analysed. Methionine and cysteine contents reported here may be slightly underestimated due to oxidation, even if acid hydrolysis was performed after nitrogen flushing of test vials. Values are means (n 3).
Main ingredients were ground (below 250 μm) in a micro pulverizer hammer mill (SH1; Hosokawa Micron, B.V.). Powder ingredients and oils were then mixed according to the target formulation in a paddle mixer (RM90; Mainca, S.L.). All diets were manufactured by temperature-controlled extrusion (pellet sizes: 1·5 mm) by means of a low-shear extruder (P55; Italplast, S.r.l.). Upon extrusion, all feed batches were dried in a convection oven (OP 750-UF; LTE Scientifics) for 4 h at 45°C. Formulation of experimental diets is presented in Table 1. Proximate composition analysis was conducted by the following methods: DM, by drying at 105°C for 24 h; ash, by combustion at 550°C for 12 h; crude protein (N × 6·25), by a flash combustion technique followed by gas chromatographic separation and thermal conductivity detection (LECO FP428); fat, after petroleum ether extraction, by the Soxhlet method; total phosphorus, according to the ISO/DIS 6491 method, using the vanadomolybdate reagent; and gross energy, in an adiabatic bomb calorimeter (IKA).
Diets were analysed for total AA content at Aquagroup/CCMAR laboratory. Diet samples were hydrolysed in 6 m HCl at 116°C for 2 h in nitrogen-flushed glass vials to prevent methionine and cysteine oxidation. Samples were then pre-column derivatised with a Waters AccQ Fluor Reagent (6-aminoquinolyl-N-hydroxysuccinimidyl carbamate) using the AccQ Tag method (Waters). Analyses were done by ultra-high-performance liquid chromatography in a Waters reversed-phase AA analysis system, using norvaline as an internal standard. During acid hydrolysis, asparagine is converted to aspartate and glutamine to glutamate, so the reported values for these AA represent the sum of the respective amine and acid. Since it is partially destroyed by acid hydrolysis, tryptophan was not determined. The resultant peaks were analysed with EMPOWER software (Waters). The AA profile of the experimental diets and the relative percentage of methionine supplementation are presented in Table 2.
Experimental design
European seabass (Dicentrarchus labrax) juveniles were acquired from a certificated hatchery (MARESA, Spain) and maintained in quarantine for 2 weeks at the Ramalhete research station (Centre of Marine Sciences of Algarve, University of Algarve) fish holding facilities under the rearing conditions described below. After this period, fish were weighed (10·34 (sd 0·19) g) and randomly distributed into fifteen tanks (1000 litres; five groups with three replicates of fifty fish each) of a land-based flow-through system with a supply of 2 litres/min of seawater. The trial was performed between May and August, and average temperature was 22 (sd 2·0) °C, dissolved O2 in seawater was 92·7 (sd 4·5) % of saturation, salinity 35 (sd 0·3) ppt and natural photoperiod May–August 2018. Ammonium and nitrite levels were kept below 0·025 and 0·3 mg/l, respectively.
European seabass juveniles were acclimated during 1 week to the experimental rearing conditions and fed the same commercial diet used in the acclimatisation period. Thereafter, the five previously described dietary treatments were evaluated in triplicate groups in a complete randomised design.
At 2 and 12 weeks after feeding the experimental diets, forty-five fish from each group (fifteen per replicate) were euthanised by an overdose of anaesthetic (Tricaine methanesulfonate; Sigma) and weighed and blood and head-kidney samples were collected.
Blood was collected from the caudal vein using heparinised syringes. A drop of blood from three fish per replicate (n 9) was used to perform blood smears for peripheral differential leucocytes counting, whereas the remaining sample was centrifuged at 10 000 g for 10 min at 4 °C and the plasma was collected, frozen on dry ice and stored at –80 °C for evaluating innate humoral immune parameters. Plasma samples were pooled from every three individuals (five pools per replicate). Head-kidney tissues were collected from two fish per replicate (n 6), immediately frozen on dry ice and stored at –80 °C until processed for gene expression analysis.
The trial was conducted according to the guidelines on the protection of animals used for scientific purposes from the European directive 2010/63/UE. CCMAR facilities and their staff are certified to house and conduct experiments with live animals (Group-C licences by the Direção Geral de Alimentação e Veterinária, Ministério da Agricultura, Florestas e Desenvolvimento Rural). The protocol was approved by the CCMAR Animal Welfare Committee.
Differential leucocyte counting
Immediately after blood collection, blood smears were performed and air-dried. After fixation with formol-ethanol (10 % of 37 % formaldehyde in absolute ethanol), detection of peroxidase was carried out as described by Afonso et al. (Reference Afonso, Silva and Lousada32) in order to facilitate identification of neutrophils. Blood smears were then stained with Wright’s stain (Haemacolor; Merck). Slides were examined (1000×), and at least 200 leucocytes were counted and classified as the relative percentage (%) of thrombocytes, lymphocytes, monocytes and neutrophils.
