Introduction
The use of remote camera traps to survey wildlife has become increasingly popular due to cost-effectiveness, non-invasiveness, and because multiple study questions can be addressed simultaneously with the data collected (Sunarto et al. Reference Sunarto, Mohamed and Kelly2013, Monterroso et al. Reference Monterroso, Rich, Serronha, Ferreras and Alves2014, Trolliet et al. Reference Trolliet, Huynen, Vermeulen and Hambuckers2014, Welbourne et al. Reference Welbourne, MacGregor, Paull and Lindenmayer2015). In particular, the application of occupancy analysis to presence-absence data collected via camera traps allows researchers to determine factors that influence distribution (Erb et al. Reference Erb, McShea and Guralnick2012, Gerber et al. Reference Gerber, Karpanty and Randrianantenaina2012) and monitor population trends over multiple years (Karanth et al. Reference Karanth, Nichols, Kumar, Jathanna, O’Connel, D Nichols and Karanth2011, O’Connell and Bailey Reference O’Connell, Bailey, O’Connell, Nichols and Karanth2011). Although a majority of camera-trapping surveys are focused on medium- to large-sized mammals, they generate extensive observations on non-target species, such as ground dwelling birds (O’Brien and Kinnaird Reference O’Brien and Kinnaird2008, Davis et al. Reference Davis, Kelly and Stauffer2011, Beaudrot et al. Reference Beaudrot, Ahumada, O’Brien, Alvarez-Loayza, Boekee, Campos-Arceiz, Eichberg, Espinosa, Fegraus, Fletcher, Gajapersad, Hallam, Hurtado, Jansen, Kumar, Larney, Lima, Mahony, Martin, McWilliam, Mugerwa, Ndoundou-Hockemba, Razafimahaimodison, Romero-Saltos, Rovero, Salvador, Santos, Sheil, Spironello, Willig, Winarni, Zvoleff and Andelman2016), which can be used to fill gaps in ecological knowledge and further inform conservation and management decisions.
Ground-dwelling forest birds disperse seeds (Caves et al. Reference Caves, Jennings, Hillerislambers, Tewksbury and Rogers2013), regulate pest species, create burrows or cavities that other species use (Sekercioglu Reference Sekercioglu2006), provide protein sources for local people in the form of bushmeat (Gardner and Davies Reference Gardner and Davies2014), and attract the economic benefits of ecotourism to local communities (Sekercioglu Reference Sekercioglu2002). However, they are also often sensitive to habitat loss and degradation, particularly if they are rare, habitat specialists, or unable to use and/or disperse across matrix habitat (Thiollay Reference Thiollay1999, Lambert and Collar Reference Lambert and Collar2002, Sekercioglu et al. Reference Sekercioglu, Ehrlich, Daily, Aygen, Goehring and Sandi2002, Korfanta et al. Reference Korfanta, Newmark and Kauffman2012). Such forest birds also tend to be elusive (O’Brien and Kinnaird Reference O’Brien and Kinnaird2008), especially outside of the breeding season, making standard methods of detecting them—such as point count or line transects—not as efficient as camera traps, which are commonly used to monitor other elusive taxa (e.g. large carnivores; Sunarto et al. Reference Sunarto, Mohamed and Kelly2013). Although camera-trapping can be useful in answering questions regarding the distribution (Jeganathan et al. Reference Jeganathan, Green, Bowden, Norris, Pain and Rahmani2002), habitat use (Winarni et al. Reference Winarni, O’Brien, Carroll and Kinnaird2009), abundance/occupancy (Ramesh and Downs Reference Ramesh and Downs2014), and behaviour of ground-dwelling birds (Delibes-Mateos et al. Reference Delibes-Mateos, Diaz-Ruiz and Ferreras2014), relatively few studies have used camera traps to study ground-dwelling forest birds in tropical ecosystems (O’Brien and Kinnaird Reference O’Brien and Kinnaird2008, Burton et al. Reference Burton, Neilson, Moreira, Ladle, Steenweg, Fisher, Bayne, Boutin and Stephens2015, Beaudrot et al. Reference Beaudrot, Ahumada, O’Brien, Alvarez-Loayza, Boekee, Campos-Arceiz, Eichberg, Espinosa, Fegraus, Fletcher, Gajapersad, Hallam, Hurtado, Jansen, Kumar, Larney, Lima, Mahony, Martin, McWilliam, Mugerwa, Ndoundou-Hockemba, Razafimahaimodison, Romero-Saltos, Rovero, Salvador, Santos, Sheil, Spironello, Willig, Winarni, Zvoleff and Andelman2016).
