Introduction
The SCFA constitute a group of molecules that contain from one to seven carbon atoms and which exist as straight- or branched-chain compounds. They mainly originate from the intestinal bacterial fermentation of plant materials such as celluloses, fibres, starches and sugars for which mammals lack the necessary enzymes to split these compounds. Among the SCFA, acetic, propionic and butyric acid are the predominant forms. Because SCFA are weak acids with a pK of ≤ 4·8 and the pH of the gastrointestinal (GI) tract (GIT) fermentation chambers is nearly neutral, 90–99 % of the SCFA are present in the GIT as anions rather than as free acids(Reference Bergman1). SCFA have several beneficial effects for animals. As an example, acetic and propionic acids, due to their physical and chemical properties, are used as acidifiers in pig diets in order to help overcome problems in the post-weaning lag period. Moreover, dietary acidification is known to be beneficial for the performance of fattening animals(Reference Bergman1, Reference Partanen and Mroz2). For ruminants and herbivorous animals, SCFA constitute the major substrates for energy production. Evidence also indicates that there is considerable fermentation in the caecum and colon of both human and non-herbivorous animals.
Among the SCFA, butyric acid has received particular attention. It is a natural substance present in the GIT, in milk as well as in the sweat and faeces of most mammals. Butyric acid is available as the Na, K, Mg or Ca salt. The advantage of salts over free acids is that they are generally odourless and easier to handle in the feed manufacturing process owing to their solid and less volatile form. For the purpose of the present review, the term ‘butyrate’ is used interchangeably for the acid, the salt and the anion forms. In the GIT, butyrate is naturally present in high concentration in the lumen of the large intestine. It is preferentially taken up by the colonic epithelium where it is actively metabolised to produce energy(Reference Poirier, Degrace and Niot3, Reference Roediger4). Much attention has been devoted to the anticancerous properties of butyrate in human nutrition despite numerous papers suggesting its role as a growth factor. The effects of butyrate, however, depend on the experimental model (in vivo, in vitro), the state of the cells (normal or cancerous)(Reference Pender, Quinn and Sanderson5), the degree of inflammation(Reference Inagaki and Sakata6) and the doses used(Reference Velazquez, Jabbar and DeMatteo7).
In addition to the recognised effects on intestinal metabolism, butyrate shows indirect effects that contribute to the general metabolism of animals. In all tissues butyrate is a natural component of cellular metabolism. It may also act as a growth promoter when added to diets at low doses (0·1–0·5 g/kg)(Reference Guilloteau, Zabielski and David8).
The purpose of the present paper is to give an overview of the properties of butyrate either originating from bacterial intestinal fermentation or added to the rations of animals. In addition to the recognised effects on intestinal metabolism, butyrate shows indirect effects that contribute to the general metabolism of animals.
Production and delivery of butyrate in the gut
Butyrate production patterns in the gut at birth and during development
In utero, the intestine of the mammalian fetus is sterile. At the time of delivery, the intestinal microbiota are acquired by swallowing maternal anal or vaginal organisms(Reference Stark and Lee9). In poultry, hatching through the eggshell has a similar effect. The effect of the first inoculum may be lifelong, directing the development of the immune system and the normal intestinal microbiota. The bacterial composition of the inoculum received at birth, the structure of the host's intestinal epithelium and the diet all affect the density and composition of the microbiota(Reference Hold, Pryde and Russell10). These microbiota, particularly in the large intestine, have a fundamental role in supplying energy to the host through anaerobic fermentative processes producing SCFA(Reference Duncan, Louis and Flint11). Another source of SCFA in general and butyrate in particular is the milk. Butyric acid is a natural component of mammalian milk, except for human and sows' milk where only traces can be found. In cows' milk butyrate concentration is about 0·16 g/l(Reference Alais12), which seems to be insufficient to cover the energy needs of the GI epithelium in the newborn calf but it may be enough to stimulate GI development together with milk-borne hormones and growth factors such as insulin, leptin, glucagon-like peptide (GLP)-2, insulin-like growth factors, epithelial growth factor and many others(Reference Guilloteau, Biernat, Wolinski, Zabielski, Gregory and Westrom13). Another source of butyrate in suckling neonates is the hydrolysis of milk lipids by salivary and gastric lipases. This source of butyrate is difficult to estimate but it may be utilised in the upper GIT. In newborn rats, very high levels of butyrate β-oxidation are detected in colonic epithelial cells. The high oxidation levels may be explained by the fact that the animal is geared to use luminal substrates (high-fat milk diet) with maximum efficiency(Reference Krishnan and Ramakrishna14). The stools of normal full-term infants contain less than 100 mm-SCFA(Reference Ogawa, Ben and Pons15), with acetate being the major and butyrate the minor acid found in the faeces of formula fed-babies, while virtually no butyrate is found in the faeces of breast-fed infants(Reference Edwards, Parrett and Balmer16). In premature infants deficient in intestinal lactase, high concentrations of SCFA are produced due to carbohydrate malabsorption, bacterial overgrowth and poor intestinal motility and can significantly lower the pH of the intestinal luminal contents. When specific pathogens, such as Clostridium difficile, proliferate in this environment, they can cause necrotising enterocolitis(Reference Li, Myeroff and Smiraglia17). In healthy infants, fermentation is lower than in adults and butyrate production is established at a slower rate than for acetate and propionate but, by 2 years, an adult SCFA profile has emerged(Reference Midtvedt and Midtvedt18). Similar SCFA production patterns have been detected in animals. In broilers aged 1 d, SCFA increased from undetectable levels to high concentrations in broilers aged 15 d, and then stabilised(Reference van Der Wielen, Biesterveld and Notermans19).
Butyrate production in the gut of adult animals
Large bowel fermentation: the main source of available butyrate
In ruminants, the resident microbiota components are essential, as they convert the feed components into nutrients that are readily available for the animal. In single-stomached animals, less microbial involvement is needed, as most nutrients in the diet are directly available to the host. Whilst bacteria affect the performance of the host by competing for dietary compounds, they also provide energy to the host through fermentation. Based on the location of fermentation, animals can be classified into forestomach fermenters and hindgut fermenters. Forestomach fermenters such as ruminants, camels, hippopotamuses and some monkeys have a fermentation chamber cranial to the acid-secreting part of the stomach. Rodents, horses, elephants and most omnivores and carnivores are hindgut and large intestine and/or caecum fermenters, with the GIT serving as a chamber for microbial action and SCFA production(Reference Bergman1). Elsden et al. (Reference Elsden, Hichcock and Marshall20), 60 years ago, compared total SCFA concentration at different sites in the GIT of a number of herbivorous and omnivorous species and noticed the highest concentrations in the caecum, and a progressive decline along the colon. In mice and rats, a higher concentration of SCFA was reported in the caecum compared with the colon(Reference McKay and Eastwood21). While in ruminants the highest concentration of SCFA was found in the rumen, another peak was measured in the caecum and colon, indicating that even in forestomach fermenters considerable further digestion occurs in the large intestine(Reference Bergman1). The intestine contains several different bacterial species. Saccharolytic bacteria, leading to linear SCFA, CO2 and H2, predominate in the proximal colon, while the majority of proteolytic bacteria, yielding branched SCFA, CO2, CH4, H2, phenols and amines, are mainly in the distal colon where fermentable carbohydrates are depleted on transit(Reference Hamer, Jonkers and Venema22, Reference Roberfroid23).
Polysaccharides, oligosaccharides and disaccharides resistant to the action of hydrolytic enzymes in the upper GIT enter into the caeco-colon. Here, the carbohydrates are depolymerised to their constituent sugars by a wide range of bacterial cell-associated (Fig. 1) and secreted hydrolytic enzymes such as β-fructosidase, β-galactosidase, xylanase and other hydrolases, and are then fermented(Reference Savage24). Energy is gained in the form of ATP by substrate-level phosphorylation during oxidative substrate breakdown via the fermentation process. The transfer of the resulting reducing equivalents into the metabolic intermediates leads to the formation of large amounts of reduced endproducts such as butyrate, which acts as a terminal electron acceptor in an environment limited in O2. There are two distinct pathways for the production of intracellular butyrate in bacteria. In the first pathway butyryl-CoA is converted to butyrate with the intermediate formation of butyryl phosphate by the enzymes phosphotransbutyrylase and butyrate kinase(Reference Miller and Jenesel25). In the second pathway the enzyme butyryl-CoA:acetate CoA transferase is involved and transfers the CoA moiety from butyryl-CoA to external acetate, which leads to the formation of acetyl-CoA and butyrate. The CoA transferase route is shown to be more dominant in the human butyrate-producing microbiota(Reference Duncan, Barcenilla and Stewart26). In vitro data show that strains possessing the CoA transferase convert 75 % of the supplied glucose into lactate when acetate is absent. In the presence of acetate, mainly butyrate is produced(Reference Diez-Gonzalez, Bond and Jennings27).