Analyses of plasma innate immune parameters
Peroxidase activity
Total peroxidase activity in plasma was measured following the procedure described by Quade & Roth(Reference Quade and Roth33). In triplicates, 15 µl of plasma was diluted with 135 µl of Hanks’ balanced salt solution (HBSS) without Ca2+ and Mg2+ in flat-bottomed ninety-six-well plates. Then, 50 µl of 20 mm 3,3′, 5,5′-tetramethylbenzidine hydrochloride (Sigma) and 50 µl of 5 mm H2O2 were added. After 2 min, the colour change reaction was stopped by adding 50 µl of 2 m sulphuric acid and the optical density was read at 450 nm in a Synergy HT microplate reader. Wells without plasma were used as blanks. The peroxidase activity (units/ml plasma) was determined by defining one unit of peroxidase as that which produces an absorbance change of 1 OD (optical density).
Lysozyme activity
Lysozyme activity was measured using a turbidimetric assay as described by Costas et al. (Reference Costas, Conceicao and Dias22). A solution of Micrococcus lysodeikticus (0·5 mg/ml, 0·05m sodium phosphate buffer, pH 6·2) was prepared. In triplicates, 15 µl of plasma was added to a microplate and 250 µl of the above suspension was pipetted to give a final volume of 265 µl. The reaction was carried out at 25°C, and the absorbance (450 nm) was measured after 0·5 and 4·5 min in a Synergy HT microplate reader. Serial diluted, lyophilised hen egg white lysozyme (Sigma) in sodium phosphate buffer (0·05m, pH 6·2) was used to develop a standard curve. The amount of lysozyme in the sample was calculated using the formula of the standard curve.
Anti-protease activity
The anti-protease activity was determined as described by Ellis(Reference Ellis, Stolen, Andrerson and Roberson34) with some modifications(Reference Machado, Azeredo and Diaz-Rosales20). Briefly, 10 µl of plasma was incubated with the same volume of a trypsin solution (5 mg/ml in NaHCO3, 5 mg/ml, pH 8·3) for 10 min at 22°C in polystyrene microtubes. To the incubation mixture, 100 µl of phosphate buffer (NaH2PO4, 13·9 mg/ml, pH 7·0) and 125 µl of azocasein (20 mg/ml in NaHCO3, 5 mg/ml, pH 8·3) were added and incubated for 1 h at 22°C. Finally, 250 µl of trichloroacetic acid was added to each microtube and incubated for 30 min at 22°C. The mixture was centrifuged at 10 000 g for 5 min at room temperature. Afterwards, 100 µl of the supernatant was transferred to a ninety-six-well plate containing 100 µl of NaOH (40 mg/ml) per well. The OD was read at 450 nm in a Synergy HT microplate reader. Phosphate buffer in place of plasma and trypsin served as blank, whereas the reference sample was phosphate buffer in place of plasma. The percentage inhibition of trypsin activity compared with the reference sample was calculated. All analyses were conducted in triplicates.
Protease activity
The protease activity was determined as described by Ross et al. (Reference Ross, Firth and Wang35). Briefly, 10 µl of plasma was incubated in polystyrene microtubes, with 100 µl of phosphate buffer (NaH2PO4, 13·9 mg/ml, pH 7·0) and 125 ml of azocasein (20 mg/ml in NaHCO3, 5 mg/ml, pH 8·3) for 24 h at 22°C. Finally, 250 µl of trichloroacetic acid was added to each microtube and incubated for 30 min at 22°C. The mixture was centrifuged at 10 000 g for 5 min at room temperature. Afterwards, 100 µl of the supernatant was transferred to a ninety-six-well plate containing 100 µl of NaOH (40 mg/ml) per well. The OD was read at 450 nm in a Synergy HT microplate reader. Phosphate buffer in place of plasma and trypsin served as blank, whereas the reference sample was trypsin (5 mg/ml in NaHCO3, 5 mg/ml, pH 8·3) in place of plasma. The percentage of protease activity was calculated by comparison with the reference sample. All analyses were conducted in triplicates.
