Aquaculture is currently supplying increasing proportions of fish for global human consumption, resulting in an increasing demand for feeds for farmed fish. The use of fishmeal (FM) and fish oil (FO) in fish nutrition, particularly for carnivorous species such as salmonids, has been common practice for years. This is due to the fact that FM and FO constitute excellent sources of essential amino acids and fatty acids, particularly highly unsaturated fatty acids( Reference Sargent and Tacon 1 – Reference Gatlin, Barrows and Brown 3 ). However, the current stagnation of FM and FO production from wild fisheries might limit the growth of aquaculture unless effective alternative ingredients are found.
Terrestrial plant-based products are thus nowadays increasingly used as substitutes for marine resources in feeds for farmed fish( Reference Gatlin, Barrows and Brown 3 , Reference Hardy 4 ). Studies conducted with diets containing little or no FM and high levels of plant protein sources have shown lower growth performance in rainbow trout, possibly linked to reduced feed intake( Reference Pierce, Palti and Silverstein 5 ). With regard to dietary FO replacement, several studies carried out in salmonids( Reference Bell, McGhee and Campbell 6 , Reference Richard, Kaushik and Larroquet 7 ) have shown that complete replacement of FO in the diet by vegetable oils does not affect growth or feed efficiency when the n-3 PUFA requirements are met by lipids contained in FM. Indeed, one of the major consequences of the replacement of marine ingredients by plant-based products is the drastic modification of the fatty acids (FA) content of the diets, because none of the plant-based products contain n-3 long chain (LC)-PUFA such as EPA (20 : 5n-3) and DHA (22 : 6n-3), which are known to play a key role in fish reproduction and development( Reference Salze, Tocher and Roy 8 , Reference Sargent, McEvoy and Estevez 9 ). A few studies have also been conducted on the concomitant replacement of FM and FO. These studies showed lower growth performance in fish fed the plant-based diet, an effect mainly linked to FM replacement( Reference Panserat, Hortopan and Plagnes-Juan 10 , Reference Le Boucher, Vandeputte and Dupont-Nivet 11 ).
The metabolic consequences of FM and FO replacement with alternative protein or fatty acid sources are numerous and mediated by several interacting pathways. Nutrigenomic tools (i.e. transcriptomics) are increasingly used to investigate molecular events taking place in a genome receiving nutritional signals and responding to them through characteristic metabolic processes in the organism( Reference Zduńczyk and Pareek 12 ). Nutrigenomics studies in farmed fish have addressed the replacement of different percentages of FM and/or FO with plant ingredients in diets( Reference Leaver, Bautista and Björnsson 13 – Reference De Santis, Bartie and Olsen 15 ), and the effects of such replacement are well characterised in the hepatic transcriptome of salmonids( Reference Panserat, Hortopan and Plagnes-Juan 10 , Reference Jordal, Torstensen and Tsoi 16 – Reference Morais, Pratoomyot and Taggart 18 ) and marine species such as sea bass( Reference Geay, Ferraresso and Zambonino-Infante 14 ). For example, the replacement of fish oil by vegetable oils was found to be mainly associated with modification of genes involved in cholesterol and fatty acid biosynthesis( Reference Jordal, Torstensen and Tsoi 16 , Reference Leaver, Villeneuve and Obach 19 ), whereas the substitution of fishmeal by plant proteins was found to be associated with a decreased capacity for protein biosynthesis and variation in N metabolism in rainbow trout( Reference Panserat, Kolditz and Richard 17 ). The replacement of both fishmeal and fish oil by plant-based ingredients in the diet of rainbow trout was associated with changes in nucleic acid and glucose metabolism, in addition to the aforementioned changes in lipid and protein metabolism( Reference Panserat, Hortopan and Plagnes-Juan 10 ). Other studies have investigated the intestinal gene expression profile in response to different levels of dietary replacement of marine ingredients by plant products in several fish species such as Atlantic salmon (Salmo salar)( Reference Morais, Silva and Cordeiro 20 – Reference Frøystad, Lilleeng and Bakke‐Mckellep 22 ), gilthead sea bream (Sparus aurata L.)( Reference Calduch-Giner, Sitjà-Bobadilla and Davey 23 ) and Atlantic cod (Gadus morhua)( Reference Morais, Edvardsen and Tocher 24 ). However, most of these studies were carried out on growing fish, and there is still a gap in the understanding of the effects of plant-based diets on the rest of the life cycle (broodstock and early stages). In addition to the already-recognised importance of broodstock nutrition on progeny survival and development, nutrients contained in the yolk sac, transmitted by broodstock to developing progeny, are also known to influence the characteristic gene expression of offspring by modifying or interacting with transcription factors or DNA structure( Reference Bougas, Audet and Bernatchez 25 ). The effects of the maternal dietary history on reproduction and metabolic capacities of the progeny are still poorly documented, especially when broodstock are fed a totally plant-based diet without any FM and FO, and thus devoid of n-3 LC-PUFA, over the whole life cycle. In an earlier trial, we showed that broodstock produced viable offspring even when reared exclusively with a plant-based diet( Reference Lazzarotto, Corraze and Leprevost 26 ). We also showed that trout are capable of synthesising n-3 LC-PUFA from the dietary precursor (α-linolenic acid, 18 : 3n-3) and of incorporating them into ova, which in fish represent the main sources of nutrients utilised by the embryo( Reference Brooks, Tyler and Sumpter 27 ) and later by the developing alevin.
The early life stages of fish represent a transitional ontogenetic period of simultaneous growth and organ/tissue differentiation, during which fish undergo the transition from endogenous to exogenous feeding – that is, from yolk consumption to ingestion of external food( Reference Gisbert, Ortiz-Delgado and Sarasquete 28 ). Moreover, previous studies carried out on developing larvae( Reference Mennigen, Skiba-Cassy and Panserat 29 , Reference Darias, Zambonino-Infante and Hugot 30 ) showed that gene expression, and the subsequent activation of the related metabolic pathways, is differentially regulated with advancing ontogenesis. Thus, regulation of gene expression during this phase is considered to be a key mechanism underlying the management of the biological process required for harmonious development over this phase of life, during which nutritional input is of great importance.
In order to characterise the effects of broodstock nutritional history as well as those of first-feeding diets with different proportions of FM and FO and plant ingredients, the whole-body transcriptome of rainbow trout alevins was characterised at two different developmental stages: (i) before first feeding (end of endogenous feeding period) to assess the effects of maternal nutritional history and (ii) after 3 weeks of feeding (exogenous feeding alevins) to assess both the effects of maternal nutritional background and those of first-feeding diets.