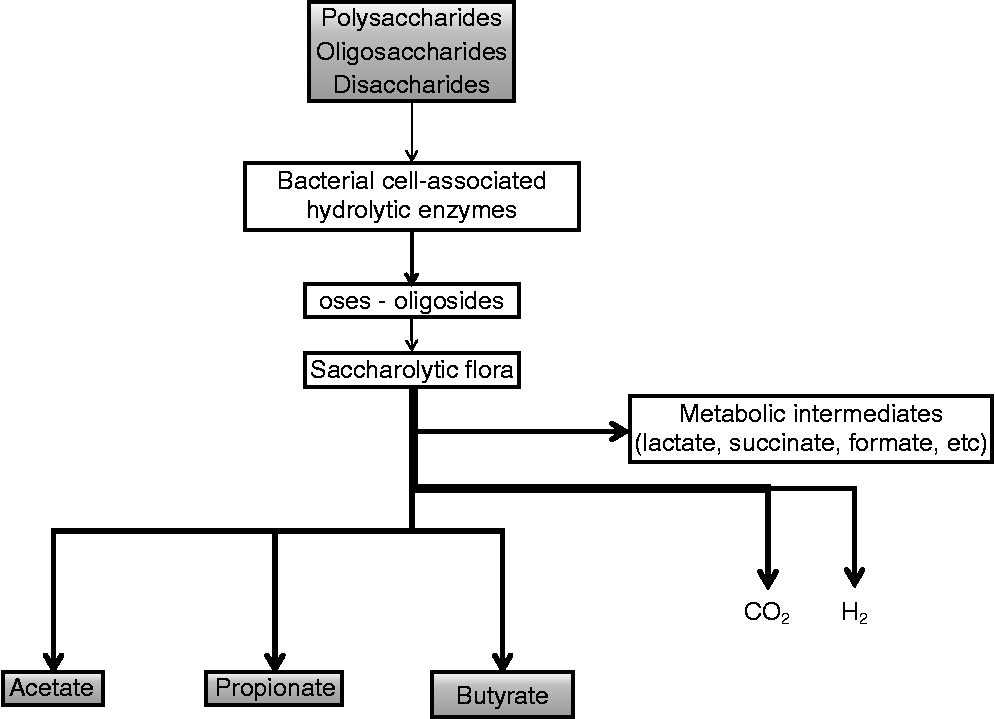
Fig. 1 Overview of butyrate production in the gut of adult animals.
Substrates used for bacterial fermentation with butyrate as endproduct
In the large intestine, the ubiquitous nutrient glucose is only of secondary importance. Enterocytes use both glutamine and glucose as the favoured metabolic fuels, while colonocytes preferentially use butyrate(Reference Roediger4). The rate and amount of butyrate produced along the colonic lumen depends on the microbiota composition, the chemical composition, the physical form and the amount of substrates available in the diet. Substrates can be indigestible or digestible carbohydrates and, additionally, sloughed cells, mucus and endogenous secretions may also provide some fermentable substances. Examples of indigestible carbohydrates are oligosaccharides (for example, raffinose, oligofructose, inulin) and NSP which can be soluble (for example, -glucans) or insoluble (for example, cellulose and hemicellulose). The former are highly fermentable and hence generate greater quantities of SCFA in the colon while the latter have a rather low fermentability but increase the faecal bulking and decrease the colonic transit time. The most important digestible carbohydrate is starch, known as resistant starch (RS; d-glucose units connected by α-1,4/α-1,6 glucosidic bonds) when it has escaped digestion and/or absorption in the small intestine. RS can be subdivided into four types: physically trapped starch (in coarse grains), RS granules naturally rich in amylose (i.e. raw potato flour), retrograded starch (i.e. cooked and cooled potato) and chemically modified starch (i.e. processed foods)(Reference Englyst, Kingman and Cummings28).
As a result of increasing concentrations of acidic fermentation products, the luminal pH in the proximal colon is lower. This pH seems to boost the formation of butyrate, as mildly acidic pH values allow butyrate-producing bacteria to compete against Gram-negative carbohydrate-utilising bacteria such as Bacteroides spp.(Reference Walker, Duncan and William Leitch29). An in vitro fermentation study, using faecal inoculum, showed that the population of butyrate producers, as well as the concentration of butyrate, was significantly higher at pH 5·5 while Bacteroidetes dominated as the fermenter was at pH 6·5. Lowering the pH value further, for example, to pH 5·2, resulted in the loss of lactate-utilising bacteria, such as Eubacterium hallii, leading to the accumulation of lactic acid(Reference Walker, Duncan and William Leitch29). Although all dietary fermentable carbohydrates reaching the hindgut have the potential to be converted to butyrate, not all are equally butyrogenic. In vitro (Reference Weaver, Krause and Miller30), animal(Reference Marsono, Illman and Clarke31) and human(Reference Topping, Illman and Clarke32) studies have shown that RS fermentation generates relatively more butyrate than NSP fermentation. Butyrogenic substrates may affect the fermentative metabolism in individual bacteria, as more substrate leads to enhanced butyrate formation, which provides a route for the disposal of excess reducing equivalents (i.e. butyrate is used as a hydrogen sink). Butyrogenic substrates may also affect the microbial population by stimulating butyrate-producing species that are efficient primary degraders of these substrates(Reference Gottschalk and Starr33). Indirect stimulation of butyrate production occurs by metabolic cross-feeding. The first mechanism is via cross-feeding of oligosaccharide breakdown products released by active polysaccharide-metabolising bacteria. These breakdown products are then fermented by butyrate producers. This was demonstrated in in vitro cultures between bifidobacteria and butyrate-producing species(Reference Falony, Vlachou and Verbrugghe34). The second mechanism is via cross-feeding of fermentation products such as lactate, ethanol and succinate. These compounds are intermediates in the global fermentation process in the microbiota and are detected in very low concentrations in healthy subjects. In vitro, the accumulation of lactate is prevented when human butyrate-producing strains such as Eubacterium hallii and Anaerostipes caccae are grown in co-culture with lactate-producing strains such as Bifidobacterium adolescentis (Reference Duncan, Louis and Flint35).
Scientific reports support the view that RS is the most powerful butyrogenic substrate(Reference Bird, Brown and Topping36). Both in vitro as well as in vivo fermentation of RS generally results in butyrate production in the order of 20 to 28 mol% compared with about 10 to 15 mol% for NSP(Reference Brouns, Kettlitz and Arrigoni37). In rats fed RS-containing diets, there was a significant increase of the number of bifidobacteria and lactobacilli. Although these bacteria are predominantly acetate and lactate producers and not butyrate producers, butyrate production was significantly enhanced. Therefore it was accepted that the increased number of lactic acid bacteria induced a higher production of lactate that was efficiently converted to butyrate by lactic acid-utilising bacteria(Reference Silvi, Rumney and Cresci38). Due to the variable structure and complexity of starch, the exact starch type has a considerable impact(Reference Brouns, Kettlitz and Arrigoni37). Among RS, the retrograded RS (RS3) seems to be the most powerful butyrogenic substrate(Reference Brouns, Kettlitz and Arrigoni37). The butyrogenic properties of RS3 is linked to the length of the 1,4-α-d-glucans chain. Chain lengths with a degree of polymerisation of about 20–35 units of glucose are optimal for a high output of butyrogenic RS3(Reference Jacobasch, Dongowski and Schmiedl39). It is possible to increase the butyrate production from RS3 fermentation by an appropriate hydrothermal treatment. In a recent study, Jacobasch et al. (Reference Jacobasch, Dongowski and Schmiedl39) compared the physiological effects of three RS3 in rats: Novelose 330, annealing-Novelose and heat moisture-treated Novelose. In the caecum, proximal colon and distal colon treated with RS3, the digestive concentration of butyrate significantly increased and in the distal colon, heat moisture-treated Novelose yielded the highest butyrate concentration. This result is of great importance because a high butyrate concentration in the distal part of the colon is considered to be essential for colonocyte health and may have many implications in the prevention and the treatment of some chronic digestive diseases such as diverticulosis. The ability to produce butyrate from RS is also a dose-related effect and may vary greatly among individuals(Reference Brouns, Arrigoni and Langkilde40).