Bactericidal activity
The bactericidal activity assay was performed using (P. damselae subsp. piscicida, Phdp) strain PP3. Bacteria were cultured in tryptic soy broth (Difco Laboratories) supplemented with NaCl to a final concentration of 2 % (w/v) (tryptic soy broth-2), and exponentially growing bacteria were resuspended in sterile HBSS and adjusted to 1 × 106 colony-forming units (cfu)/ml. Plating serial dilutions of the suspensions onto tricloroacetic acid (TSA-2) plates and counting the number of cfu following incubation at 22°C confirmed bacterial concentration of the inoculum. Plasma bactericidal activity was then determined following the method described by Graham & Secombes(Reference Graham and Secombes36) with modifications(Reference Machado, Azeredo and Diaz-Rosales20). Briefly, 20 µl of plasma was added to duplicate wells of a U-shaped ninety-six-well plate. HBSS was added to some wells instead of plasma and served as positive control. To each well, 20 µl of Phdp (1 × 106 cfu/ml) was added and the plate was incubated for 2·5 h at 25°C. Then, 25 µl of 3-(4,5-dimethyl-2-yl)-2,5-diphenyl tetrazolium bromide (1 mg/ml; Sigma) was added to each well and incubated for 10 min at 25°C to allow the formation of formazan. Plates were then centrifuged at 2000 g for 10 min, and the precipitate was dissolved in 200 µl of dimethyl sulphoxide (Sigma). The absorbance of the dissolved formazan resulting from the reduction of 3-(4,5-dimethyl-2-yl)-2,5-diphenyl tetrazolium bromide in direct proportion to the number of viable bacteria present was measured at 560 nm. Viable bacteria were expressed as percentage, calculated from the difference between the dissolved formazan in samples and the one formed in the positive controls (100 %). The bactericidal activity was calculated as the percentage of non-viable bacteria.
Gene expression analysis
Total RNA isolation was conducted with a NZY Total RNA Isolation kit (NZYTech) following manufacturer’s specifications. Samples were checked for RNA integrity through gel electrophoresis, which is indicative of clean and intact RNA, prior to complementary DNA (cDNA) synthesis. First-strand cDNA was synthesised with a NZY First-Strand cDNA Synthesis Kit (NZYTech). Quantitative PCR assays were performed with an Eppendorf Mastercycle ep realplex, using 1 µl of diluted cDNA (1:5 dilution) mixed with 10 µl of NZYSpeedy qPCR Master Mix and 0·4 µl (10 µm) of each specific primer in a final volume of 20 µl. cDNA amplification was carried out with specific primers (Table 3) for genes that have been selected for their involvement in immune responses and methionine metabolism (Table 3). Primers were designed with a NCBI Primer Blast Tool according to known qPCR restrictions (amplicon size, Tm difference between primers, GC content and self-dimer or cross-dimer formation). Sequences encoding European seabass, casp8, C-C chemokine receptor type 3 (ccr3), mechanistic target of rapamycin (mtor), macrophage colony stimulating factor 1 receptor 1 (mcsf1r1), cluster of differentiation 8 beta (cd8β) and spermine synthase (sms) were identified after carrying out a search in the databases v1.0c seabass genome(Reference Tine, Kuhl and Gagnaire37) and designed as previously described. Serial, 5-fold dilutions of cDNA were used to analyse the efficiency of the primer pairs by calculating the slope of the regression line of the cycle thresholds (Ct) v. the relative concentration of cDNA.
Table 3. Forward and reverse primers for real-time PCR

* Efficiency of PCR reactions was calculated from serial dilutions of tissue RT reactions in the validation procedure.
† Annealing temperature (°C).
‡ Amplicon (nt).
Accession number, efficiency values, annealing temperature, product length and primers sequences are presented in Table 3. Melting curve analysis was also performed to verify that no primer dimers were amplified. The standard cycling conditions were 94°C initial denaturation for 2 min, followed by forty cycles of 94°C denaturation for 30 s, primer annealing temperature (Table 3) for 30 s and 72°C extension for 30 s. All reactions were carried out as technical duplicates. The expression of the target genes was normalised using the expression of European seabass ribosome 40s subunit (40s).
Data analysis
All results are expressed as mean values and standard deviations. Data were analysed for normality and homogeneity of variance and, when necessary, transformed before statistical analysis (all gene expression data were log-transformed). All data expressed as percentage were arcsine transformed(Reference Zar38). Data were analysed by two-way ANOVA, with time and diet as factors and followed by Tukey’s post hoc test to identify differences in the experimental treatments. All statistical analyses were performed using the computer package STATISTICA 12 for Windows. The level of significance used was P ≤ 0·05 for all statistical tests.
Results
Peripheral leucocyte response
The blood of nine fish from each dietary group (three per replicate), sampled after 2 and 12 weeks of feeding, was used to perform a differential counting of each leucocyte type (Table 4). A feeding time effect was observed with an increase in the relative proportion of peripheral lymphocytes at 12 weeks regardless of dietary treatment, whereas the opposite pattern was observed for circulating thrombocytes. A diet effect was observed in seabass fed M1·5 and FM dietary treatments by presenting a higher percentage of lymphocytes than those fed M0·65, regardless of the weeks of feeding. Moreover, the relative proportion of circulating neutrophils increased in fish fed M1·25, M1·5 and FM compared with seabass fed both M0·65 and CTRL dietary treatments and particularly after 12 weeks of feeding.