Methods
Diets
Broodstock
The broodstock diets were the same as those previously described by Lazzarotto et al. ( Reference Lazzarotto, Corraze and Leprevost 26 ). In brief, broodstock were fed either a commercial (COM) diet composed of FM, FO and plant-based ingredients (45 % FM and 50 % FO replaced by plant ingredients), or an experimental plant-based (VEG) diet, completely free of marine FM and FO, which were replaced by plant protein sources (22 % maize gluten, 26 % soyabean meal, 33 % wheat gluten, 7 % durum wheat, 8 % white lupin and 4 % dehulled peas) and vegetable oils (50 % rapeseed oil, 30 % linseed oil and 20 % palm oil), respectively.
Alevins
Three different first-feeding experimental diets with different dietary levels of FM and FO replacement were formulated and manufactured (INRA-NuMéa): a marine (M) diet based on marine resources (no replacement), a commercial-like (C) diet containing both marine and plant-based ingredients (replacement of 46 % FM and 69 % of FO) and a completely plant-based diet (V) with total replacement of marine FM and FO by plant-based proteins and vegetable oils. The ingredients and composition of the three diets are provided in Table 1. In order to obtain total replacement of fish products, only plant-based proteins and vegetable oils (7 % rapeseed oil, 7 % linseed oil and 4 % palm oil) were used in the V diet, whereas the M and C diets contained FO (12 and 8 %, respectively). Consequently, the V diet contained no n-3 LC-PUFA, whereas it contained high levels of 18 : 3n-3, mainly derived from linseed oil, compared with the other two experimental diets (Table 2).
Table 1 Ingredients and composition of first-feeding diets

FM, fishmeal; FO, fish oil; Diet M, marine FM-FO-based diet; Diet C, commercial-like FM-FO and plant-based diet; Diet V, experimental 100 % plant-based diet.
* Origin co-fishery products – all species.
† Origin co-fishery products – sardines.
Table 2 Fatty acid composition (percentage of total fatty acids) of the experimental diets
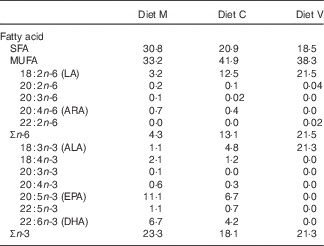
FM, fishmeal; FO, fish oil; Diet M, marine FM-FO-based diet; Diet C, commercial-like FM-FO & plant-based diet; Diet V, experimental 100 % plant-based diet; LA, linoleic acid; ARA, arachidonic acid; ALA, α-linolenic acid.
Animals and experimental plan
The experiment was carried out in strict accordance with EU legal frameworks relating to the protection of animals used for scientific purposes (Directive 2010/63/EU) and according to the National Guidelines for Animal Care of the French Ministry of Research (Decree no. 2001-464, 29 May 2001). It was approved by the Ethics Committee of INRA (INRA 2002-36, 14 April 2002) and the scientist in charge of the experimentation received training and personal authorisation (no. B64 10 003). Female rainbow trout were produced at the INRA facilities (Pisciculture Expérimentale INRA des Monts d’Arrée; PEIMA – permit no. B29-277-02). During the trial, they were reared under natural photoperiod and temperature conditions. At the beginning of the trial, female fish were randomly divided into two groups, fed from first feeding and through a 3-year life cycle, with either the broodstock COM diet or the broodstock plant-based (VEG) diet( Reference Lazzarotto, Corraze and Leprevost 26 ). At spawning, ova produced by ten female trout/group (3-year-old females) of similar body weight from each dietary treatment were fertilised with a pool of sperms from males fed a commercial diet. Eggs were transferred to our experimental hatchery (INRA – permit no. A64-104-1) where the water temperature is about 7 °C all year long. Just before first feeding (62 d post-fecundation), body weights and survival rates of alevins were recorded and whole-body samples of fry were collected.
The remaining alevins from both cohorts were subsequently split into three groups of fish. Each group (four replicates) received one of the three experimental diets from first feeding – that is diet M, diet C or diet V. After 3 weeks of feeding, survival rates and body weights of alevins were recorded and whole-body alevin samples were collected for subsequent analysis. All the samples were frozen in liquid N2 and stored at −80°C until analysis.
Lipid and fatty acid analysis
Total lipids of whole-body alevins collected before first feeding (pool=15 alevins/maternal group) and after 3 weeks of feeding (pool=15 alevins/dietary group) were extracted and quantified gravimetrically according to Folch et al. ( Reference Folch, Lees and Sloane Stanley 31 ). Neutral (NL) and polar (PL) lipid fractions were separated on silica cartridges (Sep-Pak, Waters)( Reference Juaneda and Rocquelin 32 ), and fatty acid methyl esters (FAME) were prepared according to Shantha & Ackman( Reference Shantha and Ackman 33 ). FAME were then analysed by GC as previously described in detail( Reference Lazzarotto, Corraze and Leprevost 26 ).
RNA extraction
Total RNA was extracted from individual whole-body swim-up fry (n 8/maternal group) and alevins (n 8/dietary group) using the TRIzol® reagent method (Invitrogen), according to the manufacturer’s recommendations. The quantity and quality of extracted RNA were analysed using a spectrophotometer (ND-1000; NanoDrop) and a Bioanalyzer (Agilent Technologies), respectively.
Complementary RNA synthesis, labelling and purification
Cyanine 3-CTP (Cy3)-labelled experimental complementary RNA (cRNA) samples (eight samples per treatment) were generated using the Agilent ‘One-Color Microarray-based Gene Expression Analysis’ (Low Input Quick Amp Labeling (LIQA)) kit, according to manufacturer’s instructions. The method uses T7 RNA Polymerase Blend (Agilent), which simultaneously amplifies target material and incorporates Cy3. For each sample, 150 ng of total RNA was used to generate fluorescent cRNA. Agilent Spike-In (Agilent) was included in each reaction. After the denaturation step (10 min in circulating bath at 65°C) and cRNA synthesis step (2 h at 40°C), the reactions were incubated at 70°C for 15 min to inactivate the AffinityScript enzyme (Agilent). To perform the labelling reaction, cRNA samples were each mixed with 6 µl of Transcription Master Mix cocktail (Agilent), containing Cy3-dye, and then incubated at 40°C for 2 h. Purification was performed using Quiagen RNeasy mini spin columns (Quiagen), eluted in 30 µl of RNase-free water.