Oligofructose compounds are also associated with greater butyrate production. Although oligofructose is thought to be selectively fermented by bifidobacteria and, to a lesser extent, by lactobacilli, due to their production of β-fructosidase that cleaves the β-(2-1) bonds present in oligofructose and inulin, stimulation of butyrate-producing bacteria is observed(Reference Damian, Van den Mooter and Samyn41).
Besides dietary fibres, other substrates using different mechanisms are known to increase butyrate concentration. An example is acarbose, an oligosaccharide that increases the amount of starch entering the colon by acting as an α-glucosidase inhibitor(Reference Weaver, Tangel and Krause42). Also, the kinetics of butyrate production varies widely between substrates. For example, fructo-oligosaccharides are known to be rapidly fermented, whereas RS is more slowly degraded and can reach the distal part of the colon(Reference Le Blay, Michel and Blottière43, Reference Le Blay, Michel and Blottière44).
There is little information to explain why bacterial fermentation of specific carbohydrates, such as RS and oligofructose, selectively increases butyrate production, but it is assumed that interconversion reactions from primarily lactate and acetate are largely involved. In the rumen up to 60 % of butyrate is synthesised directly from extracellular acetate through interconversion reactions(Reference Linington, Meyer and van der Walt45). Acetate and butyrate are interconvertible, but due to the net gain of ATP, the conversion of butyrate to acetate is more favourable for the microbial metabolism(Reference Leng and Leonard46).
Butyrate-producing microbiota
Culture-independent surveys of the adult human gut microbiota have revealed two bacterial phyla, the Bacteroidetes and the Firmicutes(Reference Turnbaugh and Gordon47), commonly dominating this ecosystem. This is also the case for the gut of at least sixty mammalian species(Reference Ley, Hamady and Lozupone48). The dominant groups found in the large intestines of human subjects(Reference Eckburg, Bik and Bernstein49), pigs(Reference Pryde, Richardson and Stewart50) and horses(Reference Daly, Stewart and Flint51) and in the rumen of cattle are the Cytophaga, Flavobacterium, Bacteroides (CFB) group of Gram-negative bacteria, and the Clostridium cluster XIVa (Clostridium coccoides) and cluster IV (Clostridium leptum) group of Gram-positive bacteria(Reference Hold, Pryde and Russell10). In poultry, relatives of Clostridium leptum and Clostridium coccoides groups dominate the caecum(Reference Apajalahti, Kettunen and Graham52). A high frequency of the Sporomusa group (Clostridium cluster IX), which includes major propionate producers, was reported while no numbers of the CFB group were detected in the avian caecum(Reference Lu, Idris and Harmon53). Sonnenburg et al. (Reference Sonnenburg, Xu and Leip54) demonstrated that members of the Bacteroidetes phylum harbour a large set of genes which encode functions necessary to detect, bind, degrade and import carbohydrates encountered in the gut habitat – either from the diet or from host glycans associated with mucus and the surfaces of epithelial cells. The substrate breakdown products released by those primary degraders may be used by a myriad of other bacterial groups including the butyrate-producer group. The butyrate-producing bacteria cultured so far are strictly anaerobic firmicute bacteria, generally regarded as difficult to grow in vitro. The bacteria are Gram-positive, having a low mol% guanine-cytosine (G-C) content and are widely distributed across several clusters within the order Clostridiales. Those clostridial clusters (I–XIX) form a new nomenclature that rearranges the grouping of bacteria based on studies of the evolutionary relationships by 16S rRNA sequencing(Reference Collins, Lawson and Willems55). The bulk of the butyrate-producers belong to cluster IV and cluster XIVa and include some potentially important butyrate producers related to Faecalibacterium prausnitzii, Eubacterium rectale and Roseburia species, respectively(Reference Louis and Flint56). In humans, the butyrate-producing bacteria related to F. prausnitzii comprise 5–15 % of the total microbiota(Reference Eckburg, Bik and Bernstein49) and 5–10 % of the total microbiota species are related to Eubacterium rectale and Roseburia (Reference Aminov, Walker and Duncan57). In recent studies of poultry, two butyrate producers were described: Butyricicoccus pullicaecorum (Reference Eeckhaut, Van Immerseel and Teirlynck58), related to F. prausnitzii, and A. butyraticus (Reference Eeckhaut, Van Immerseel and Pasmans59), closely related to A. caccae, the lactate-utilising bacteria routinely detected in faecal samples from healthy individuals(Reference Hold, Pryde and Russell10). In the rumen of cattle and sheep, predominating butyrate-producing bacteria belong to cluster XIVa and are represented by Butyrivibrio fibrisolvens strains(Reference Bryant, Sneath, Mair and Sharpe60, Reference Stewart, Flint, Bryant, Hobson and Stewart61). Butyrivibrio fibrisolvens is also present in the faecal flora of man, rabbits and horses(Reference Hespell, Balows, Truper and Dworkin62).
Depending on the environmental conditions, fermentative metabolism of butyrate producers can result in butyrate, formate, H2, CO2, while lactate and acetate can be either produced or consumed(Reference Louis and Flint56). Butyrate producers are considered to play an important role in maintaining gut health mainly through the production of butyrate. Other traits beneficial for the hosts' health are their capacity to stabilise the luminal pH by consumption of lactate or the anti-inflammatory effect of some butyrate producers(Reference Sokol, Pigneur and Watterlot63). Given the importance of butyrate to colonic epithelial metabolism, it is reasonable to assume that butyrate-producing bacteria are well tolerated by the innate immune system(Reference Morrison, Mackay and Edwards64).
Absorption, transport and metabolism of butyrate
Absorption of butyrate, SCFA membrane receptor and transport proteins
Earlier studies have demonstrated that SCFA are absorbed in both the small and large intestine by similar mechanisms(Reference Ruppin, Bar-Meir and Soergel65, Reference Schmitt, Soergel and Wood66). More recent studies suggest the existence of species differences and different transporter isoforms expressed in enterocytes along the intestine(Reference Gill, Saksena and Alrefai67). On the apical membrane of the epithelial cell, distinct transport mechanisms have been reported, i.e. non-ionic diffusion and mechanisms involving SCFA/HCO3− exchangers, monocarboxylate transporter (MCT) type 1(Reference Ritzhaupt, Wood and Ellis68, Reference Ritzhaupt, Ellis and Hosie69), and Na-coupled MCT(Reference Ganapathy, Thangaraju and Gopal70) (SMCT or SLC5A8/12). On the basolateral membrane, a carrier-mediated, HCO3− -gradient-dependent anion–butyrate exchange system (Fig. 2) is found(Reference Tyagi, Venugopalakrishnan and Ramaswamy71). The human intestine constitutes MCT3, MCT4 and MCT5 isoforms (the MCT6 expression was not found), with low expression of MCT3 in the ileum, and high expression of MCT4 and MCT5 found predominantly in distal colon(Reference Gill, Saksena and Alrefai67). In ruminant species, the SCFA-transporting mechanisms in the rumen epithelium are highly efficient, and lead to absorption of nearly all volatile fatty acids. Anion competition experiments in the washed ovine reticulorumen segments revealed that SCFA can be transported by both bicarbonate-dependent and bicarbonate-independent protein-coupled mechanisms(Reference Aschenbach, Bilk and Tadesse72). The latter was not coupled with MCT.