Madagascar is a biodiversity hotspot and home to numerous endemic species, many of which are threatened due to continuing habitat loss and intense anthropogenic pressures (Myers et al. Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000, Brooks et al. Reference Brooks, Mittermeier, Mittermeier, da Fonseca, Rylands, Konstant, Flick, Pilgrim, Oldfield, Magin and Hilton-Taylor2002, Reference Brooks, Mittermeier, da Fonseca, Gerlach, Hoffmann, Lamoreux, Mittermeier, Pilgrim and Rodrigues2006). Fifty-one percent of Madagascar’s birds are endemic and only 15% of Madagascar’s endemic ground-dwelling birds can live in open habitats (Hawkins and Goodman Reference Hawkins, Goodman, Goodman and Benstead2003). Despite the numerous threats facing Madagascar’s ground-dwelling forest birds—including habitat loss and degradation (Scott et al. Reference Scott, Brown, Mahood, Denton, Silburn and Rakotondraparany2006, Irwin et al. Reference Irwin, Wright, Birkinshaw, Fisher, Gardner, Glos, Goodman, Loiselle, Rabeson and Raharison2010), bushmeat hunting (Gardner and Davies Reference Gardner and Davies2014), and predation by exotic carnivores (Irwin et al. Reference Irwin, Wright, Birkinshaw, Fisher, Gardner, Glos, Goodman, Loiselle, Rabeson and Raharison2010)—they are under-studied. We detected ground-dwelling forest birds during camera trap surveys originally designed for carnivores and tenrecs (Lipotyphla: Tenrecidae) at seven sites in Madagascar’s largest contiguous area of protected forest, the Masoala-Makira protected area complex. Our study takes advantage of abundant detections of non-target species from camera traps to (a) examine the response of Madagascar’s little-studied, ground-dwelling forest birds to habitat degradation and exotic predator presence and (b) examine trends over time in ground-dwelling forest bird occupancy, local colonisation, and local extirpation.
Methods
Study area
Our study was conducted in north-eastern Madagascar, the Masoala-Makira protected area complex (6,124 km2, excluding community-managed buffer; hereafter, Masoala-Makira landscape, Figure 1) is the largest contiguous area of protected forest in Madagascar and is home to six native carnivores (fosa Cryptoprocta ferox; spotted fanaloka Fossa fossana; falanouc Eupleres goudotii; ring-tailed vontsira Galidia elegans; broad-striped vontsira Galidictis fasciata; and brown-tailed vontsira Salanoia concolor) and three exotic (dogs, feral cats and the small Indian civet Viverricula indica) (Farris et al. Reference Farris, Golden, Karpanty, Murphy, Stauffer, Ratelolahy, Andrianjakarivelo, Holmes and Kelly2015b) and 85 bird species (Thorstrom and Watson Reference Thorstrom and Watson1997).
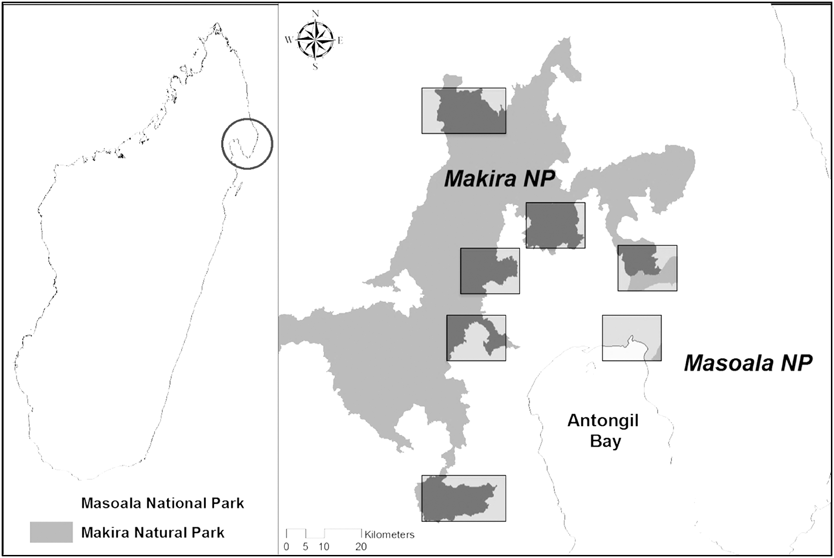
Figure 1. Location of the seven study sites (S01–S07) that were surveyed with camera traps across the Masoala-Makira protected area complex from 2008 to 2013. Site locations occur within the regions outlined by the boxes, which are used to protect the identities and locations of villages that provided sensitive hunting data.