Microarray hybridisation and scanning
Cy3-labelled cRNA sample yields (>0·825 µg cRNA) and specific activity (>6 pmol of Cy3/µg of cRNA) were verified using a NanoDrop ND-1000: 600 ng of Cy3-cRNA was fragmented and hybridised on a sub-array, following the LIQA kit instructions (Agilent). The transcriptomic analysis was conducted using a custom-commercial 8×60K oilgoarray (Agilent Technologies; Gene Expression Omnibus (GEO) accession no. GPL15840). The hybridisation reactions were allowed to continue for 17 h in a rotating hybridisation oven (65°C) before washing according to the manufacturer’s instructions. Slides were scanned with an Agilent scanner (Agilent DNA Microarray Scanner; Agilent Technologies) using the standard parameters for a gene expression 8×60K oligoarray (3 µm–20 bits). Data were then obtained using the Agilent Feature Extraction software (10.7.1.1), according to the appropriate gene expression (GE) protocol (GE1_107_Sep09). The data are deposited in NCBI’s GEO (GSE74271).
Quantitative real-time PCR
Six individual samples (single whole-body swim-up fry or alevin) per experimental condition were used as biological replicates. Total RNA (1 µg) was reverse-transcribed to cDNA with SuperScript III RNase H RT (Invitrogen) using oligo dT Primers. Real-time PCR was performed in the iCycler iQ™ (Bio-Rad). Quantitative PCR (qPCR) analyses for gene expression were performed using the Roche Lightcycler 480 system (Roche Diagnostics). The assays were performed using 2 µl of diluted cDNA mixed with 3 µl of Light cycler 480 SYBR® Green I Master mix in a total volume of 6 µl, using forward and reverse primers at a final concentration of 400 nm. Primer designing was performed using Primer 3 software. Specific primer pairs were designed with an overlapping intron, when possible, using known trout sequences in nucleotide databases (GeneBank and INRA-Sigenae). Database accession numbers and the sequences of forward and reverse primers used to test each gene are provided in the online Supplementary Table S1(a–b).
Thermal cycling was initiated with incubation at 95°C (10 min) for hot-start iTaq™ DNA polymerase activation. In total, forty-five cycles of PCR were performed, each consisting of a heating step at 95°C (15 s) for denaturing, a second step at 60°C (10 s) for annealing and a third extension step at 72°C (15 s). Following the final cycle of the PCR, melting curves were systematically monitored (with a gradient of 0·5°C/10 s from 55 to 94°C) to ensure that only one target fragment was amplified. Samples without RT and samples without RNA were run for each reaction as negative controls. mRNA levels of all target genes were normalised with the housekeeping gene α-elongation factor 1, previously used as a reference gene in salmonids( Reference Olsvik, Lie and Jordal 34 ). The expression levels were calculated according to the threshold cycle (ΔΔC T ) method( Reference Pfaffl 35 ).
Statistical analysis and data mining
Data on weight, survival, lipid content and fatty acids of whole-body alevins (collected before 1st feeding and after 3 weeks of feeding) are presented as mean values and standard deviations. Data were analysed statistically using R-software (version 2.14.0) and Rcmdr package. The normality and homogeneity of variance of the variables were tested using Shapiro–Wilk’s test and Levene’s test, respectively. Data for alevins collected before first feeding were analysed by an independent sample t test to assess the effects of the different broodstock nutritional histories, when both conditions were satisfied. The variables with non-parametric distribution were either normalised with an arcsin transformation or, if the criteria were still not met (some fatty acids), compared using a non-parametric paired Wilcoxon’s test. Data for alevins collected after 3 weeks of feeding were analysed using a two-way ANOVA (P<0·05) to assess the effects of the nutritional broodstock history and the first-feeding diets. The variables with non-parametric distribution were normalised with an arcsin transformation. Data from microarray analysis were normalised and analysed statistically using GeneSpring software (12.6; Agilent). Data were scale-normalised using the median value of each array to identify differentially expressed genes between conditions. An unpaired t test was performed to determine the effects of the nutritional broodstock history on the transcriptome of alevins collected before first feeding (Benjamini–Hochberg false discovery rate correction, P cut-off 0·05). For analysis of whole-body alevins collected after 3 weeks of feeding, differentially expressed genes were obtained by two-way ANOVA, with the different broodstock nutritional histories and first-feeding diets as independent variables (Benjamini–Hochberg correction, P cut-off 0·05). For all genes found to be differentially expressed, gene ontology (GO) annotations (biological process, cellular component, molecular functions) were obtained using Expression Analysis Systematic Explorer (EASE) software version 2.0( Reference Hosack, Dennis and Sherman 36 ). Significant enrichment of GO was tested using EASE software and the Benjamini correction (score<0·05). Gene expression data obtained by RT-qPCR were tested for normality and homogeneity of variances using Shapiro–Wilk’s test and Levene’s test, respectively. When variances were not normally distributed, a logarithmic transformation was performed. To assess the effects of the nutritional broodstock history and the first-feeding diets, gene expression was analysed by two-way ANOVA (P<0·05). Post hoc comparisons were made using Tukey’s range test, and differences were considered statistically significant at P<0·05. Correlation of the mRNA measurement by microarray with that by RT-PCR for two of the tested genes, chosen as examples, is provided in the online Supplementary Fig. S1.
Results
Growth performance
Survival rates and weight of alevins before first feeding and after 3 weeks of feeding are given in Table 3. No statistically significant differences in survival were found in alevins from the different experimental groups, either before first feeding or after the 3-week feeding challenge.
Table 3 Survival rates and weights of alevins collected before first feeding and after 3 weeks of feeding (Mean values and standard deviations)
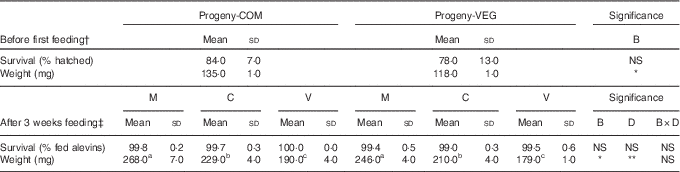
COM, commercial diet; VEG, plant-based diet; B, broodstock nutritional history effect; D, first-feeding diet effect; B×D, interaction.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0.05; Tukey’s comparison test). * P<0·05, ** P<0·01, *** P<0·001.
† Statistical difference is determined by independent sample t test followed by Tukey’s honestly significant difference comparison test, when appropriate.
‡ Statistical difference is determined by two-way ANOVA followed by Tukey’s honestly significant difference comparison test, when appropriate.
Before first feeding, alevins developing from VEG-fed females had significantly lower body weights (−13 %, P<0·001) compared with those from COM-fed females. The initial slight difference in weight resulting from the maternal nutritional history (VEG v. COM) was maintained after 3 weeks of feeding, irrespective of the diets fed to the alevins. After the 3-week feeding trial, alevins responded to the three dietary treatments (M, C or V) irrespective of maternal nutritional history, with lower growth when fed the V diet (V v. M: −27 %; V v. C: −15 %).