Fig. 2 Absorption of n-butyrate (nC4) in the large intestine and subsequent metabolism. Butyrate transport with monocarboxylate transporters (MCT) is saturable and coupled with H+ transport. Several receptors for butyrate have been identified and detected in a variety of tissues including fat tissue, but the highest expression has been found in immune cells. Butyrate prevents obesity and decreases body fat mass in mice, but the exact mechanism is unknown. After the intestine, butyrate can be metabolised in the liver to produce fatty acids, cholesterol and ketone bodies. In the peripheral blood, no significant concentration of butyrate is found. Oestr, oestrone sulfate; OAT7, organic anion transporter 7; GPR, orphan G protein-coupled receptor for SCFA; SMCT, Na-coupled monocarboxylate transporter. Adapted from Gupta et al. (Reference Gupta, Martin and Prasad73)
MCT are involved in butyrate transport in pig and human colonic luminal membrane. Ritzhaupt et al. (Reference Ritzhaupt, Wood and Ellis68, Reference Ritzhaupt, Ellis and Hosie69) demonstrated that butyrate uptake is via a pH-activated, electroneutral anion exchange system. The optimal pH for the activity of the colonic butyrate transporter seems to be 5·5. Butyrate transport with MCT is saturable, coupled with H+ and inhibited by several monocarboxylates such as acetate, propionate, pyruvate, l-lactate and α-ketobutyrate. More recently, a second class of MCT was identified(Reference Ganapathy, Thangaraju and Gopal70), named SMCT. Two proteins have been cloned: SLC5A8 or SMCT1 and SLC5A12 or SMCT2(Reference Gupta, Martin and Prasad73). Conversely to MCT, SMCT transport mechanism involves Na+ uptake by the transport cycle and also uses nicotinate and ketone bodies as substrates. In the colon, Li et al. (Reference Li, Myeroff and Smiraglia17) showed that SLC5A12 (or SMCT2) functions as a tumour suppressor. Ganapathy et al. (Reference Ganapathy, Thangaraju and Gopal70) demonstrated that a non-malignant colon cell line expresses the transporter contrary to malignant cells. So, exposure of non-malignant cells to butyrate does not induce apoptosis. However, when SLC5A12 (SMCT2) is ectopically expressed in malignant cells and when butyrate is added in the culture medium, cells undergo massive apoptosis. In the normal colon, SLC5A8 is claimed to have less importance in butyrate transport than MCT1(Reference Thibault, Blachier and Darcy-Vrillon74) but it plays a key function in intestinal lactate absorption. In humans, SLC5A8 is considered a tumour suppressor(Reference Ganapathy, Thangaraju and Prasad75). During the transformation of non-malignant cells into malignant ones, expression of SLC5A8 is silenced perhaps to avoid butyrate inhibition of histone deacetylase. But in the presence of butyrate, expression of SLC5A8 is again enhanced and it increases cell apoptosis(Reference Ganapathy, Thangaraju and Prasad75). Nevertheless, the lack of susceptibility for colon cancer in SLC5A8 − / − mice and the failure to detect a significant uptake of butyrate by SLC5A8 question the role of SLC5A8 both in butyrate transport and colon cancer suppression(Reference Frank, Groger and Diener76).
Two orphan G protein-coupled receptors (GPR) for SCFA, GPR41 (or FFA3) and GPR43 (or FFA2) have been isolated from the intestine(Reference Tazoe, Otomo and Kaji77–Reference Brown, Goldsworthy and Barnes82). FFA2 mRNA was detected in a variety of tissues, but the highest expression was found in immune cells, including polymorphonuclear cells, suggesting that SCFA might be involved in the activation of leucocytes. Indeed, recent studies in (FFA2) GPR43-deficient mice (Gpr43− / − ) indicated that acetate administration may help in resolution of the inflammatory response(Reference Maslowski, Vieira and Ng83). FFA3 has an even more widespread expression pattern than FFA2, including adipose tissues, pancreas, spleen, lymph nodes, bone marrow and peripheral blood mononuclear cells(Reference Le Poul, Loison and Struyf81). Abundant FFA2 and FFA3 expression was detected in the rat distal ileum and colon, and the human ascending colon(Reference Tazoe, Otomo and Karaki78, Reference Karaki, Tazoe and Hayashi84, Reference Karaki, Mitsui and Hayashi85). Expression was limited to mucosal cells, and absent in enteric neurons and smooth muscle cells. Immunohistochemical staining with anti-FFA2 and anti-FFA3 sera showed FFA2 and FFA3 immunoreactivity in enterocytes and enteroendocrine cells of open type(Reference Tazoe, Otomo and Karaki78, Reference Karaki and Kuwahara86). The FFA3 immunoreactive enteroendocrine cells were less numerous than the FFA2 cells, and double-immunostaining for FFA2 and FFA3 revealed no common localisation in one cell. Both the FFA2- and FFA3-immunoreactive enteroendocrine cells exhibited peptide YY, but no 5-hydroxytryptamine, expression, supporting the evidence for stimulation of peptide YY release by SCFA(Reference Tazoe, Otomo and Karaki78). The FFA2 and FFA3 distribution and physiological role in the GIT, involving sensing of luminal content, intestinal motility, secretion and innate immunity, have been recently reviewed by Karaki & Kuwahara(Reference Karaki and Kuwahara86).
In healthy rats fed a fibre-free or a RS-enriched diet and using [1-13C]butyrate intra-caecal perfusion, the flux of butyrate production was 0·7–1·8 μmol/min whereas portal flux was from 0·2 to 0·4 μmol/min and parietal utilisation from 0·9 to 1·8 μmol/min(Reference Moreau, Champ and Goupry87). In unfed pigs with an intra-caecal perfusion of [1-13]C-butyrate, the parietal utilisation of butyrate varied from 60 to 120 μmol/min when the concentration of perfused [1-13]C-butyrate varied from 0 to 160 μmol/min (L Martin, unpublished results).
Energy source for colonocytes
In vitro studies have demonstrated that butyrate also represents the preferred energy-providing substrate for the colonic cells(Reference Roediger4, Reference Sakata and Von Engelhardt88). Colonocytes exhibit a great capacity to rapidly metabolise butyrate. Through fatty acid oxidation, butyrate is entirely oxidised into CO2 or used as a precursor for lipid synthesis(Reference Del Castillo, Muniz, Sulbaran-Carrasco, Binder, Cummings and Soergel89). In fact, butyrate is able to increase lipogenesis from acetyl-CoA or ketone bodies synthesis via the hydroxyl-methyl-glutaryl-CoA pathway(Reference Roediger90). Consequently, the synthesis of many key components of the intestinal epithelial tissue depends on butyrate metabolism (see later).
As butyrate is a key substrate for colonocytes, very small quantities reach the general circulation or portal vein. Guilloteau et al. (Reference Guilloteau, Zabielski and David8) did not observe any changes in plasma butyrate concentration in the peripheral circulation in calves. Nevertheless, the concentration of butyrate in the portal vein may vary according to the diet. Some substrates such as RS or oligosaccharides lead to substantial increases in butyrate concentration in the portal vein(Reference Martin, Dumon and Champ91, Reference Moreau, Martin and Toquet92). Hepatic uptake of butyrate is almost total. In the liver, butyrate metabolism also yields acetyl-CoA as in colonocytes.
In contrast to single-stomached animals, in vivo studies in steers revealed important butyrate uptake in the rumen as well as limited capacity to metabolise butyrate in the ruminal epithelium and liver. In cattle, butyrate absorption is saturable and if it exceeds the metabolic capacity, it affects rumen epithelial, hepatic nutrient metabolism and the nutrient supply of peripheral tissues(Reference Kristensen and Harmon93).
Butyrate transport into the peripheral blood circulation: is there proof for physiological direct effects?
As shown by Kristensen & Harmon(Reference Kristensen and Harmon93) in cattle, substantial amounts of butyrate produced by rumen microbiota may be absorbed through the ruminal epithelium and induce a number of effects via the blood circulation. First, included at a low dose in the diet (0·3 % of DM), butyrate disappears from the upper GIT (mainly in the stomach). It is thought to be metabolised in the GIT wall because it is not found in blood(Reference Guilloteau, Zabielski and David8, Reference Manzanilla, Nofrarias and Anguita94). Nevertheless, when ingested with the diet, in specific conditions, butyrate would be measurable in peripheral blood(Reference Gao, Yin and Zhang95) and seems to be able to act on peripheral organs (skeletal muscle, brown adipose tissue, liver, etc). Second, butyrate generated from dietary fibre fermentation at a high dose in the hindgut lumen (from 3 to 70 mm)(Reference Topping and Clifton96) is quickly absorbed in rodents(Reference Egorin, Yuan and Sentz97) and transported via the portal vein to the liver. In humans the butyrate concentration in the portal vein is about 30 μm whereas it is 12 μm in the hepatic vein(Reference Bergman1). A significant amount of butyrate is detected in the portal vein but not in the peripheral circulation in pigs fed a diet enriched with resistant potato starch(Reference Martin, Dumon and Lecannu98). Similarly, using rye fibres as a RS source, butyrate concentration significantly increases in both the portal vein and peripheral circulation(Reference Bach Knudsen, Serena and Kjaer99). In pigs, the direct perfusion of sodium butyrate in the caecum induces a dose-related increase of butyrate in the portal blood (L Martin, unpublished results). Butyrate, however, is not regularly detected in the peripheral blood even after a long period of perfusion with a supra-physiological solution of butyrate in the colonic lumen (L Martin, unpublished results). Taken together, these data suggest that butyrate can be absorbed from the gut and entirely metabolised either in the gut mucosa or in the liver, which makes a direct effect of butyrate via blood circulation unlikely. Indeed, Bloemen et al. (Reference Bloemen, Venema and van de Poll100) showed that in human patients, no intestinally produced butyrate escaped the splanchnic area due to a highly efficient hepatic uptake.