Camera trap surveys
From 2008 to 2013 we conducted 15 camera trap surveys at seven sites across the Masoala-Makira landscape originally to monitor native carnivore and tenrec populations. Each survey consisted of 18 to 30 unbaited camera stations (Figure 1 and Appendix S1 in the online supplementary materials). Each station had two camera traps, which were positioned 20–30 cm above the ground on opposite sides of wildlife (0.0–0.5 m) or human-made trails (> 0.5 m) and operated 24 h/d. Across all 15 surveys, we used four different camera trap brands: one film (DeerCam DC300) and three digital (Reconyx PC85 and HC500; Moultrie D50 and D55; Cuddeback IR). To avoid detection biases based on camera trap brand, each camera station had two camera trap brands present; a study analysing data from the same surveys found no effect of camera trap brand on carnivore detection (Farris et al. Reference Farris, Golden, Karpanty, Murphy, Stauffer, Ratelolahy, Andrianjakarivelo, Holmes and Kelly2015b). Stations for 13 surveys were spaced 400–600 m apart, based on the home range of a native carnivore, the spotted fanaloka Fossa fossana (Kerridge et al. Reference Kerridge, Ralisoamalala, Goodman, Pasnick, Goodman and Benstead2003). The remaining two surveys of one site (S03 in 2013) were spaced 200–300 m apart to monitor tenrecs (Lipotyphla: Tenrecidae).
We define a ‘photographic event’ as an animal triggering a camera, by movement and body heat, which results in pictures of the animal. We define a ‘photographic capture’ to be the number of distinctly different individuals of a species detected within a 30-min period (Di Bitetti et al. Reference Di Bitetti, Paviolo and De Angelo2006, Davis et al. Reference Davis, Kelly and Stauffer2011). We calculated the activity of each species, defined as trap success (TS), as the number of photographic captures of a species divided by the total number of trap nights for that survey, multiplied by 100. Trap nights (TN) are the number of 24-h periods that a station had at least one camera trap functional. We combined the trap successes of three small carnivores—the ring-tailed vontsira, the broad-striped vontsira, and the brown-tailed vontsira—to create one ‘small carnivore’ trap success due to their similar size and likelihood of preying upon birds (Goodman Reference Goodman2012).
Landscape-level and station-level habitat sampling
We ranked our seven sites from least to most degraded using a maximum likelihood estimated (MLE) principal components analysis (PCA) of landscape-level and station-level habitat data, resulting in the classification of two intact (S01 and S02), three intermediately-degraded (hereafter, intermediate; S03, S04 and S05) and two degraded sites (S06 and S07; see Farris et al. Reference Farris, Golden, Karpanty, Murphy, Stauffer, Ratelolahy, Andrianjakarivelo, Holmes and Kelly2015b for habitat data collection methods and Appendix S2). We labelled sites based on their level of degradation (01 = least degraded; 07 = most degraded) rather than by area name to protect the identities of local villages near our sites, due to the sensitivity of hunting data used in related publications.
Single-season and multi-season occupancy analyses
We examined patterns in landscape occupancy and trends in annual occupancy for ground-dwelling forest birds across Masoala-Makira using single-season and multi-season occupancy analyses, respectively, in program PRESENCE (v 6.8; MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2005, Hines Reference Hines2006). We conducted a Pearson’s correlation on 41 possible covariates (i.e. landscape-level and station-level habitat characteristics, native/exotic species and human trap success, and the season in which the survey was conducted). We chose the most biologically relevant from any highly correlated covariates (|r| > 0.70) and discarded the others. Of the remaining uncorrelated covariates, we chose 20 to include in our landscape occupancy models based on a priori hypotheses (Appendix S3). All covariates were normalized within PRESENCE (Hines Reference Hines2006). We did not include covariates in annual occupancy models due to low sample sizes.
Based on camera trap data, we created capture histories, where we recorded whether a species was detected (‘1’ or present) or undetected (‘0’ or absent) for each trap night at each camera station. We then collapsed these capture histories so that each survey occasion was equal to nine trap nights to improve model convergence for single-season and multi-season occupancy analyses. We chose nine traps nights as the collapse interval because the total survey nights across most surveys could be evenly divided by that interval and that interval allowed us to robustly estimate our parameters of interest. Capture histories for landscape occupancy analyses were composed of data from the initial surveys of each site (n = 7 surveys and 148 camera stations); we excluded data from the repeated surveys of S02, S03, and S05 in landscape analyses (see Appendix S1 for description survey site details). Each site was considered independent from the other due to a) when we modelled site as a covariate on co-occurring carnivore occupancy and detection, we found no effect (Farris et al. Reference Farris, Golden, Karpanty, Murphy, Stauffer, Ratelolahy, Andrianjakarivelo, Holmes and Kelly2015b) and b) the distance between sites was large (minimum 17.5 km), such that if we did not find an effect on wide-ranging carnivores we did not expect to find an effect on ground-dwelling birds. Capture histories for annual occupancy analyses at intact forest site S02 and intermediate forest site S05 included data from the initial surveys of each site (2008 and 2011, respectively), and subsequent resurveys of the sites (Appendix S1). Camera station locations for the resurveys of S02 and S05 were similar or identical throughout the years.