Alevin whole-body lipid composition
Data on alevins collected before first feeding are presented in detail in the study by Lazzarotto et al. ( Reference Lazzarotto, Corraze and Leprevost 26 ) and are summarised in the online Supplementary File 1. In brief, before first feeding, there were no significant differences in lipid content between alevins originating from COM-fed (5·9 % of fresh weight) and alevins originating from VEG-fed (5·6 % of fresh weight) females. The whole-body lipid content of fry mainly comprised NL (70 %; PL: 30 %) in progeny from both broodstock groups (COM and VEG).
In alevins collected after the 3-week feeding challenge (Table 4), we observed an effect of both maternal nutritional history and first-feeding diets on the whole-body lipid content, whereas no interaction between the two factors was found. Lipid content was significantly higher in progeny from the VEG-fed females that received the M diet for 3 weeks (5 % of fresh weight), whereas the progeny from the COM-fed females that received the V diet had the lowest whole-body lipid content (4 % of fresh weight). No significant differences were found between the other treatment groups.
Table 4 Total lipid content (percentage of fresh weight) and fatty acid composition (percentage of total fatty acid) of polar and neutral lipid fractions of whole-body alevins collected after 3 weeks of feeding (Mean values and standard deviations)

VEG, plant-based diet; COM, commercial diet; FM, fishmeal; FO, fish oil; Diet M, marine FM-FO-based diet; Diet C, commercial-like FM-FO and plant-based diet; Diet V, experimental 100 % plant-based diet; B, broodstock nutritional history effect; D, first-feeding diet effect; B×D, interaction; PL, polar lipids; NL, neutral lipids; ARA, arachidonic acid.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05; Tukey’s comparison test). * P<0·05, ** P<0·01, *** P<0·001. † Statistical differences were determined by two-way ANOVA followed by Tukey’s honestly significant difference comparison test, when appropriate.
The respective proportions of NL and PL were similar in all experimental groups (70 % NL and 30 % PL), and were therefore not affected by dietary treatments.
Fatty acid composition
Alevins before first feeding
Data on FA profiles of whole-body alevins collected before first feeding are presented in detail by Lazzarotto et al. ( Reference Lazzarotto, Corraze and Leprevost 26 ) and were used in the present study (online Supplementary File 1) as a starting point for comparison with data from alevins fed for 3 weeks. We found that alevins of females fed the VEG diet had higher n-6 PUFA, 18 : 2n-6, arachidonic acid (ARA) and 18 : 3n-3 levels before the first feeding compared with those from COM-fed females. In contrast, higher percentages of n-3 PUFA, EPA and DHA were found in progeny from COM-fed females, with the exception of the PL fraction, where no significant differences were found in DHA content between groups. Lower amounts of EPA+DHA were found in alevins from VEG-fed females (1·4 mg alevin−1) than in alevins from COM-fed females (2·6 mg alevin−1) (Table 5).
Table 5 EPA and DHA contents (mg alevin−1) in whole-body alevins collected before first feeding and after 3 weeks of feeding

COM, commercial diet; VEG, plant-based diet; FM, fishmeal; FO, fish oil; Diet M, marine FM-FO-based diet; Diet C, commercial-like FM-FO and plant-based diet; Diet V, experimental 100 % plant-based diet.
Alevins after 3 weeks of feeding
After 3 weeks of feeding, the fatty acid composition of whole-body alevin samples reflected those of the respective experimental first-feeding diets M, C or V (Table 4).
Polar lipid fraction
All FA classes (except SFA) were significantly affected by both broodstock nutritional history and the dietary treatment.
Lower percentages of SFA were found in fish fed the C and V diets, compared with M-fed fish. Levels of MUFA were higher in fish fed the C and V diets, with higher values in fish from VEG-fed females. The percentage of total n-6 PUFA (reflecting mainly 18 : 2n-6) was higher when FM and FO were replaced by plant ingredients (C and V diets), with higher levels in progeny from females fed the VEG diet. On the other hand, levels of n-3 PUFA were significantly higher in progeny from COM-fed females, with EPA and DHA levels being the lowest in fish fed the V diet.
Neutral lipid fraction
Lower levels of SFA were found in alevins fed the C and V diets, the lowest levels being found in progeny from broodstock fed the VEG diet. Higher percentages of MUFA were found in alevins fed the C diet in both broodstock groups (mainly due to the higher 18 : 1 content).
Alevins originating from females fed the VEG diet exhibited higher (or equal) levels of n-6 PUFA than those from the COM-fed broodstock, with higher levels with the V diet compared with the other groups. Alevins receiving the C diet had values intermediate between the M-fed and the V-fed alevins (V>C>M). These differences were related to the greater quantities of linoleic acid in alevins fed the V diet. Higher proportions of ARA were found in alevins fed the V diet, and values in progeny of VEG-fed females were higher.
Lower n-3 PUFA levels were found in progeny from VEG-fed females compared with progeny from COM-fed females. Alevins fed the V diet had lower percentages of n-3 PUFA compared with alevins fed the C or M diet. Higher (or equal) proportions of 18 : 3n-3 were found in alevins originating from females fed the VEG diet compared with those from COM-fed females. Percentages of 18 : 3n-3 were higher in alevins fed the V diet compared with those fed the C or M diet, irrespective of broodstock nutritional history. On the other hand, lower percentages of EPA and DHA were found in alevins originating from females fed the VEG diet. Alevins fed the V diet had lower EPA and DHA values than alevins fed the other experimental diets (C or M).
Amounts of EPA+DHA
The difference in quantity of EPA+DHA (mg alevin−1) originating from the maternal nutritional history (COM v. VEG) still remained after 3 weeks of feeding, with lower levels recovered in progeny from VEG-fed females, irrespective of the first-feeding diets (Table 5). After 3 weeks of feeding, lower levels of EPA+DHA were found in progeny fed the V diet, irrespective of the broodstock nutritional history (COM, V v. M: −62 % and V v. C: −40 %; VEG, V v. M: −65 % and V v. C: −44 %).
Transcriptomics
Microarray results
Transcriptome of alevins collected before first feeding
Although 3185 genes exhibited fold changes (FC)>1·5, 624 (FC>2) and 114 (FC>3) between progeny originating for COM-fed and VEG-fed females (Table 6(a)), none of the changes was statistically significant (P>0·05, false discovery rate>5 %).