Butyrate and hepatic metabolism
Only a small fraction of luminal butyrate can reach the liver via the portal vein. Nevertheless, the effect of butyrate on hepatic cell has been studied. In the liver, butyrate leads to the production of acetyl-CoA that enters into the citric acid cycle. It has been shown that the production of acetyl-CoA via the butyryl-CoA synthase pathway consumes 1 ATP/mol but due to the reoxidation of reduced compounds, the tissue ATP content is maintained. In an experiment using isolated perfused liver of rats, Beauvieux et al. (Reference Beauvieux, Tissier and Gin101) observed that, unexpectedly, butyrate perfusion led to an impairment in energy metabolism, i.e. a decrease in net ATP content in comparison with acetate. The authors hypothesised that butyrate might impair mitochondrial activity inducing an uncoupling between the respiration chain and ATP synthesis. So, the effect of butyrate on hepatic metabolism appears to be close to that of longer-chain fatty acids. As long-chain fatty acids play a part in the pathogenesis of insulin resistance, the effect of butyrate should be considered in specific nutritional conditions (acarbose treatment, high level of dietary RS, etc)(Reference Gallis, Tissier and Gin102).
When ingested, butyrate enhances glycogen synthesis in the liver at the same rate as glucose(Reference Beauvieux, Roumes and Robert103). The authors demonstrated a clear effect on both a decrease in glucose oxidation and an increase in hepatic glycogen storage. They postulated that such a mechanism could be one of the molecular bases to explain the effect of dietary fibres on the prevention of insulin resistance.
Recently a new organic anion transporter (OAT7) was specifically identified in the liver(Reference Shin, Anzai and Enomoto104), exhibiting a significant transport activity for butyrate (Fig. 2). This transporter mediates the bidirectional transport of oestrone sulfate in exchange for butyrate. This exchange with oestrone sulfate might suggest a contribution (direct or indirect) of butyrate in liver steroid hormone metabolism. More fundamentally, the narrow substrate selectivity of OAT7 suggests that butyrate might participate in the efficient translocation of some sulfate conjugates without interference from the other anionic compounds such as bile salts. In other words, butyrate may play a role in the efficiency of liver detoxification pathways.
Effects of butyrate on gastrointestinal epithelial cell functions
Several studies have indicated that butyrate, besides providing epithelial cells with energy, markedly increases epithelial cell proliferation and differentiation, and improves colonic barrier function(Reference Mariadason, Kilias and Catto-Smith105–Reference Roediger108). Butyrate operates as a signal metabolite in the homeostasis of colonocytes, regulating the balance between proliferation, differentiation and apoptosis(Reference Jacobasch, Schmiedl and Kruschewski109) (Fig. 3).

Fig. 3 Multiple effects (local) of butyrate in the intestine.
Cell proliferation
Earlier studies suggested that trophic effects on the GI epithelium are a result of SCFA. Butyrate is considered to have the strongest effect, that of acetate is weaker, and that of propionate is the weakest(Reference Salminen, Tapiola and Korhonen110). Butyrate ingestion is known to modify the microstructure of the small and large intestines in animals and humans(Reference Le Gall, Gallois and Sève111–Reference Sakata115).
In enterocytes
In the small intestine, butyrate enhances proliferation, differentiation and maturation, and reduces apoptosis of normal enterocytes, mediated through its influence on gene expression and protein synthesis(Reference Sengupta, Muir and Gibson116). In the small intestine, the crypt depth and the villi height increase largely in butyrate-supplemented weaning or weaned pigs(Reference Manzanilla, Nofrarias and Anguita94, Reference Wang, Chen and Xang117). Interestingly, when infused in the colon, butyrate was shown to exert trophic effect on ileal and jejunal epithelial cells, presumably indirectly, through a neurohormonal mechanism(Reference Frankel, Lew and Su114, Reference Sakata115).
More recent molecular studies investigating cell proliferation, damage, and programmed death (mainly apoptosis) revealed that butyrate speeds up intestinal mucosa maturation during the development or repair after injury(Reference Kotunia, Wolinski and Laubitz112). Calves supplemented with low doses of sodium butyrate showed a significantly increased mitotic index in the upper jejunum but not in the duodenum or ileum as compared with controls(Reference Guilloteau, Zabielski and David8). However, changes in the mitotic index were not reflected in villi length and tunica mucosa thickness.
In colonocytes
In contrast to the upper intestine, in the large intestine the trophic effects appeared only at the place of butyrate administration. In rodents and swine, all studies showed that the mass of large-bowel tissue is higher in animals fed a butyrate-producing diet, compared with controls(Reference Lacorn, Goerke and Claus118–Reference Le Leu, Brown and Hu120). An increase in the length of the colon is repeatedly reported. Increased butyrate concentrations are associated with morphological changes of the mucosa(Reference Mentschel and Claus121). The crypt depth is significantly increased in pigs fed a raw potato starch diet associated with a decrease in the apoptotic activity. The secretory activity, but not the number, of epithelial growth factor-secreting goblet cells is nearly 2-fold increased compared with the controls. Simultaneously, the activity of two anti-apoptotic-regulating proteins is increased (Bcl-2; Bak). An overall reduction of apoptosis was detected without any effects on mitosis. Therefore, it was concluded that the main butyrate effect, in non-tumoural cells, is a shift of apoptosis towards the middle and basal compartments of the crypts. Conversely, in the absence of butyrate, there is massive apoptosis of colonocytes(Reference Luciano, Groos and Busche122).
Two plausible mechanisms are used to explain the increased proliferation index of colonic mucosa in physiological conditions(Reference Blottière, Buecher and Galmiche123). The first mechanism is that butyrate is the main energy source for colonocytes and more energy stimulates cell growth. The second mechanism involves the stimulation of the release of GI peptides and/or growth factors by butyrate acting on cell proliferation.
Apoptosis
In normal cells, butyrate acts as a survival factor but it is an inducer of apoptosis in human colonic carcinoma cells(Reference Luciano, Groos and Busche122). In an in vivo study in pigs, inulin-coated butyrate led to a reduction of apoptosis in the ileal mucosa and an increase in the length of ileal villi although it had no effect on cell mitosis(Reference Lacorn, Goerke and Claus118). Bailón et al. (Reference Bailón, Cueto-Sola and Utrilla124) evaluated the effects of butyrate on the proliferation and activation state of different cell types involved in inflammatory bowel disease: intestinal epithelial cells, macrophages and T-lymphocytes, using primary non-transformed cultures and established cell lines. Low concentrations of butyrate inhibited the proliferation of all immune cell types, whereas it induced apoptosis only in activated T-lymphocytes, non-differentiated epithelial cells and macrophage cell lines, but not in differentiated epithelial cells or primary macrophages. The induction of apoptosis was mediated by caspase-3/7 activation (mitochondrial pathway). Butyrate was only able to modify cell activation, measured as expression of inflammatory cytokines, in those cell types in which apoptosis was induced.
Apoptosis is an important regulatory process against cancer but various mechanisms can lead cells into a pro-apoptotic state. Zeng & Briske-Anderson(Reference Zeng and Briske-Anderson125) showed that prolonged butyrate treatment in vitro (HT1080 cells) increased the expression of both pro-metastatic and anti-metastatic genes but the net effect was an inhibition of pro-metastatic and an activation of anti-metastatic genes. Butyrate also arrested cell growth and migration and invasion of HT1080 cells by inhibiting histone deacetylases, and also by acting on non-histone proteins(Reference Bordonaro, Lazarova and Sartorelli126) at the first stages of DNA damage. Once DNA is damaged, butyrate seems to be ineffective. The absolute concentration of butyrate is also very relevant, as low amounts of butyrate enhance cell proliferation while high amounts inhibit it.
In the human colon cancer RKO cell line, butyrate induced apoptosis through a mechanism involving the c-Jun-N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) kinase pathway(Reference Zhang, Zhou and Bao127). In contrast, in neutrophils obtained from healthy human volunteers, butyrate increased apoptosis irrespective of their activation state, by factors other than MAPK and GPR, and their mechanisms probably relate to their histone deacetylase inhibition activity, which may control a1 mRNA expression(Reference Aoyama, Kotani and Usami128).