To build our landscape occupancy models in our single-season occupancy analyses, we first determined which covariates influenced bird detection while holding occupancy constant. Once we determined the best detection model, we used that model as a foundation for determining what covariates influenced occupancy. Finally, we conducted goodness of fit (GOF) tests on our most parameterised models and corrected for overdispersion (ĉ ≥ 3.0; Lebreton et al. Reference Lebreton, Burnham, Clobert and Anderson1992). To build our annual occupancy models, we used the first parameterisation for multi-season occupancy analysis in PRESENCE. In this parameterisation, detection, local colonisation, and local extirpation can vary by year or secondary survey occasion (MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2005). Local colonisation is the probability that, at year t, a species colonises a site it was absent from in the previous year (t-1; MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2005). Local extirpation is the probability that, at year t, a species is extirpated from a site where it was present in the previous year (t-1; MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2005). To determine the best multi-season occupancy models for each species, we first held local colonisation and extinction constant, and we determined whether annual detection was constant throughout the years or was different from year to year or survey occasion to survey occasion. Once we determined the best detection model, we used it as a base to examine the nature of local colonisation and local extirpation throughout the years (either constant, or varying year to year or survey occasion to survey occasion). When necessary, we fixed parameter values for local colonisation or local extinction to ‘0’ or ‘1’ in PRESENCE to aid in model convergence (MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2005).
At intermediate forest site S03, the placement and number of stations was not identical between 2009 (24 stations; 400–600 m spacing) and 2013 (25–30 stations; 200–300 m spacing). Although station locations and spacing were not the same between 2009 and 2013, the extent of the camera trap grids at S03 overlapped geographically and covered similar habitat; thus we felt it best to estimate ground-dwelling bird occupancy and detection for 2009 and 2013 using a single-season occupancy analysis to estimate occupancy for each year. We then compared the estimates between the years to see if there were any differences. We included covariates in this analysis, conducted a GOF test, and estimated ĉ. Because both camera-trapping grids overlapped geographically, we feel that our analyses can be cautiously interpreted as annual trends in ground-dwelling bird occupancy in this region.
Single-season and multi-season occupancy models were considered competing if they had a ΔAIC or a ΔQAIC ≤ 2.0 (Akaike Reference Akaike, Petran and Csaki1973). Parameter and beta estimates were model-averaged unless the top model was strongly supported (model weight ≥ 80%; Akaike Reference Akaike, Petran and Csaki1973). We did not estimate occupancy or detection probabilities for species with an estimated ĉ ≥ 3.0 (i.e. lack of model fit) in the single-season occupancy analyses, and species whose multi-season occupancy models did not converge. This measure of overdispersion (ĉ ≥ 3.0) was likely caused by sparse data and/or a violation of a model assumption (MacKenzie and Bailey Reference MacKenzie and Bailey2004).
Results
From 2008 to 2013, we accumulated 18,056 TNs and obtained 4,083 captures of 26 identifiable native bird species and two exotic species (chickens Gallus gallus domesticus and Helmeted Guineafowl Numida meleagris; Appendix S4). Landscape bird trap success, including unidentified birds, was 22.61 birds/100 TN. Our seven most commonly observed species were: the Red-breasted Coua Coua serriana, Madagascar Wood-rail Mentocrex kioloides, Madagascar Turtle-dove Nesoenas picturatus, Scaly Ground-roller Geobiastes squamiger, Madagascar Magpie-robin Copsychus albospecularis, Madagascar Crested Ibis Lophotibis cristata, and Red-fronted Coua C. reynaudii; Appendices S4 and S5). Out of the seven species, Red-breasted Coua had the highest TS (7.36/100 TN) and Red-fronted Coua had the lowest (0.69/100 TN). With the exception of the Madagascar Magpie-robin, all analysed species were ground-dwelling forest birds. All bird detections occurred during dawn, day, and/or dusk hours. The seven species ranged from 50 cm (Madagascar Crested Ibis) to 18 cm (Madagascar Magpie-robin) in size (Morris and Hawkins Reference Morris and Hawkins1998).
Landscape occupancy and detection
Of the seven species, we could estimate occupancy probability for five (Red-fronted Coua and Madagascar Turtle-dove models were overdispersed). The Madagascar Magpie-robin had the highest landscape occupancy probability (ψ = 0.75 ± SE 0.09) and the Scaly Ground-roller had the lowest (ψ = 0.25 ± SE 0.06; Table 1 and Appendix S6A). Madagascar Magpie-robin occupancy was positively related to percentage of rainforest cover (β = 0.93 ± SE 0.26) and small carnivore TS (β = 1.15 ± SE 0.57; Table 2). Madagascar Crested Ibis, Madagascar Wood-rail, and Red-breasted Coua occupancy was positively related to landscape habitat patchiness (β = 1.03 ± SE 0.45, β = 1.38 ± SE 0.28, and β = 0.78 ± SE 0.24, respectively). For all five species, mean occupancy estimates were similar among intact, intermediate, and degraded forest sites (Table 1).