Table 6 Whole-body transcriptome of alevins collected (a) before first feeding and (b) after 3 weeks of feedingFootnote *: fold changes (FC) and number of differentially expressed genes between groups (VEG-fed v. COM-fed)
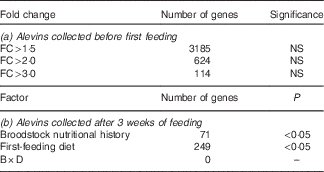
VEG, plant-based diet; COM, commercial diet; B, broodstock nutritional history; D, first-feeding diet; B×D, interaction.
* Data were obtained by two-way ANOVA (P<0·05, Benjamini–Hochberg correction, P cut-off 0·05).
Transcriptome of alevins collected after 3 weeks of feeding
Two-way ANOVA analysis of the transcriptome profile of whole-body alevins collected 3 weeks after first feeding revealed that seventy-one genes were significantly differentially expressed in response to the broodstock nutritional background, and 249 gene features in response to the first-feeding diets. No significant interaction between the nutritional background of female broodstock and first-feeding diets was detected at the level of gene expression (Table 6(b)). The GO enrichment analysis highlighted changes in expression of genes involved in different GO categories (Fig. 1(a) and (b)). In the following discussion, we will focus on the main over-represented processes, which are principally involved in metabolism-related biological processes.

Fig. 1 Whole-body alevins transcriptome: proportions of different gene ontology categories represented by differentially expressed genes obtained by a two-way ANOVA (false discovery rate 0·05). (a) Broodstock nutritional history effect. ![]() , Carbohydrate/energy metabolism (20 %);
, Carbohydrate/energy metabolism (20 %); ![]() , muscle contraction/cell motility (22 %);
, muscle contraction/cell motility (22 %); ![]() , lipid metabolism (2 %); (b) first-feeding diet effect.
, lipid metabolism (2 %); (b) first-feeding diet effect. ![]() , Amino acid/protein metabolism (17 %);
, Amino acid/protein metabolism (17 %); ![]() , cholesterol/lipid metabolism (14 %);
, cholesterol/lipid metabolism (14 %); ![]() , carbohydrate/energy metabolism (12 %);
, carbohydrate/energy metabolism (12 %); ![]() , muscle contraction (8 %);
, muscle contraction (8 %); ![]() , transport and catabolism (10 %);
, transport and catabolism (10 %); ![]() , oxidation-reduction process (7 %);
, oxidation-reduction process (7 %); ![]() , transcription/translation (7 %);
, transcription/translation (7 %); ![]() , apoptotic process (2 %);
, apoptotic process (2 %); ![]() , trans-sulphuration pathways (2 %);
, trans-sulphuration pathways (2 %); ![]() , miscellaneous (22 %).
, miscellaneous (22 %).
Effects of broodstock nutritional history
With regard to the analysis of the effects of broodstock nutritional history (VEG v. COM) on gene expression in alevins, fifty-four of the seventy-one differentially expressed probes had an assigned gene annotation. The GO enrichment analysis highlighted changes in metabolism-related biological processes (EASE score<0·05). In particular, eleven genes involved in carbohydrate metabolism and energy pathways (20 % of annotated genes) were found to be down-regulated in the transcriptome of whole-body alevins from females fed the VEG diet, compared with alevins from COM-fed broodstock. The GO enrichment also indicated differential expression of twelve genes related to muscle growth and contraction (22 % of annotated genes). For these genes, microarray analysis also revealed overall down-regulation in the transcriptome of whole bodies of progeny of females fed the VEG diet compared with those of COM-fed females (Table 7).
Table 7 Impact of broodstock nutritional history on whole-body transcriptome of alevins collected after 3 weeks of feedingFootnote *

FC, fold change; FM, fishmeal; FO, fish oil; VEG, plant-based diet; Diet M, marine FM-FO-based diet; COM, commercial diet; Diet C, commercial-like FM-FO and plant-based diet; Diet V, experimental 100 % plant-based diet.
* Fold changes refer to progeny developing from VEG-fed females compared with progeny from COM-fed females.
† Genes tested by RT-qPCR.
Effects of first-feeding diets
Of the 249 probes corresponding to genes differentially expressed in response to the first-feeding diets, 133 had an assigned gene annotation. GO enrichment for the biological process was performed to interpret this list of genes further. The GO enrichment analysis revealed over-representation of biological processes related to amino acid/protein metabolism (sixteen genes, 17 % of annotated genes), lipid/cholesterol metabolism (thirteen genes, 14 % of annotated genes), carbohydrate and energy metabolism (eleven genes, 12 % of annotated genes), transport and catabolism (nine genes, 10 % of annotated genes) and muscle contraction (seven genes, 8 % of annotated genes). The other GO processes affected by the first-feeding diets (oxidation-reduction process, transcription/translation and trans-sulphuration pathways) and their respective percentages are shown in Fig. 1(b). The microarray analysis showed up-regulation of the genes involved in both amino acid/protein metabolism and lipid and cholesterol metabolism with the introduction of plant-based ingredients in the diets (Table 8). By studying the expression of genes involved in carbohydrate and energy metabolism, we observed down-regulation of glucokinase (GCK) with the C diet, and this effect became more evident when fish were fed the V diet. In contrast, up-regulation of hexokinase (HK2) was found with the C diet, which became more pronounced with the V diet. Down-regulation of genes involved in muscle contraction was also observed in the transcriptome of fish fed the C and V diets, compared with those fed the M diet. Genes involved in transport and catabolism were up-regulated in fish fed the plant-based C and V diets. A complete list of the pathways that have been found to be affected by the first-feeding diet is provided in the online Supplementary Table S2.
Table 8 Impact of experimental first-feeding diets on whole-body transcriptome of alevins after three weeks of feeding (main Biological Processes impacted)Footnote *

FC, fold change; FM, fishmeal; FO, fish oil; COM, commercial diet; Diet C, commercial-like FM-FO and plant-based diet; Diet M, marine FM-FO-based diet; Diet V, experimental 100 % plant-based diet; VEG, plant-based diet.
* Fold changes refer to progeny fed C or V diet compared with fish fed the M diet.
† Genes tested by RT-qPCR.
Real time quantitative PCR
Effects of broodstock nutritional history on gene expression
Of the genes found to be differentially expressed by microarray approach, four of the genes involved in muscle growth and contraction were analysed by RT-qPCR (α-actin (ACTA1), creatine kinase muscle (CKM), myosin-binding proteins-C (MYBPC1, MYBPC2), and are presented in online Supplementary Fig. S2(a). The analysis revealed down-regulation of these genes in progeny from females fed the VEG diet, confirming the microarray results. In addition, RT-qPCR showed an effect of the first-feeding diets (P<0·01) on ACTA1 and an interaction between the broodstock nutritional history and the first-feeding diets on CKM (P<0·05), which were not evident on microarray analysis.