In the large intestine, cell proliferation, differentiation and positional localisation along the crypt axis are governed by several signalling pathways including the Wnt/β-catenin pathway(Reference Le, Franken and Fodde129). When Wnt signalling is activated, normal cell differentiation and proliferation are modified, leading to probable cancer genesis(Reference Le, Franken and Fodde129). Furthermore, the relative levels of Wnt signalling determine whether cells proliferate or go to apoptosis(Reference Bordonaro, Lazarova and Sartorelli126), since several in vivo studies support the evidence that increased activation of Wnt transcriptional activity enhances apoptosis in some cancerous cells. In vitro studies showed that butyrate hyper-induced Wnt transcriptional activity in some tumour cell lines(Reference Bordonaro, Lazarova and Sartorelli130). The authors conclude that both relatively high and relatively low level of canonical Wnt transcriptional activity can lead to cancerous cell apoptosis, and that cells involved in the tumorigenic process due to Wnt signalling are more vulnerable to butyrate action.
Beneficial effects on performance and intestinal health
Studies with oral administration of butyrate showed that butyrate can improve growth performance in production animals. The effective doses of sodium butyrate were fixed between 1 and 4 % of DM intake, and protection of the molecule by microencapsulation in lipid matrix improved its efficiency. Moreover, slow release of butyrate from a lipid matrix prevented its rapid metabolism in the stomach and upper small intestine, thereby increasing the area under the influence of the molecule(Reference Gorka, Kowalski and Pietrzak131, Reference Claus, Gunthner and Letzguss132). Butyrate was found to have widespread positive effects on growth, digestibility and feed efficiency through the modulation of cell proliferation, differentiation and function in the GIT, especially mucosal epithelial cells, and on defence systems (barrier function, antimicrobial potency, immune system) in healthy and sick animals(Reference Partanen and Mroz2, Reference Manzanilla, Nofrarias and Anguita94, Reference Pouillart133–Reference Mazzoni, Le Gall and De Filippi138).
Effects on growth performance and digestive efficiency
Dietary supplementation with butyrate enhanced growth rate and improved the feed conversion rate in calves before and during weaning(Reference Guilloteau, Zabielski and David8, Reference Gorka, Kowalski and Pietrzak131, Reference Guilloteau, Romé and Le Normand139) and in piglets(Reference Partanen and Mroz2, Reference Galfi and Bokori140–Reference Piva, Pizzamiglio and Morlacchini143). Studies in neonatal(Reference Kotunia, Wolinski and Laubitz112) and in weaned(Reference Le Gall, Gallois and Sève111, Reference Mazzoni, Le Gall and De Filippi138) piglets showed that the ingestion of butyrate resulted in improved performance, which was further improved if butyrate was fed sooner rather than later post-birth. Butyrate supplementation during the suckling period had no influence on ileal apparent digestibility, but it significantly improved the faecal apparent digestibility of feed components after weaning(Reference Le Gall, Gallois and Sève111). In these studies, butyrate supplementation delayed gastric emptying, which may suggest that increased gastric retention (longer-lasting gastric phase of digestion, slower digesta flow to the intestine) is beneficial for digestive processes(Reference Guilloteau, Toullec and Patureau-Mirand144, Reference Zabielski, Dardillat and Le Huerou-Luron145). In the newborn calves, the effect of butyrate supplementation both to the milk replacer and starter diet reduced diarrhoea, and led to an improved health status in the first weeks of life(Reference Gorka, Kowalski and Pietrzak131). In calves fed milk formula based on soyabean protein, butyrate supplementation improved the DM digestibility and the digestibility of major components of the diet(Reference Guilloteau, Savary and Jaguelin-Peyrault146). Moreover, the concomitant addition of butyrate to milk replacer and starter mixture may stimulate rumen development. Thus, the weight of the rumen (percentage of the whole stomach) was increased at the expense of the abomasum, and the ruminal papillae length and width were increased(Reference Gorka, Kowalski and Pietrzak131), improving the absorptive surface of the rumen.
The pancreas weight and the pancreatic protein content were not modified by butyrate supplementation (3 g/kg DM) in neonatal pigs and calves(Reference Guilloteau, Zabielski and David8, Reference Kotunia, Wolinski and Laubitz112). In contrast, the mitotic index increased and apoptotic index decreased in exocrine pancreatic cells in calves(Reference Pietrzak, Kotunia and Gorka147). These results suggest that pancreatic growth was not modified by butyrate supplementation; rather, the pancreatic cell turnover was accelerated. This corroborates with earlier studies demonstrating pro-mitotic effects of butyrate in the healthy gut of weaned pigs in vivo, though without effects on villous–crypt architecture(Reference Biagi, Piva and Moschini142) and supports the suggestion that animals should receive butyrate supplementation after birth as soon as possible. Preliminary studies by Pietrzak et al. (Reference Pietrzak, Kotunia and Wrzesinska148) demonstrated that butyrate supplementation exerted a pro-mitotic and anti-apoptotic effect in the small-intestinal mucosa in calves. This in turn elevated the mitotic:apoptotic ratio in butyrate-treated calves and resulted in increased villi height and intestinal absorptive surface.
Taking into account all of these results, butyrate may be considered as an important factor controlling early postnatal development of the GIT(Reference Scheppach and Weiler135) and it seems that the digestive process in the intestinal lumen could be more efficient in animals fed a butyrate-supplemented diet.
Effects on digestive secretions
In piglets, pre-weaning supplementation of butyrate was found to increase the densities of gastric parietal cells(Reference Mazzoni, Le Gall and De Filippi138), suggesting an increase in gastric acid secretion. Indeed, the effects of butyrate had little association with the enhanced acidity of the ingesta, as suggested by Manzanilla et al. (Reference Manzanilla, Nofrarias and Anguita94). Intra-ileal administration of butyrate in pigs caused an acute increase of trypsin and protein outputs(Reference Sileikiene, Mosenthin and Tafaj149). Duodenal butyrate infusion, however, did not change the mean flow rate of juice and proteins for 1 h after initiating administration, in contrast to observations after oral supplementation. Moreover, in contrast to ingestion, butyrate infusion decreased the mean flow rate of chymotrypsin(Reference Guilloteau, Savary and Jaguelin-Peyrault146).
In calves, butyrate supplementation in milk formula increased total daily pancreatic secretions. For pancreatic juice, this increase was most important from 12 to 17 h after the morning meal(Reference Guilloteau, Savary and Jaguelin-Peyrault146). In the same experiment, it was shown that during the 3 h postprandial period, oral butyrate supplementation led to a physiological postprandial decrease of pancreatic secretion. Likewise, butyrate supplementation was accompanied by a 50 % increase in activity of elastase II in pancreatic tissue(Reference Guilloteau, Zabielski and David8). These effects may be relevant for overall digestive processes, because during the postprandial 2–3 h the arrival of gastric digesta in the duodenal lumen is the highest(Reference Guilloteau, Toullec and Sauvant150, Reference Guilloteau, Zabielski and Garnsworthy151). These observations help to explain, at least in part, the increase of nutrient digestibility by butyrate supplementation. This hypothesis is supported by the fact that lipase is the pancreatic component that was the most affected, in parallel with fat digestibility(Reference Guilloteau, Savary and Jaguelin-Peyrault146).
In calves and pigs, the activities of brush-border dipeptidylpeptidase IV, lactase and maltase in the mid-jejunum and aminopeptidases A and N in the distal jejunum were increased(Reference Guilloteau, Zabielski and David8, Reference Galfi, Gabel and Martens141). Similar results were reported in other species (i.e. mouse, rat, guinea-pig, vole, pig, sheep and goat) when butyrate was used in in vitro preparations, as an additive in the diet as well as after intravenous (in conscious or anaesthetised animals) or intraduodenal administration. Although it is realised that the response can vary between species(Reference Galfi, Gabel and Martens141, Reference Demol and Sarles152–Reference Claus, Losel and Lacorn157), the data as a whole suggest that butyrate in mammalian species acts directly at the GIT level rather than after its absorption. After oral administration of physiological doses of butyrate, butyrate could act directly on the GIT secretions in the stomach and proximal duodenum but indirectly in the lower small intestine.
Effects on the defence system
In the GIT, the digestive defence system includes the mucous layer in the gut (physical barrier), the innate and adaptative immune systems and the expression of heat-shock proteins (HSP). Butyrate has been found to have a profound impact on the immune system of human subjects and rodents(Reference Weber and Kerr158) and to exert anti-inflammatory properties in vivo and in vitro (Reference Andoh, Bamba and Sasaki159–Reference Venkatraman, Ramakrishna and Shaji161). Surprisingly, butyrate supplementation reduced the small intestine mucosal mass(Reference Le Gall, Gallois and Sève111). This would suggest that butyrate can interfere with intestinal protein metabolism(Reference Jiang, Elson-Schwab and Courtemanche162, Reference Pirman, Ribeyre and Mosoni163), following a reduced bacterial load and possibly a lower inflammation locally.