Table 1. Occupancy and detection probabilities (SE) from landscape single-season analyses of five ground-dwelling forest birds detected by camera traps across the Masoala-Makira landscape, northeastern Madagascar (2008–2013).
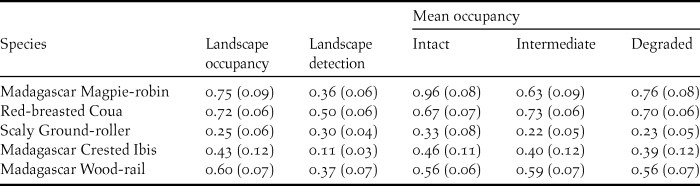
Table 2. Beta estimates (SE) from landscape single-season analyses for five ground-dwelling bird species—Madagascar Magpie-robin (MMR), Red-breasted Coua (RBC), Scaly Ground-roller (SGR), Madagascar Crested Ibis (MCI), and Madagascar Wood-rail (MWR)— in northeastern Madagascar (2008–2013). Only covariates that strongly influence occupancy or detection probability (i.e., 95% CIs do not overlap 0) are shown.
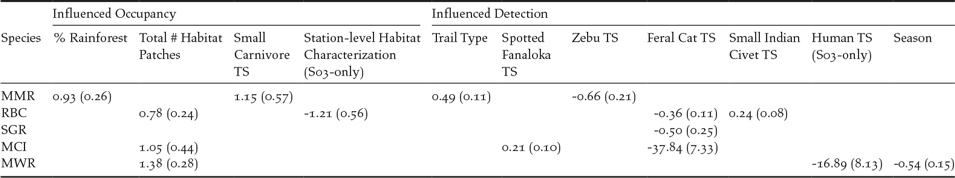
Red-breasted Couas had the highest landscape detection probability (P = 0.51 ± SE 0.06) and Madagascar Crested Ibises had the lowest (P = 0.11 ± SE 0.03; Table 1). Madagascar Crested Ibis detection was positively related to spotted fanaloka TS (β = 0.23 ± SE 0.1; Table 2). Scaly Ground-roller, Red-breasted Coua, and Madagascar Crested Ibis detection was negatively related to feral cat TS (β = -0.50 ± SE 0.25, β = -0.36 ± SE 0.12 and β = -37.15 ± SE 7.24, respectively). Red-breasted Coua detection was positively related to small Indian civet TS (β = 0.24 ± SE 0.08). Madagascar Magpie-robin detection was positively related to trail width (β = 0.49 +/- SE 0.11) and negatively related to zebu (domestic livestock; Bos primigenius) TS (β = -0.66 +/- SE 0.21; Table 2 and Appendix S6A).
Annual trends in ground-dwelling bird occupancy
Out of the five species we were able to estimate annual occupancy and average local extirpation/colonisation probabilities for intact S02, three species showed declines in occupancy probabilities from 2008 to 2013 (Figure 2 and Appendix S6B). Red-breasted Coua occupancy showed the largest decline (76%) between 2008 and 2013. At intermediate S05, we were only able estimate annual occupancy and average local extirpation/colonisation probabilities for three species; only the Madagascar Magpie-robin showed a decline (74%) in occupancy probability from 2011 to 2013 (Figure 3 and Appendix S6B).
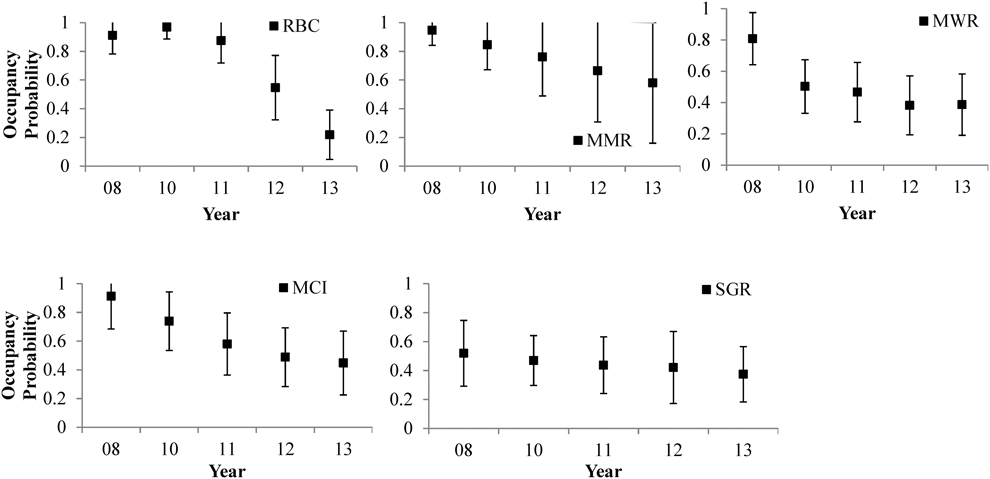
Figure 2. Annual (2008–2013) occupancy trends of five ground-dwelling bird species at intact site S02 as estimated by multi-season occupancy models in PRESENCE. Species abbreviations are: Madagascar Magpie-robin (MMR), Red-breasted Coua (RBC), Scaly Ground-roller (SGR), Madagascar Crested Ibis (MCI), and Madagascar Wood-rail (MWR). Black lines are 95% CIs.