Of the genes involved in carbohydrate metabolism and energy pathways (online Supplementary Fig. S2(b)), phosphoglycerate kinase 1 (PGK1) was up-regulated in progeny from VEG-fed females, not confirming the microarray analysis. Expression levels of five other genes (phosphorylase glycogen, muscle (PYGM), phosphorylase glycogen, liver (PYGL), phosphofructokinase, muscle (PFKM), succinate dehydrogenase complex subunit A (SDHA) and glycerol-3-phosphate dehydrogenase 1 (GPD1)) were not significantly changed when measured by RT-qPCR.
Effects of first-feeding diets on gene expression
A number of genes involved in amino acid and protein metabolism (isoleucyl-tRNA synthetase (IARS), leucyl-tRNA synthetase (LARS), glutamyl-prolyl-tRNA synthetase (EPRS) and aspartyl-tRNA synthetase (DARS)) were assayed by RT-qPCR, confirming the up-regulation with the V diet observed by microarray analysis (online Supplementary Fig. S3(a)). With regard to cholesterol metabolism, two genes involved in cholesterol synthesis were analysed by RT-qPCR (3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and 3-hydroxy-3-methylglutaryl-CoA synthase 1 (HMGCS1)), and the results are presented in the online Supplementary Fig. S3(b). Up-regulation of these genes was observed with the introduction of plant-based ingredients in the diet (C diet and V diet). Among the genes involved in carbohydrate metabolism that showed changed expression in the array analysis, three were also analysed by RT-qPCR (GCK, HK2 and lactate dehydrogenase A (LDHA)). GCK and LDHA were down-regulated with the V diet, whereas up-regulation of HK2 expression was observed, confirming the microarray results (online Supplementary Fig. S3(c)).
Discussion
This study is to our knowledge the first investigation into the effects of a totally plant-based diet (no FM or FO) on the whole-body transcriptome of rainbow trout alevins. It is also one of the first studies investigating the consequences of long-term feeding broodstock (3 years) a totally plant-based diet on the ability of progeny to respond to different first-feeding diets with a replacement of marine ingredients rate of up to 100 %. The relatively low values of FC found in this study (although statistically significant) suggest that the modifications induced by the diets, and therefore the metabolic consequences of the dietary replacement, are not so drastic. It is also important to bear in mind that one of the limitations of transcriptomic analysis in early stages might be linked to the use of RNA extracted from whole-body fish, because such sample types include a mixture of different organs. The use of this kind of sample thus does not provide information about the regulation of expression in a specific organ and/or tissue.
Plant-based diets do not have detrimental effects on survival but affect growth of alevins
We recently demonstrated that feeding broodstock the VEG diet throughout a 3-year life cycle had no detrimental effects on survival but resulted in lower body weight of fry before first feeding compared with those originating from COM-fed females( Reference Lazzarotto, Corraze and Leprevost 26 ). Survival levels after the 3-week feeding challenge did not differ between alevins fed any of the three experimental diets (M, C or V), irrespective of the broodstock nutritional history (COM or VEG). We found that a 50 % replacement rate (C diet) resulted in lower body weights and the effect was more pronounced with total replacement (M>C>V), irrespective of the maternal nutritional history. The concomitant replacement of marine ingredients by plant-protein sources and vegetable oils is known to be responsible for a reduction in feed intake and feed efficiency( Reference Lim, Webster and Lee 37 – Reference Corraze and Kaushik 40 ), resulting in reduced growth performance( Reference Drew, Ogunkoya and Janz 41 ). This effect is believed to be mainly related to the replacement of FM but not to FO substitution in rainbow trout( Reference Panserat, Kolditz and Richard 17 , Reference Kaushik, Cravedi and Lalles 42 ), European seabass( Reference Kaushik, Coves and Dutto 43 ) or gilthead sea bream( Reference Gómez-Requeni, Mingarro and Calduch-Giner 44 ).
Maternal nutritional history has no visible effect on whole-body transcriptome of alevins before first feeding
Early embryonic development in teleosts is governed until the start of zygotic transcription by maternally supplied mRNA that is incorporated into the oocyte during oogenesis( Reference Lubzens, Young and Bobe 45 ). Maternal mRNA is critical to embryonic development as it implements basic biosynthetic processes, directs first mitotic divisions and defines initial cell fate and embryonic patterning( Reference Dworkin and Dworkin‐Rastl 46 ). Given the previous findings on the effects of a plant-based diet on the transcriptome of adult fish and the importance of broodstock nutrition for the development of progeny, our hypothesis was that broodstock nutritional history can affect progeny transcriptome. However, our results did not demonstrate any significant regulation in the whole-body transcriptomic profile of alevins before the first feeding, despite differences in body weight and FA profile. These results suggest that no trans-generational effects linked to maternal nutritional background are present or visible at a molecular level at this specific developmental stage. One possible explanation could be that the transcriptional differences are governed by specific tissues such as the liver that are present in smaller proportions in whole individuals at this specific stage of development. The liver represents only a small proportion (approximately 1 %) of alevin whole-body components. Such a small proportion might have prevented detection of the transcriptional differences at the level of the whole individual. Nevertheless, we used the same type of sample to analyse the transcriptome of alevins after 3 weeks of feeding, and we found a number of genes linked to intermediary metabolism that were differentially expressed – that is, according to the broodstock origin. Thus, the hypothesis of a ‘whole-body diluted effect’ related to the sample type probably cannot fully explain the absence of a significant maternal effect.
Maternal nutritional history and first-feeding diets affect the whole-body transcriptome of alevins after 3 weeks of feeding
Muscle growth/contraction and metabolism-related biological processes constitute the largest group among the GO terms associated with the genes found to be differentially expressed in response to both broodstock nutritional history and first-feeding diets of exogenous feeding alevins. In the following discussion, we therefore focus on specific actors involved in metabolism from a nutrigenomic point of view in relation to different levels of FM/FO dietary replacement. However, as no interaction was found between the two factors, the effects of broodstock nutritional history and first-feeding diets are discussed separately.
Effects of broodstock nutritional history
In contrast to what was observed in alevins collected before first feeding, we found a significant effect of the maternal dietary background on the transcriptomic profile of alevins after 3 weeks of exogenous feeding. One of the possible reasons to explain these results can be found in the switch of alevins from endogenous (vitellus) to exogenous feeding (external feeding). Indeed, the initiation of exogenous feeding is known to alter gene expression, through the activation of different metabolic pathways( Reference Mennigen, Skiba-Cassy and Panserat 29 ).