Butyrate administration into the lumen of isolated vascularly perfused rat colon (5 mm) enhanced mucin secretion, but increasing the concentration up to 100 mm provoked a gradual decrease in mucus discharge(Reference Barcelo, Claustre and Moro164). In piglets fed a butyrate-supplemented diet, the number of colonic goblet cells was significantly increased(Reference Manzanilla, Nofrarias and Anguita94). In milk-fed calves, butyrate enhanced HSP27 and HSP70 expression in the abomasum and in the colon, but not in the small intestine(Reference Guilloteau, Zabielski and David8). Perhaps the heat-shock response was induced indirectly through increasing the numbers of microbials in GI segments (prebiotic activity of butyrate). This finding suggests that butyrate used at low doses may have cytoprotective effects.
Effects on digesta composition (microbiota)
There are few publications studying the effects of inclusion of butyrate at a low dose in diets for animal and human species on intestinal microbiota. The relationship between lactobacilli and enterobacteria populations has traditionally been considered as an index of desirable or undesirable bacteria in pigs, relating a high index with a greater resistance to intestinal disorders(Reference Ewing and Cole165). Adding butyrate to diets promoted greater mean values in this ratio(Reference Castillo, Martin-Orue and Roca166). Galfi & Bokori(Reference Galfi and Bokori140) observed changes in the ileal microbiota with a decrease in the proportion of coliform bacteria and a simultaneous increase of lactobacilli. Van Immerseel et al. (Reference Van Immerseel, Fievez and De Buck167), using microencapsulated butyrate in young chickens, also demonstrated a decrease in the caecum colonisation of Salmonella after an experimental infection. It was reported that oral butyrate supplementation would not influence the number of total bacteria inhabiting different sections of the GIT but rather promote the largest changes in the composition of the jejunal microbiota and in the ecological structure and metabolic activity of the microbial ecosystem of the large intestine(Reference Castillo, Martin-Orue and Roca166). These authors suggested that such changes in the upper GIT could potentially modify the fermentable material reaching the large intestine and, therefore, modify the microbial activity at the faecal level.
Protecting effect against inflammatory intestinal diseases
In humans, effects of lower butyrate concentrations have been reported in patients with ulcerative colitis compared with healthy subjects and there is evidence to suggest that the metabolism of butyrate is impaired in ulcerative colitis(Reference Roediger168). In experimental colitis, colonocyte oxidation of butyrate is markedly suppressed(Reference Ahmad, Krishnan and Ramakrishna169), leading to reduced cellular ATP levels and decreased cell metabolism. Many studies have demonstrated that delivery of butyrate through local enema or diet results in a protective or therapeutic effect against inflammatory bowel diseases(Reference Luhrs, Gerke and Muller170, Reference Okamoto, Sasaki and Tsujikawa171). The role of butyrate in colitis, however, remains complex. Butyrate exerts trophic effects on the colonic epithelium and is able to modify the inflammatory response by inhibiting, for instance, NFκB activation and HSP expression. In dextran sulfate sodium-treated rats, it has been shown that butyrate is efficient in reducing inflammation and in restoring intestinal permeability. The mechanisms of action are numerous and, among them, the inhibition of HSP70 synthesis has been indicated(Reference Venkatraman, Ramakrishna and Shaji161). As mentioned earlier, the effects of butyrate could be different in normal and cancerous cells, such as for apoptosis. In HT-29 cell lines, butyrate seems to be able to prevent inflammation by reducing both cellular permeability in the colon through PPARγ activation and the expression of IL-8 genes(Reference Kinoshita, Suzuki and Saito172). Nevertheless, mechanisms for butyrate action are not always well known. Butyrate can affect several components of the colonic barrier, leading to an improvement in epithelial colonic defence mechanism: mucin (MUC) production, improvement of intestinal epithelial permeability and modulation of pro-inflammatory cytokines via impairment in NFκB activation. In vitro, butyrate increases expression of the MUC gene(Reference Gaudier, Jarry and Blottière173) but the effect seems to be dose dependent(Reference Barcelo, Claustre and Moro164). In Caco-2 cells, a decrease in butyrate increases intestinal permeability(Reference Peng, Li and Green174) and butyrate up-regulates lipoxygenase 5-LOX, 12-LOX and 15-LOX mRNA expression(Reference Ohata, Usami and Miyoshi175). The authors suggested that LOX activated by butyrate may induce cellular differentiation and in addition it could control tight-junction permeability. A recent paper showed that one possible mechanism of action of butyrate on tight junctions involves the AMP-activated protein kinase, but how this kinase is activated by butyrate remains unclear(Reference Peng, Li and Green174). One hypothesis could be that butyrate enhances the energetic state of the cell (increase of cellular ATP content).
In animals and in humans, dietary fibre and SCFA are stimulators of both GLP-1 and GLP-2 synthesis. Butyrate appears to be the strongest stimulator of GLP-2(Reference Mangian and Tappenden176). There is already a great number of GLP-2 effects recognised in the intestine: suppression of gastric acid secretion, decrease in gastric motility, enhancement of intestinal nutrient transport, increase in intestinal blood flow, increase in intestinal cell proliferation, enhancement of intestinal barrier function and inhibition of crypt cell apoptosis. Recently, GLP2 has emerged as a possible treatment option against intestinal bowel diseases(Reference Yazbeck, Howarth and Abbott177).
Mechanisms of action of butyrate in physiological and pathological conditions
In physiological conditions
Action on regulatory peptides
Following ingestion of a butyrate- or butyrate precursor-enriched diet, a common effect of butyrate on the architecture of the GIT wall can be found since it stimulates proliferation, differentiation and maturation, and reduces apoptosis in the cells. The effects on the forestomach, stomach and hindgut seem to be direct(Reference Gorka, Kowalski and Pietrzak131, Reference Mentschel, Leiser and Mulling178), whereas the effects on the pancreas and small intestine are probably indirect. It is increasingly recognised that intestinal development (growth and maturation) and digestive functions depend on a unique neuro-hormonal and immune-regulatory system mediated by a multitude of regulatory substances(Reference Guilloteau, Le Meuth-Metzinger and Morisset179). Thus, it has been suggested that the possible effect of butyrate on GIT development is by its effects on bioactive peptides or hormones secreted into the blood and/or locally produced in the GIT(Reference Guilloteau, Zabielski and David8, Reference Guilloteau, Savary and Jaguelin-Peyrault146) (J Flaga, unpublished results). Two candidate regulatory peptides are gastrin and cholecystokinin, known for their trophic effects in the upper gut. Cholecystokinin and secretin are also known to control the secretion of pancreatic juice and bile. However, no change in the plasma concentration of gastrin, cholecystokinin and secretin or in the expression of cholecystokinin or gastrin receptors was observed in calves fed with a butyrate-enriched diet. These results are in contrast to an enhanced efficiency of duodenal digestive functions and suggest that these peptides do not mediate the butyrate effects(Reference Guilloteau, Zabielski and David8, Reference Guilloteau, Savary and Jaguelin-Peyrault146). These findings disagree with data obtained from in vitro cell-culture studies showing increased gastrin mRNA expression and gastrin secretion during butyrate treatment(Reference Simon, Merchant and Eissele180). Inhibition of postprandial circulating somatostatin in calves(Reference Guilloteau, Zabielski and David8) may be explained by a direct effect of butyrate on somatostatin-secretin cells. Plasma pancreatic polypeptide, which inhibits pancreas and gut secretions, was reduced in newborn piglets following the ingestion of a butyrate-supplemented milk replacer(Reference Kotunia, Wolinski and Laubitz112).
The effects of butyrate on the upper small intestine could involve the GLP-2 pathway in piglets(Reference Bartholome, Albin and Baker113) and in calves(Reference Gorka, Kowalski and Pietrzak131) in agreement with the fact that GLP-2 is considered an important endocrine signal activating intestinal adaptation, cell survival and proliferation in newborn mammals(Reference Burrin, Guan and Stoll181, Reference Burrin, Stoll and Guan182). Also, an increase in plasma enteroglucagon levels was correlated with the increase in the villus height and crypt depth in the small intestine(Reference Andoh, Bamba and Sasaki159). Finally, there was an increase in the expression of insulin-like growth factor-1 receptors in the jejunum of butyrate-supplemented calves(Reference Guilloteau, Zabielski and David8). These results suggest that part of the effects of butyrate is likely to be mediated by insulin-like growth factor-1(Reference Tsubaki, Choi and Ingermann183).