Table 4. Relative proportion of peripheral blood leucocytes (i.e. neutrophils, monocytes, lymphocytes and thrombocytes) of European seabass juveniles fed dietary treatments during 2 and 12 weeks (n 9)*
(Mean values and standard deviations)

MET0·65, 0·65 % methionine in feed; CTRL, control; MET1·25, 1·25 % methionine in feed; MET1·5, 1·5 % methionine in feed; FM, fishmeal.
* P values from two-way ANOVA (P ≤ 0·05) (n 9). If interaction was significant, Tukey’s post hoc test was used to identify differences in the experimental treatments. Unlike lowercase letters stand for significant differences among dietary treatments for the same time, while unlike capital letters indicate differences among diets regardless of time.
Plasma innate humoral parameters
For the evaluation of the innate humoral immune response, forty-five fish were collected from each experimental group (fifteen per replicate) and, due to technical constrains, the plasma from each three fish was pooled. Humoral innate immune parameters assessed in plasma are presented in Table 5.
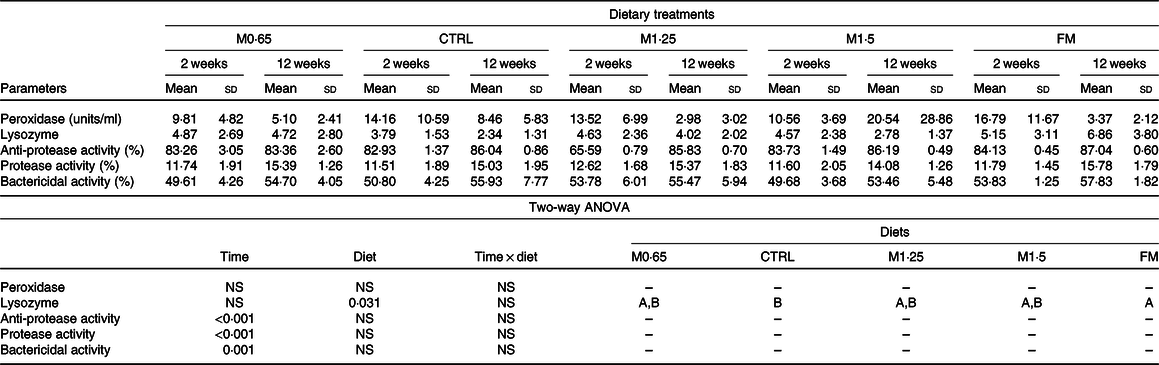
Table 5. Plasma peroxidase, lysozyme, anti-proteases, proteases and bactericidal activities in European seabass juveniles fed dietary treatments during 2 and 12 weeks (n 15)*
(Mean values and standard deviations)

MET0·65, 0·65 % methionine in feed; CTRL, control; MET1·25, 1·25 % methionine in feed; MET1·5, 1·5 % methionine in feed; FM, fishmeal.
* P values from two-way ANOVA (P ≤ 0·05). If interaction was significant, Tukey’s post hoc test was used to identify differences in the experimental treatments. Unlike capital letters indicate differences among diets regardless of time.
An increased activity of several humoral parameters was observed, with anti-proteases, proteases and bactericidal activity increasing from 2 to 12 weeks regardless of dietary treatments. Lysozyme activity increased in seabass fed FM compared with those fed the CTRL diet regardless of weeks of feeding.
Head-kidney gene expression
With the aim to evaluate the expression of genes with key roles in both immune response and methionine metabolism, cDNA was transcribed from head-kidney samples collected from six fish from each group (two per replicate) and the normalised expression of each gene is presented in Table 6.