A set of genes related to different aspects of muscle development and contraction was found to be down-regulated in progeny from females fed the VEG diet compared with those from COM-fed broodstock. In particular, we observed down-regulation of creatine kinases (CKM-creatine kinase, brain (CKB)) and myomesins (MYOM1 and MYOM2), which are involved in the structure of the contractile muscles, as well as down-regulation of ACTA1, which is the major constituent of the contractile apparatus. Down-regulation of myosin (myosin heavy chain (MYH2)) and slow- and fast-type MYBPC1 and MYBPC2, respectively was also observed in alevins from VEG-fed females. In fish, as in other vertebrates, skeletal muscle formation (myogenesis) involves the specific control of several myogenic regulatory factors that control processes such as specification, activation and differentiation of myogenic cells( Reference Fuentes, Valdés and Molina 47 ). Once myogenic cells are activated, they proliferate and differentiate; finally, in the later stage of differentiation, the expressions of different genes that encode structural muscle proteins such as myosin light chain, actin and MYH2 are up-regulated, marking sarcomeric assembly( Reference Johnston 48 ). The down-regulation of the major muscular actors observed in the present study in progeny originating from VEG-fed females could therefore be mainly related to the delayed growth, and specifically muscle mass growth and development, rather than to the metabolism. Moreover, after 3 weeks of feeding, the differences in body weight observed between groups in response to broodstock nutritional background became more evident. This increased difference could thus have helped in making the transcriptional changes detectable.
Furthermore, an overall decrease in expressions of genes related to carbohydrate and energy metabolism was found. For example, a specific form of muscular phosphofructokinase (PFKM), an actor of glycolysis, the main pathway providing energy for swimming activity in fish white muscle, was less strongly expressed in fish fed diets containing plant ingredients. Another gene encoding creatine kinase was also associated with the glycolysis-related gene expression pattern. Previous studies on larva development of European sea bass( Reference Darias, Zambonino-Infante and Hugot 30 ) showed that these genes were increasingly expressed throughout larva growth, linked to the development of skeletal muscle( Reference Wieser 49 ). These findings suggest that the delayed growth recorded in fish from VEG-fed females in our study may be linked to delayed muscle differentiation.
Considering the expressions of genes involved in carbohydrate metabolism and energy pathways, the results obtained by microarray analysis were not confirmed by RT-qPCR. This might be due to the fact that the primers designed for RT-qPCR do not necessarily match exactly the probes on the array, as it has been previously observed in a study on Atlantic salmon liver( Reference Morais, Pratoomyot and Taggart 18 ). Indeed, due to the whole-genome duplication that occurred in salmonids( Reference Allendorf and Thorgaard 50 ), transcriptomic and gene expression studies are often more challenging because of the presence of duplicated and highly similar genes whose transcripts might be differentially regulated.
Effects of first-feeding diets
The dietary replacement of both marine proteins and oil sources by plant ingredients has been shown to result in changes in protein metabolism( Reference Panserat, Hortopan and Plagnes-Juan 10 , Reference Tacchi, Secombes and Bickerdike 21 ). Interestingly, we found up-regulation of eleven aminoacyl-transfer RNA (tRNA) synthetases, which catalyse the ligation of specific amino acids to their cognate tRNA, and thereby assemble the building blocks of RNA translation and protein synthesis( Reference Ibba and Söll 51 ), with the plant-based diets. The results thus showed concomitantly higher expressions of three initiation factors and a translation elongation factor in fish fed the V and C diets. Taken together, these results seem to suggest that the replacement of FM and FO dietary sources by plant-based ingredients led to higher levels of protein synthesis. Previous studies in fish have shown that protein synthesis rates differ between tissues( Reference Fauconneau and Arnal 52 , Reference Carter and Houlihan 53 ). In our study, we focused on the early stages, a period of major changes in development, during which fish go through differential rates of relative growth of organs, called allometry( Reference Fuiman 54 ), in order to meet the specific needs of this critical developing stage and to ensure that the most essential organs for primary functions are developed first, followed by the development of organs with lower priority for survival( Reference Osse and Van den Boogaart 55 ). According to these assumptions, and considering the delay in (muscle) growth found in fish fed the plant-based diets, we can hypothesise that the differences in gene expressions between groups were mostly linked to the delay in development of the plant-fed groups. However, as a number of processes have key roles in protein and amino acid metabolism, the biological significance of the changes in gene expression observed is limited and we prefer to treat this hypothesis with caution.
As for the broodstock nutritional history-related effects, down-regulation of genes involved in muscle contraction was also found in response to the first-feeding diets in progeny receiving diets containing increasing levels of plant ingredients. These findings seem to confirm our previous hypothesis, reflecting the delay in growth and muscle development induced by plant-based diets.
Another metabolic pathway significantly affected by dietary FM and FO replacement was that of sterol metabolism. Our results suggest a general up-regulation in the expression levels of genes involved in cholesterol metabolism in fish fed diets containing increased levels of plant ingredients – namely, the C diet and the V diet. Among the genes we found to be differentially expressed, HMGCR, a transmembrane glycoprotein involved in the rate-limiting step of sterol biosynthesis, was increased, as reported in European sea bass fed a diet where fish oil was replaced by vegetable oils( Reference Geay, Ferraresso and Zambonino-Infante 14 ). In previous studies with Atlantic salmon( Reference Leaver, Villeneuve and Obach 19 ) and rainbow trout( Reference Panserat, Hortopan and Plagnes-Juan 10 ), the authors found up-regulation of genes involved in cholesterol biosynthesis. Plant ingredients are in fact rich in phytosterols that can interfere with cholesterol metabolism, whereas diets based on marine FM and FO contain greater amounts of cholesterol( Reference Tocher, Bendiksen and Campbell 56 ). The positive effects on genes of cholesterol biosynthesis pathways found in our study confirmed that trout fed the plant-based diets were capable of responding to the reduced dietary cholesterol levels as early as 3 weeks from first feeding. Indeed, the cholesterol content in our experimental diets was lower in the V diet (0·34 %) and the C diet (0·52 %) than in the M diet (0·66 %).