Figure 3. Annual (2011–2013) occupancy trends of three out of the five focal ground-dwelling bird species at intermediate site S05 as estimated by multi-season occupancy models in PRESENCE. Species abbreviations are: Madagascar Magpie-robin (MMR), Red-breasted Coua (RBC), and Scaly Ground-roller (SGR). Black lines are 95% CIs.
There were no differences in ground-dwelling bird occupancy estimates in 2009 and 2013 at intermediate forest site S03 (Appendix S6C). However, Red-breasted Coua occupancy was higher at stations located in intact microhabitat (ψ = 0.72 ± SE 0.12) than in degraded microhabitat (ψ = 0.09 ± SE 0.11; β = -1.21 ± SE 0.56) and Madagascar Wood-rail detection was lower at stations with high human TS (β = -16.89 ± SE 8.13; Table 2 and Appendix S6C).
Discussion
Camera trap surveys and occupancy analyses can provide a wealth of information on elusive species; however, researchers must be careful to consider study design and model assumptions when interpreting results. For example, our camera trap surveys did not target ground-dwelling forest birds, which could introduce sampling biases that influence the estimation of their occupancy and/or detection probabilities (MacKenzie et al. Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002, Burton et al. Reference Burton, Neilson, Moreira, Ladle, Steenweg, Fisher, Bayne, Boutin and Stephens2015). However, to detect Madagascar’s small-bodied native carnivores and tenrecs, we used high camera trap sensitivity and located our camera traps close to the ground, which increased our ability to detect ground-dwelling forest birds. We also located our camera stations on game and human-made trails, as carnivores often use trails to move throughout their territory (Cusack et al. Reference Cusack, Dickman, Rowcliffe, Carbone, Macdonald and Coulson2015). This could bias our ground-dwelling forest bird detections to species that would also use trails to move through habitat or for foraging; however, additional surveys comparing on and off trail detections are needed to explore this further.
Occupancy analyses assume that there are no false positives (i.e. a species is recorded as present when it is actually absent) and that the occupied status of sampling sites does not change during the survey. To avoid false positives, we were conservative in our species identifications, using only high-quality photos where the species could be seen clearly. The assumption of constant occupancy at sampled sites was likely met because our surveys were short (48.7 ± SE 4.34 TN) relatively to the lifespan of bird species studied. Camera station spacing is a key component in determining sampling site independence (MacKenzie et al. Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002). If camera stations are spaced too close together, the probability of site independence will decrease and the likelihood of detecting an individual or species at multiple camera stations increases (Wegge et al. Reference Wegge, Pokheral and Jnawali2004, Dillon and Kelly Reference Dillon and Kelly2007). Our camera station spacing was based on the home range of the spotted fanaloka (Kerridge et al. Reference Kerridge, Ralisoamalala, Goodman, Pasnick, Goodman and Benstead2003) with the goal of estimating spotted fanaloka density. Unfortunately, there is no information available on the home range sizes of our five analysed bird species; however, it is unlikely that any of the birds have a larger home range than the spotted fanaloka based on their body sizes and ranging habits (Morris and Hawkins Reference Morris and Hawkins1998, Kerridge et al. Reference Kerridge, Ralisoamalala, Goodman, Pasnick, Goodman and Benstead2003).