Table 6. Quantitative expression (normalised mRNA expression) of immune-related genes in the head-kidney of European seabass juveniles fed dietary treatments during 2 and 12 weeks (n 6)*
(Mean values and standard deviations)
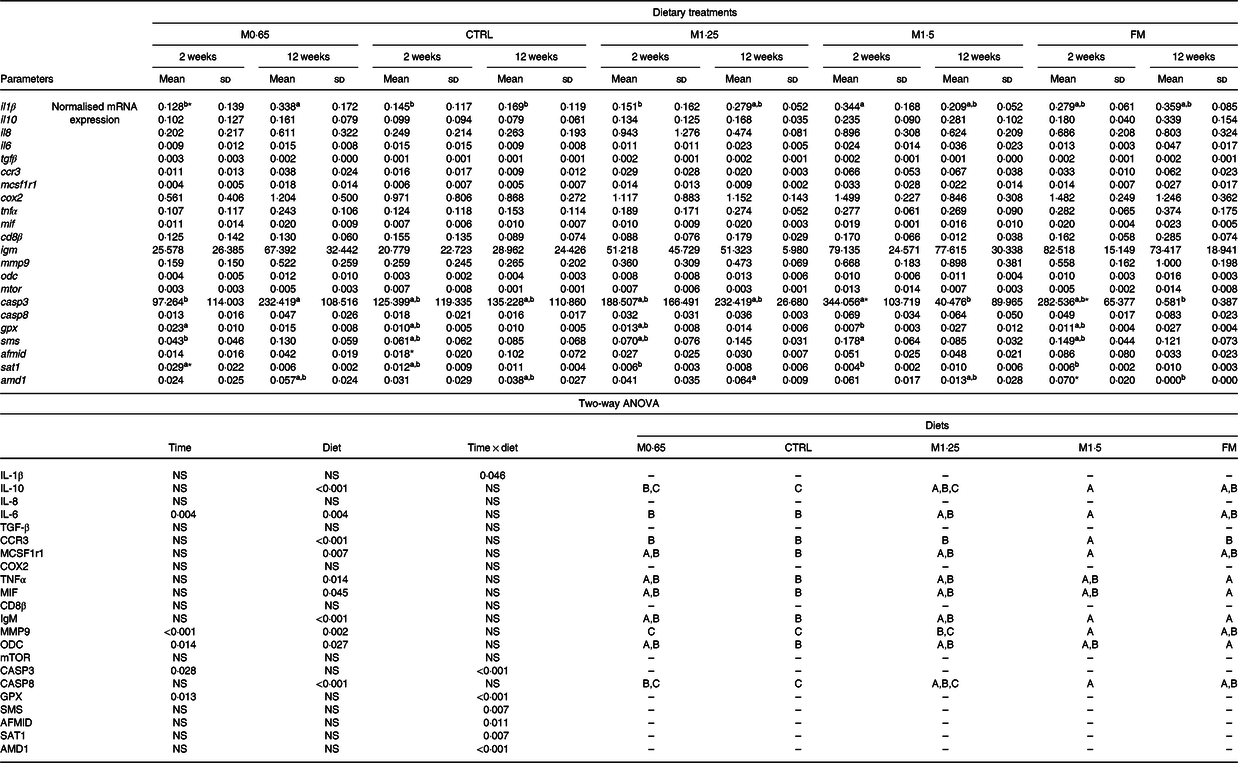
MET0·65, 0·65 % methionine in feed; CTRL, control; MET1·25, 1·25 % methionine in feed; MET1·5, 1·5 % methionine in feed; FM, fishmeal; ccr3, C-C chemokine receptor type 3; mcsf1r1, macrophage colony stimulating factor 1 receptor 1; mif, macrophages migration inhibitory factor; mmp9, matrix-metalloproteinase 9; odc, ornithine decarboxylase enzyme; mtor, mechanistic target of rapamycin; casp3, caspase 3; casp8, caspase 8; gpx, glutathione peroxidase; sms, spermine synthase; afmid, arylformamidase-like; sat1, spermine/spermidine N-(1)-acetyltransferase; amd1, adenosylmethionine decarboxylase 1.
* P values from two-way ANOVA (P ≤ 0·05). If interaction was significant, Tukey’s post hoc test was used to identify differences in the experimental treatments. Unlike lowercase letters stand for significant differences among dietary treatments for the same time, while asterisks stand for significant differences between times for the same diet. Unlike capital letters indicate differences among diets regardless of time.
The expression of il6, matrix-metalloproteinase 9 (mmp9) and ornithine decarboxylase enzyme (odc) levels showed an increase with the increase of weeks of feeding. The expression of the genes coding for IL-10, TNFα, macrophages migration inhibitory factor (MIF), IgM, ODC and CASP8 increased in seabass fed FM compared with fish fed the CTRL diet, whereas mmp9 transcripts were higher in those fed FM than in seabass fed both CTRL and M0·65 dietary treatments. An increased expression of il10, il6 and casp8 was observed in seabass fed M1·5 compared with those fed CTRL and M0·65 dietary treatments, while fish fed M1·5 presented higher expression of mcsf1r1 and igm compared only with their counterparts fed the CTRL diet. European seabass fed the M1·5 dietary treatment presented a higher expression of mmp9 compared with fish fed the remaining FM-free diets (i.e. M0·65, CTRL and M1·25) and of ccr3 compared with all dietary treatments (Table 6).
An increase in il1β expression was observed in seabass fed the M1·5 diet compared with their counterparts fed M0·65, CTRL and M1·25 dietary treatments after 2 weeks of feeding, while seabass fed the M0·65 diet augmented il1β transcript levels compared with fish fed the CTRL and FM diets after 12 weeks (Fig. 1(A)). Casp3 (Fig. 1(B)) and sms (Fig. 1(C)) expression levels were higher in fish fed the M1·5 diet than those fed the M0·65 diet at 2 weeks, whereas casp3 expression increased in seabass fed the M0·65 diet compared with their counterparts fed M1·5 and FM dietary treatments at 12 weeks. Moreover, a decrease with feeding time was also observed for casp3 expression levels in fish fed both dietary treatments, M1·5 and FM. In contrast, glutathione peroxidase (gpx) (Fig. 1(D)) transcripts decreased in seabass fed the M1·5 diet compared with those fed M0·65 after 2 weeks of feeding. Similarly, spermine/spermidine N-(1)-acetyltransferase (sat1) (Fig. 1(E)) mRNA expression was lower in seabass fed the M1·25, M1·5 and FM dietary treatments than in those fed the M0·65 diet at 2 weeks. The expression of the gene coding SAT1 was also found to decrease with feeding time. It was also observed a decrease of adenosylmethionine decarboxylase 1 (amd1) (Fig. 1(F)) expression levels in seabass fed the FM diet compared with fish fed the M1·25 diet at 12 weeks. An increase in time of the arylformamidase-like (afmid) expression was observed for the CTRL dietary treatment (Fig. 1(G)).