Our findings also suggest differential regulation of genes involved in different steps of glucose metabolism with the introduction of plant ingredients in the diet. In alevins fed the V diet, we observed up-regulation of HK2, a gene involved in the first step of the glycolysis pathway( Reference Pilkis and Granner 57 ), and down-regulation of GCK, which is involved in maintaining the hepatic glucose balance. Focusing on the latter, a previous study with rainbow trout, gilthead sea bream and common carp( Reference Panserat, Médale and Blin 58 ) showed that nutritional induction of GCK gene expression and activity was associated with a high dietary carbohydrate (starch) intake. In our study, the down-regulation of GCK may have been linked to the lower level of dietary starch in the C and V diets (about 10 v. 13·5 % in the M diet). The low level of expression could also explain the absence of induction of genes involved in lipogenesis, this process being induced when glucose is in excess. α-Enolase, which participates in the conversion of glucose to pyruvate, a key intermediate at the intersection of multiple metabolic pathways including lipogenesis, was slightly down-regulated in fish fed the C and V diets, as previously observed in salmon fed rapeseed oil compared with those fed FO( Reference Jordal, Torstensen and Tsoi 16 , Reference Morais, Pratoomyot and Taggart 18 ).
Effects of broodstock nutritional history and first-feeding diets on fatty acid profile of alevins
In a previous study, we showed that feeding broodstock a totally plant-based diet (VEG) throughout the life cycle affects the fatty profile of progeny (before first feeding) with regard to both PL and NL fractions( Reference Lazzarotto, Corraze and Leprevost 26 ). In the present study, the analysis of whole-body FA composition of alevins showed higher percentages of 18 : 2n-6 and 18 : 3n-3 in those originating from broodstock fed the VEG diet and in response to the V diet. These results reflected the higher dietary content of these FA and were consistent with findings in many studies on feeding fish vegetable oils( Reference Morais, Edvardsen and Tocher 24 , Reference Izquierdo, Obach and Arantzamendi 59 – Reference Mourente, Good and Bell 61 ). Moreover, non-negligible amounts of n-3 LC-PUFA (EPA and DHA) were found in both PL and NL fractions, although the dietary intake was nil with the plant-based diet. These results suggest active bioconversion from dietary precursor 18 : 3n-3, and subsequent activation of the LC-PUFA biosynthesis pathway. Previous studies analysing fish transcriptome responses after dietary substitution of FO with vegetable oils have shown that lipid metabolism is highly affected( Reference Morais, Pratoomyot and Taggart 18 , Reference Leaver, Villeneuve and Obach 19 , Reference Calduch-Giner, Sitjà-Bobadilla and Davey 23 , Reference Morais, Pratoomyot and Torstensen 62 , Reference Limtipsuntorn, Haga and Kondo 63 ), regardless of the vegetable oil used. For instance, in these studies, genes involved in LC-PUFA biosynthesis were over-represented among the differentially expressed genes in Atlantic salmon post-smolts( Reference Leaver, Villeneuve and Obach 19 ) and in juvenile rainbow trout( Reference Panserat, Hortopan and Plagnes-Juan 10 ). The biosynthesis of n-3 LC-PUFA in vertebrates involves consecutive desaturation and elongation reactions, which convert the 18 : 3n-3 (α-linolenic acid) to longer-chain more-unsaturated FA of the same series, including EPA and DHA( Reference Tocher 2 ). Two types of enzymes are responsible for this conversion – namely, fatty acid desaturases and elongases. The former introduce a double bond in the fatty acyl chain from the carboxyl group, and elongases account for the condensation of activated fatty acids with malonyl-CoA in the FA elongation pathway. The analysis of our transcriptomic data on alevins did not show any significant changes in the expressions of genes involved in this pathway. A possible explanation of this result may be that we used RNA extracted from whole-body alevins, including a mixture of different organs. Indeed, the use of this kind of sample does not allow unambiguous interpretation of the diet-induced regulation of gene expression, because regulation of genes in the liver and intestine, the main tissues in which the bioconversion of LC-PUFA occurs, can be masked by the mean expression pattern throughout the other organs/tissues of whole fish, especially the muscle. Moreover, when comparing the amounts of EPA+DHA (mg alevin−1) in whole-body alevins at our starting point (before first feeding) and at the end of the trial (after 3 weeks of feeding), we observed a decrease in their relative quantities in alevins fed the V-diet, irrespective of the broodstock nutritional history. Indeed, during the 3-week feeding trial, V fed alevins from both COM- and VEG-fed females used about 54 and 36 % of the amounts of EPA+DHA they had at the beginning of the trial, respectively. These results suggest that the reserves in terms of n-3 LC-PUFA provided by the mother through the egg (vitellus) are enough to satisfy the needs of alevins during early development, and therefore they do not need to activate the bioconversion pathway at this stage.
The present study confirmed that increasing replacement of fishmeal and fish oil by plant ingredients (up to total replacement) in the rainbow trout diet allowed fish to survive and grow, but with slight differences in terms of weight. The replacement of marine sources by plant-based ingredients in both broodstock and first-feeding diets resulted in significant effects on the transcriptome of whole-body alevins after 3 weeks of feeding. However, the relatively low values of FC found in this study (although statistically significant) suggest that the modifications induced by the diets, and therefore the metabolic consequences of the dietary replacement, are not too drastic. An organ-dedicated approach would be more informative and precise to improve understanding of the effects of external input, and specifically the replacement of FM and FO by plant ingredients.
Overall, these results improve the understanding of mechanisms and pathways activated by concomitant FM and FO replacement in diets for rainbow trout. These results also provide a framework for additional research on the consequences of maternal nutrition with reduced levels of fishmeal and fish oil on the physiological and metabolic responses of progeny to different replacement rates in the first-feeding diets. These results open up avenues for further reduction of the reliance of aquaculture on marine fishery resources by using plant-based diets over the full life cycle of fish, including broodstock and the early stages. Indeed, the limited negative consequences, despite the suppression of FM and FO, suggest that larger proportions of FM and FO can be replaced by plant ingredients in diets for trout broodstock and alevin, compared with what is currently practiced.
Acknowledgements
The authors thank the team at the PEIMA experimental facilities (INRA, Sizun, France) for rearing broodstock and providing eggs, P. Maunas and N. Turonnet from the INRA experimental facilities of Lées Athas (Pyrénées Atlantique, France) for rearing fish and F. Sandres and F. Terrier for manufacturing the experimental diets (INRA, Donzacq, France). The authors thank A. Le Cam and J. Montfort (INRA-LPGP, Rennes, France) and T. Cerezo for technical assistance.
This work was supported by the European Advanced Research Initiatives for Nutrition and Aquaculture no. 288925 project and by the ‘Region Aquitaine’. The funders had no role in the design, analysis or writing of the article.
Formulated research questions and designed the study: F. M., G. C. Performed the experiments: V. L., L. L., F. M., G. C. Analysed the data: V. L., G. C., D. M., F. M. Wrote the paper: V. L. G. C., F. M.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001252