Factors influencing ground-dwelling bird occupancy/detection
We predicted that bird occupancy would be positively related to percentage of rainforest cover and negatively related to distance to forest edge (Morris and Hawkins Reference Morris and Hawkins1998, Scott et al. Reference Scott, Brown, Mahood, Denton, Silburn and Rakotondraparany2006), yet only the Madagascar Magpie-robin’s occupancy was positively influenced by percentage rainforest cover. The Madagascar Magpie-robin’s need for contiguous forest is corroborated by studies in south-eastern Madagascar (H. Boone and Z. Farris pers. comm.). Distance to forest edge had no strong effect on occupancy for any species. This is especially surprising for the Madagascar Crested Ibis, which has been found to avoid forest edge in south-eastern Madagascar (Watson et al. Reference Watson, Whittaker and Dawson2004). In addition, there were no differences in occupancy estimates for any species across the habitat degradation gradient. It might be that Madagascar’s ground-dwelling forest birds are more tolerant to habitat patchiness and heterogeneity than previously thought. However, it is also possible that the sites we surveyed did not vary enough in habitat degradation characteristics (i.e. percentage rainforest cover and distance to forest edge) for us to detect a response in ground-dwelling bird occupancy. Further research should survey a wider range of sites—from intact and core primary to highly degraded forests—to determine the true relationships between ground-dwelling forest bird occupancy and landscape-level characteristics.
We predicted that trail characteristics and season would affect ground-dwelling bird detection, yet only Madagascar Magpie-robin detection was positively influenced by trail width and Madagascar Wood-rail detection by season. Our camera stations were set up specifically on trails that were of similar size (0.61 ± SE 0.03 m), thus there may not have been enough variance to thoroughly explore the influence of trail width on ground-dwelling bird detection. The lack of seasonal effects on ground-dwelling bird detection suggests that changes in bird activity were not appreciable enough to be detected via camera traps. We found one other factor, human trap success, to negatively influence Madagascar Wood-rail detection at one site (S03) only, suggesting that the response of ground-dwelling birds to certain factors might only be detectable at a local level.
At the landscape level, Red-breasted Coua, Madagascar Crested Ibis, and Scaly Ground-roller were all detected less often at camera stations with high feral cat trap success. Feral or domestic cats are thought to have caused at least 14% of global mammal, avian, and reptilian extinctions, and are primary threats to 8% of critically endangered species (Medina et al. Reference Medina, Bonnaud, Vidal, Tershy, Zavaleta, Josh Donlan, Keitt, Corre, Horwath and Nogales2011). Low ground-dwelling bird detection in areas of high feral cat activity could mean that birds reduce activity or use of trails to avoid feral cats. Meanwhile, native carnivore presence was positively associated with occupancy and detection of Madagascar Magpie-robin and Madagascar Crested Ibis, respectively. This effect is likely habitat-mediated, with native carnivore and ground-dwelling bird occupancy/detection being influenced by similar resources or exotic species in similar ways. The Madagascar Magpie-robin had a lower detection probability at stations with high zebu trap success, which might be due to the negative impact zebu have on the understorey while traveling through the forest, or because they are more common near forest edges. Red-breasted Coua detection was positively related to small Indian civet trap success, but due to differences in activity patterns between the diurnal birds and nocturnal small Indian civet (Farris et al. Reference Farris, Gerber, Karpanty, Murphy, Andrianjakarivelo, Ratelolahy and Kelly2015a), this relationship is likely also be habitat-mediated.
Annual occupancy trends of Masoala-Makira’s ground-dwelling forest birds
At S02, Red-breasted Coua, Madagascar Crested Ibis, and Madagascar Wood-rail occupancy declined dramatically from 2008 to 2013. At S05, the Madagascar Magpie-robin was the only species to show a decline in occupancy. Declines in Madagascar Magpie-robin occupancy have been seen at other resurveyed sites in south-eastern Madagascar (H. Boone and Z. Farris pers. comm.). It is possible that the Madagascar Wood-rail and Madagascar Magpie-robin occupancy trends could be explained by habitat degradation at the two sites that we were unable to measure. However, we believe that the trends in Red-breasted Coua and Madagascar Crested Ibis occupancy cannot be explained solely by habitat degradation. Red-breasted Coua and Madagascar Crested Ibis are highly prized by locals as bushmeat (Goodman and Wilmé Reference Goodman, Wilmé, Goodman and Benstead2003, Gardner and Davies Reference Gardner and Davies2014). Although we did not measure local bushmeat hunting rates, overexploitation of these two ground-dwelling birds by humans, and increases in feral cat occupancy (from 0 in 2008 to 0.68 ± SE 0.14 in 2013) at S02, could be the reason why Red-breasted Coua and Madagascar Crested Ibis occupancy is declining at S02 (Farris et al. in review). Future surveys at these sites should focus on determining the cause of ground-dwelling bird occupancy declines, with a particular focus on the Red-breasted Coua, which is vulnerable to extinction due to its restricted geographic range (Morris and Hawkins Reference Morris and Hawkins1998, Langrand and Sinclair Reference Langrand and Sinclair2003).