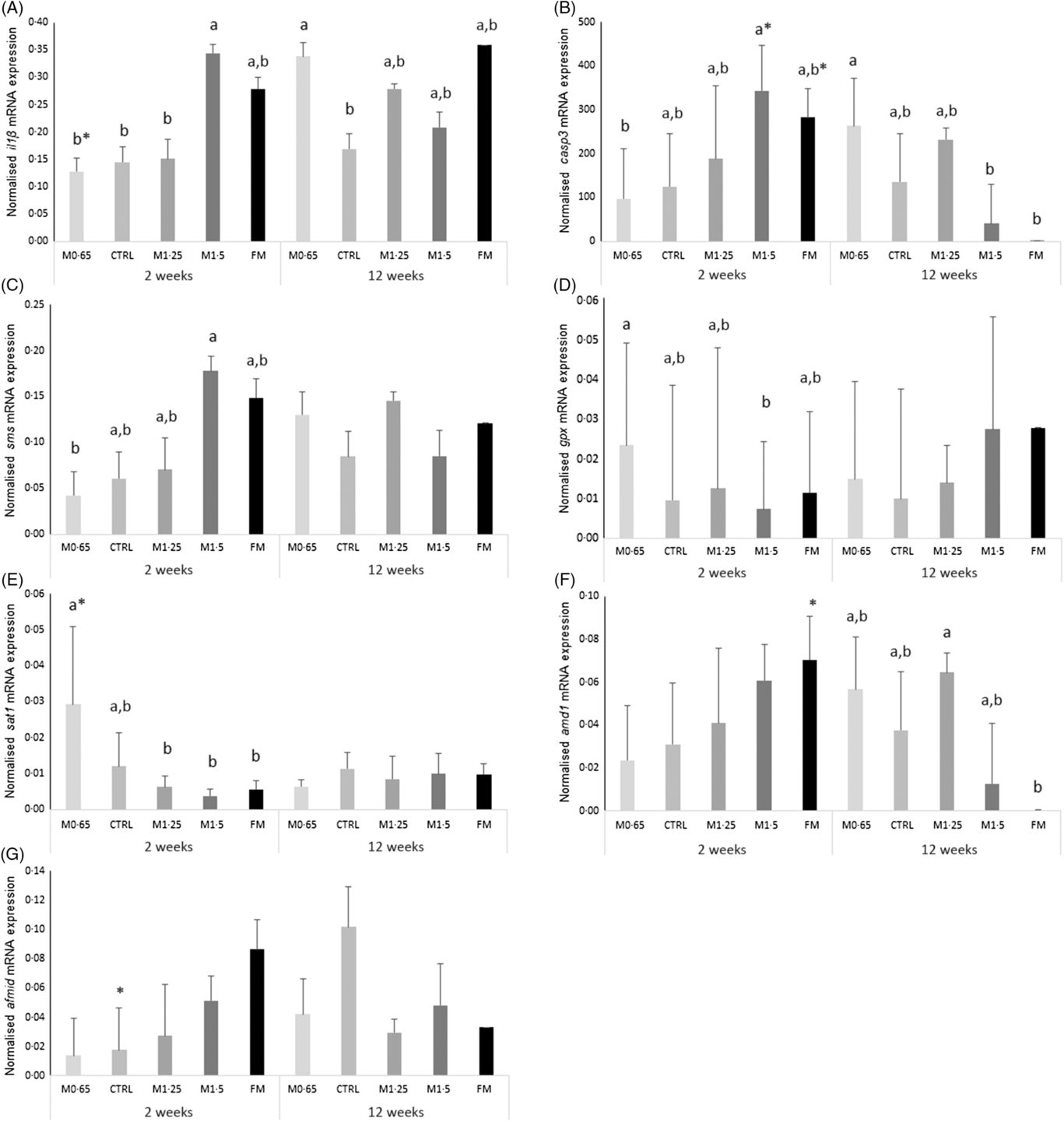
Fig. 1. Quantitative expression (A) IL-1β, (B) caspase 3 (casp3), (C) spermine synthase (sms), (D) glutathione peroxidase (gpx), (E) spermine/spermidine N-(1)-acetyltransferase (sat1), (F) adenosylmethionine decarboxylase 1 (amd1) and (G) arylformamidase-like (afmid) in the head-kidney of European seabass juveniles fed dietary treatments during 2 and 12 weeks. Values are presented as means and standard deviations (n 6). P values from two-way ANOVA (P ≤ 0·05). If interaction was significant, Tukey’s post hoc test was used to identify differences in the experimental treatments. Unlike letters stand for significant differences among dietary treatments for the same time, while * stands for significant differences between times for the same diet. MET0·65, 0·65 % methionine in feed; CTRL, control; MET1·25, 1·25 % methionine in feed; MET1·5, 1·5 % methionine in feed; FM, fishmeal.
Discussion
Methionine is the first limiting AA in aquafeeds containing high levels of PP sources(Reference Mai, Wan and Ai24), and its supplementation is essential to satisfy the nutritional requirements of farmed species(Reference Espe, Lemme and Petri9). The requirement level established for growth may overlook the metabolic need for fish optimal health since methionine participates in a wide range of pathways important for cell homoeostasis and immune response. The present study was designed in a way to seed more knowledge on the specific role of dietary methionine within the context of an alternative feed formulation (0 % FM). Dietary methionine deficiency as well as two levels beyond its estimated requirement was tested in the European seabass immune mechanisms after short and prolonged feeding periods. A FM-based diet was also evaluated as the ideal, and even not practical, diet for seabass leading to a good immune status scenario.
To the best of our knowledge, this is the first study to explore the role of dietary methionine levels in the context of a FM-free diet for the European seabass. Previous studies reported a clear modulatory effect of dietary methionine supplementation in FM-based diets in the European seabass immune status after only 2 or 4 weeks of feeding(Reference Machado, Azeredo and Diaz-Rosales20,Reference Machado, Azeredo and Fontinha21) . The latter study showed that methionine can enhance the peripheral cellular immune status without triggering pro-inflammatory humoral indicators as well as down-regulate pro-inflammatory genes. In contrast, in a PP dietary scenario, results from the present study only showed most changes at the transcriptional level in fish fed the highest methionine level (i.e. M1·5), and particularly after 2 weeks of feeding compared with those fed M0·65, where methionine was found below the requirement level. Genes coding for the pro-inflammatory cytokine IL-1β and the enzyme SMS that converts spermidine into spermine previously provided by S-adenosylmethionine through the aminopropylation pathway(Reference Neidhart and Neidhart39) were found up-regulated in response to the progressive increase of dietary methionine. This could be the result of methionine participation on polyamine (i.e. spermidine and spermine) biosynthesis, required for cell proliferation(Reference Igarashi and Kashiwagi25). This hypothesis was further reinforced with the decrease in the expression of gpx. Since the latter gene encodes the antioxidant enzyme glutathione peroxidase, it could indicate an improved consumption of S-adenosylmethionine through the aminopropylation route rather than by the transsulphuration pathway by which methionine is a precursor of cysteine for the formation of glutathione(Reference Grimble and Grimble14). This is further supported by the decreased expression of sat1, regulated by the intracellular concentration of polyamines, observed in fish fed both M1·25 and M1·5 dietary treatments. As previously reported for European seabass after 4 weeks of feeding, a methionine-supplemented diet (i.e. 1 % of feed above the requirement level)(Reference Machado, Azeredo and Fontinha21), a drop in the expression of sat1 could be understood as a negative feedback mechanism to the cellular high polyamine content(Reference Pegg40) avoiding non-specific deleterious effects in host tissues and, in fact, there seems to be no cellular modulation after 2 weeks of feeding. In contrast to that observed by Machado et al. (Reference Machado, Azeredo and Fontinha21), the present study showed an augmentation of casp3 mRNA expression levels in response to methionine availability, which could also be interpreted as a cell level control mechanism in response to the pro-inflammatory signals at the transcription level since a positive correlation was found between the increase of casp3 and the pro-inflammatory cytokine mRNA expression tnfα (r 2 = 0·97, y = 1229x – 33·109) and il1β (r 2 = 0·96, y = 1048·7x – 11·894).