Conservation implications and future research
Camera traps provide abundant non-target species detections that are rarely used, but could increase our ecological knowledge of little-known species. In this study we show that these observations can provide much-needed data on landscape and annual occupancy trends of ground-dwelling forest birds in Madagascar. There are little to no data on the ecology of Madagascar’s ground-dwelling birds, including home range size, habitat use, behaviour, or interactions with exotic predators (Morris and Hawkins Reference Morris and Hawkins1998, Hawkins and Goodman Reference Hawkins, Goodman, Goodman and Benstead2003, IUCN 2015), despite the possible ecosystem services that they provide. Despite what is believed about the Madagascar Magpie-robin’s tolerance of habitat degradation (Morris and Hawkins Reference Morris and Hawkins1998, Langrand and Sinclair Reference Langrand and Sinclair2003) and its IUCN status as ’Least Concern’ (IUCN 2015), our analyses and the analyses of our collaborators in south-eastern Madagascar have shown that the Madagascar Magpie-robin might be particularly sensitive to habitat degradation.
In Madagascar and across the world, fragmented and degraded habitat patches are becoming more vulnerable to the pressures of hunting and exotic species invasion (Chapin III et al. Reference Chapin, Zavaleta, Eviner, Naylor, Vitousek, Reynolds, Hooper, Lavorel, Sala, Hobbie, Mack and Díaz2000, Corlett Reference Corlett2007, Bradshaw et al. Reference Bradshaw, Sodhi and Brook2009, Gardner et al. Reference Gardner, Barlow, Chazdon, Ewers, Harvey, Peres and Sodhi2009, Newbold et al. Reference Newbold, Hudson, Phillips, Hill, Contu, Lysenko, Blandon, Butchart, Booth, Day, De Palma, Harrison, Kirkpatrick, Pynegar, Robinson, Simpson, Mace, Scharlemann and Purvis2014). It is predicted that 13% of the world’s bird species will go extinct before 2100 and tropical biodiversity hotspots like Madagascar will become extinction hotspots (Brooks et al. Reference Brooks, Mittermeier, Mittermeier, da Fonseca, Rylands, Konstant, Flick, Pilgrim, Oldfield, Magin and Hilton-Taylor2002, Sodhi et al. Reference Sodhi, Liow and Bazzaz2004, Bradshaw et al. Reference Bradshaw, Sodhi and Brook2009, Gardner et al. Reference Gardner, Barlow, Chazdon, Ewers, Harvey, Peres and Sodhi2009). To improve our ability to conserve Madagascar’s endemic ground-dwelling birds, we suggest expanded surveys at sites representing a wider spectrum of habitat degradation. Additionally, estimation of consumption rates of Madagascar’s native forest birds by local people is critical. In Madagascar, bushmeat hunting—which is important to local nutrition and livelihoods—can occur at unsustainable rates (Golden Reference Golden2009, Golden et al. Reference Golden, Fernald, Brashares, Rasolofoniaina and Kremen2011). Finally, based on our findings that multiple species are negatively affected by feral cat presence, we need detailed ecological studies on feral cats in the Masoala-Makira landscape to inform management practices and potential removal programs (Medina et al. Reference Medina, Bonnaud, Vidal, Tershy, Zavaleta, Josh Donlan, Keitt, Corre, Horwath and Nogales2011, Recio et al. Reference Recio, Mathieu, Maloney and Seddon2011, Farris et al. Reference Farris, Golden, Karpanty, Murphy, Stauffer, Ratelolahy, Andrianjakarivelo, Holmes and Kelly2015b).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270917000107
Acknowledgements
We thank the Madagascar Government and Madagascar National Parks (MNP) for approving this research (permit # 128/11, 128/12 and 123/12). We thank the Wildlife Conservation Society, Madagascar Program (WCS-MP, Christopher Holmes and Vonjy Andrianjakarivelo) and Antongil Conservation for logistical aid. This research was funded by: National Science Foundation Graduate Research Fellowship Program (Grant No. DGE 0822220), National Science Foundation (Grant No. GEO 1115057), Sigma Xi Grants-in-Aid of Research, Cleveland Metroparks Zoo, European Association for Zoos and Aquaria, Idea Wild, National Geographic Society-Waitts Grant (#W96-10), People’s Trust for Endangered Species, Virginia Tech Chapter of Sigma Xi, Virginia Tech Department of Fish and Wildlife Conservation, and WCS-MP. We thank our Malagasy field assistants (B. L. J. Donah, Marika Helin, R. Willison, B. J. R. Rasolofoniaina, E. J. G. Anjaraniaina, Nam Didice and Randriamaniry Augustin), numerous Malagasy collaborators, and our many field volunteers and data entry undergraduate volunteers. Finally, we wish to thank the Editor, Associate Editor and two anonymous reviewers for their helpful suggestions, which improved this manuscript. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation.