In the present study, both dietary methionine deficiency and two levels beyond its requirement were tested in the context of an extreme feed formulation during a prolonged feeding period. This is an important issue in modern fish farming since methionine requirement level was established considering optimal growth in fish fed FM-based diets. Therefore, considering a challenging feed formulation scenario, established requirements may oversee increased metabolic needs for seabass optimal health. In a PP-based diet, the dietary level of methionine concentration led to a clear modulation of the percentage of peripheral neutrophils found. After 12 weeks of feeding, the number of this phagocytic cell increased in seabass fed methionine-supplemented diets above the theoretical requirement, supporting the methionine role in the polyamine synthesis pathway and thus leading to an improved cellular proliferation(Reference Igarashi and Kashiwagi25). Moreover, the latter fish did not show evidences of cell activation (e.g. neutrophils degranulation in response to a stimulus) since no plasma humoral parameter modulation was observed. Nonetheless, this blood neutrophilia was accompanied by the reduction of caspase 3 (casp3) mRNA expression, a gene coding a protein essential for processes associated with the formation of apoptotic bodies and associated with the role of methionine on the control of inflammation and apoptotic mechanisms(Reference Porter and Janicke41). In spite of the described results, a reduced expression of the AMD1 was observed. AMD1 is essential for biosynthesis of the polyamines being responsible for decarboxylation of S-adenosylmethionine(Reference Waterland26). Its reduced expression could be understood as a negative feedback mechanism in response to the superior and prolonged methionine availability or even a sparing effect of methionine from the aminopropylation route to the transsulphuration pathway since, contrary to the results found at 2 weeks, the levels of gpx, appear to be increased by dietary content of methionine.
The present study also observed the immune-modulatory role of methionine since the increase of its dietary content, regardless of feeding time, led to a clear lymphocytosis and increased expression of cytokines (il10 and il6), the mcsf1r1, a receptor for chemokines (ccr3), the igm, the mmp9 gene that encodes an enzyme involved in the degradation of the extracellular matrix during cell migration and caspase 8 (casp8), involved in the programmed cell dead. The progressively improved immune status displayed by the increase in methionine dietary content could be important upon inflammatory activation since former studies reported the positive effect of methionine supplementation, in a FM context, in response to infection with the enhancement of the inflammatory mechanism(Reference Machado, Azeredo and Diaz-Rosales20) and disease resistance against Phdp (Reference Machado, Azeredo and Fontinha21) after 15 d and 4 weeks feeding period, respectively. Moreover, juvenile Jian carp fed graded levels of methionine hydroxyl analogue, a synthetic methionine source, showed an increased survival rate after injection with Aeromonas hydrophila (Reference Kuang, Xiao and Feng42). Finally, the authors could not exclude the possible and recognised dietary methionine surplus effect, on the improvement of digestive and nutrient absorption functions that could counteract the adverse effects of antinutritional compounds found in plant-derived nutrient sources(Reference Wu, Tang and Jiang43).
Even though CTRL and FM dietary treatments presented similar methionine contents, the dietary protein source seems to present a clear impact on fish immune status since CTRL presented a reduced expression of several immune-related genes such as the cytokines il10 and tnfα, mcsf1r1, igm, casp8, mif, mmp9 an enzyme involved in the degradation of the extracellular matrix during cell migration and odc. This further supports the proposed possibility that in a practical PP-based diet scenario, the requirement level of methionine needed for immune support could be higher.
The overall results point to changes in the expression of genes directly related to methionine pathways, and particularly for cell proliferation, after only 2 weeks of feeding a FM-free diet with an increased methionine dietary content. The immune-modulatory role of methionine was more evident after 12 weeks of feeding with an enhancement of the immune status of fish fed a methionine-supplemented FM-free diet, without triggering an inflammatory response.
The general activation of inflammatory mechanisms by FM-free diets suggests and reinforces the importance of methionine in extreme dietary scenarios(Reference Montero, Benitez-Dorta and Caballero1,Reference Geay, Ferraresso and Zambonino-Infante44,Reference Montero, Mathlouthi and Tort45) . Moreover, it can be suggested that in an alternative diet formulation scenario, the supplementation of methionine could not only be important for the enhancement of European seabass immune status, as observed, but also as a strategy for increasing fish disease resistance.
In conclusion and in spite of the unclear results observed after 2 weeks, after a prolonged feeding period, methionine supplementation above the theoretical requirement level to a 0 % FM diet led to an enhancement of immune status without evidences of cell activation and with a gradual tendency to present values close to those observed by fish fed the FM diet. These results may suggest that the requirement level of methionine needed for immune support in a PP-based diet may possibly be higher compared with a FM.
Acknowledgements
This work was supported by Projects ALISSA (reference ALG-01-0247-FEDER-3520), IF/00197/2015, IF/00482/2014, UID/Multi/04423/2019 and UID/Multi/04326/2019 financed by Portugal and the European Union through FEDER, COMPETE 2020 and CRESC Algarve 2020, in the framework of Portugal 2020, and through the COMPETE and Operational Human Potential Programmes and national funds through Fundação para a Ciência e a Tecnologia (FCT, Portugal). M. M., B. C. and S. E. were supported by FCT, Portugal (SFRH/BD/108243/2015, IF/00197/2015 and IF/00482/2014, respectively).
M. M., S. E., L. E. C. C. and B. C. conceived the experiments; R. C. and S. E. conducted the experimental trial. M. M. directed most laboratory techniques and wrote the manuscript under the supervision of S. E., L. E. C. C. and B. C. J. D. formulated and produced the experimental diets. All authors contributed to and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.