Patients fed dietary fibre have modified acute postprandial responses for glucose, insulin and gut hormones(Reference Weickert and Pfeiffer184). Among gut hormones, GLP-1 stimulates insulin secretion, reduces the rate of gastric emptying, increases insulin sensitivity and decreases energy intake(Reference Freeland, Wilson and Wolever185). As butyrate is able to increase ileal and colonic proglucagon mRNA expression and postprandial GLP-1 concentrations in rats, it probably contributes indirectly via GLP-1 to glucose and energy metabolism.
Action on the defence system
Butyrate decreases in vitro lymphocyte proliferation in mice(Reference Kyner, Zabos and Christman186) and in rats(Reference Cavaglieri, Nishiyama and Fernandes187). In T helper 1 cells butyrate acts as a cell anergy factor. When activated, T helper 1 cells enter into the G1 phase of the cell cycle. However, when antigen-presenting cells are in the presence of butyrate, T helper 1 cell proliferation is blocked(Reference Jackson, DeLoose and Gilbert188). In this case, butyrate requires T-cell receptor ligation and Ca mobilisation(Reference Jackson, DeLoose and Gilbert188). Moreover, butyrate increases the secretion of IL-10(Reference Nancey, Bienvenu and Coffin189, Reference Saemann, Bohmig and Osterreicher190), and decreases the secretion of interferon-γ(Reference Nancey, Bienvenu and Coffin189) by activated human lymphocytes in vitro. Butyrate also decreases the ex vivo production of inflammatory cytokines in intestinal biopsies of humans suffering from Crohn's disease, and reduces the severity of 2,4,6-trinitrobenzenesulfonic acid-induced colitis in rats(Reference Segain, Raingeard de la Blétière and Bourreille160). Butyrate decreases intestinal expression levels of TNFα, IL-1β and IL-6 in patients with Crohn's diseases(Reference Weber and Kerr158). In piglets aged 40 d, Le Gall et al. (Reference Le Gall, Gallois and Sève111) have observed a tendency to a reduced expression of IL-8, in agreement with the results of Gibson & Rosella(Reference Gibson and Rosella191) who have demonstrated that, in vitro, IL-8 secretion by colonic crypt cells is suppressed by butyrate.
Members of the HSP family have been implicated in an ever-growing number of cellular activities including the effects of microbial agents(Reference Narberhaus192). It is reasonable to think that butyrate may affect (1) microbial composition in the intestinal lumen and bacterial number (probiotic effect) and this action may influence HSP expression in the gut tissues, and (2) the HSP expression in the gut mucosa by itself. Thus, cell protection is possibly enhanced because of chaperone activity, mostly ascribed to HSP70 family members(Reference Sikora and Grzesiuk193). It was shown that HSP25/27 need SCFA for basal expression in epithelial cells of the colon and in the distal small intestine in vivo in the rat(Reference Ren, Musch and Kojima194, Reference Arvans, Vavricka and Ren195), which is in agreement with the results obtained in the calf(Reference Guilloteau, Zabielski and David8). In vitro, SCFA enhance the expression of HSP25/27 in a dose- and time-dependent manner, an effect that is transcriptionally regulated(Reference Ropeleski, Tang and Walsh-Reitz196), and protect rat intestinal epithelial cells from injury(Reference Ren, Musch and Kojima194). The HSP response is a universal response to cell injury(Reference Musch, Ciancio and Sarge197) that could be associated with a stress response of the GIT epithelium.
In pathological conditions
Butyrate can hardly escape the splanchnic area; it seems able to act as a systemic mediator in several conditions (Fig. 4).

Fig. 4 Overview of the multiple effects of butyrate as a systemic factor. GLP, glucagon-like peptide; POMC, pro-opiomelanocortin.
Obesity and diabetes
In obese mice fed a high-fat diet, addition of butyrate to the diet (5 % of the meal) reduced insulin resistance and increased energy expenditure(Reference Gao, Yin and Zhang95). In the treated group, butyrate prevented obesity because there was no increase in fat mass after 16 weeks of treatment on the high-fat diet. Butyrate increased thermogenic function in the mice, and treated mice had smaller brown adipocytes than their control counterparts. The expression of two thermogenesis-related genes (PPARγ coactivator-1α (PGC-1α) and uncoupling protein-1 (UCP-1)) was increased in the butyrate group. Fasting insulin was also 50 % lower in the butyrate group, and treated mice showed much better response to insulin during the intraperitoneal insulin tolerance test. In addition, butyrate administration through food supplementation was effective in treating obesity in dietary obese mice. In agreement with these results, several studies showed that rats fed RS (a ‘butyrogenic’ dietary fibre) have lower body fat than their control counterparts. Moreover, dietary RS up-regulated hypothalamic pro-opiomelanocortin (POMC) expression in rats(Reference Shen, Keenan and Martin198). Thus, one mechanism of decreased body fat is probably linked to POMC expression.
Several neuroendocrine factors are involved in the regulation of food intake. Among them, peptide YY, GLP-1 and leptin have been widely studied. Moreover, both GLP-1 and leptin are implicated in the pathogenesis of obesity and diabetes. Butyrate directly increases peptide YY/proglucagon gene expression in vitro (Reference Zhou, Hegsted and McCutcheon199). Such a result is observed in vivo in RS-fed rats. Such findings may lead to the hypothesis that butyrate could also act as a pathogenic factor in some diseases by increasing food intake. Leptin is thought to be a sensor of energy stores in the body via the body fat mass. Numerous data demonstrate that leptin is a probable humoral link between metabolism and several neuroendocrine systems. In vitro, butyrate down-regulates leptin expression in rat anterior pituitary cells(Reference Yonekura, Senoo and Kobayashi200). In fact, in vitro, butyrate seems to be able to regulate the synthesis of some pituitary hormones. Nevertheless, to date these effects of butyrate have not been confirmed in in vivo studies and there is also apparent conflict between the results of clinical studies and animal performance studies. In clinical trials (inflammatory bowel disease, Crohn's disease, ulcerative colitis, etc) it was shown that the body weight of patients treated with butyrate increased. It was confirmed in patients with Crohn's disease who received microencapsulated butyrate (R Zabielski, unpublished results). Concerning animal production, body-weight gain increases following butyrate treatment. The appetite is probably increased and the intestinal microstructure could be improved. Thus, it is possible that in ‘healthy overweight’ individuals the response to butyrate may be different from that in patients with inflammatory bowel disease.
Protection against oxidative damage
Inflammatory bowel diseases are characterised by recurrent chronic inflammation. The inflammatory cells produce large quantities of reactive oxygen species in the deep layers of the bowel wall that pave the way for colorectal cancer(Reference Roessner, Kuester and Malfertheiner201). Butyrate could modulate the level of oxidative stress both in vitro and in vivo. In isolated human colonocytes and in HT29 tumour cells, butyrate shows a protective effect against oxidative DNA damage induced by H2O2(Reference Rosignoli, Fabiani and De Bartolomeo202). In healthy colon cells, butyrate modulates the expression of genes associated with oxidative stress(Reference Sauer, Richter and Pool-Zobel203). In humans, the expression of human catalase, human cyclo-oxygenase 2 and human metallothionein 2A is enhanced, whereas that of glutathione reductase, PG-endoperoxide synthase 2 and superoxide dismutase 2 is lowered. The authors concluded that changes could reduce oxidant-damaging effects and protect cells from cancerogenesis.
Nevertheless, the response to butyrate treatment showed large variability among donors. Rectal administration of butyrate enhances the colonic antioxidant capacity of healthy patients(Reference Hamer, Jonkers and Bast204). The rectal infusion clearly increases total glutathione production in perfused biopsies. The expression of several genes is also modified: increase of glutathione peroxidase 1, glutathione peroxidase 3 and glutamate-cysteine ligase catalytic sub-unit, and decrease of glutathione peroxidase 2, glutathione reductase, glutathione synthetase and xanthine dehydrogenase. Notably, the effects of butyrate are not long lasting and disappeared soon after finishing the infusions.
Mechanism of pathogen control against bacteria
Acetate, propionate and butyrate are weak acids with pKa values of approximately 4·8. They have bacteriostatic and bactericidal properties depending on the physiological status of the bacteria and the physico-chemical characteristics of the external environment(Reference Ricke205). The concentration of the undissociated form, which can diffuse freely across the bacterial lipid membrane into the bacterial cell, is affected by pH. Therefore pH is a key determinant of effectiveness(Reference Davidson, Doyle, Beuchat and Montville206). A pH < 4·8 increases the availability of the undissociated acid that once internalised into the more alkaline cytoplasm, dissociates into anions and protons, thereby reducing the internal pH(Reference Russell and Diez-Gonzalez207). But bacteria need to maintain a near-neutral pH in their cytoplasm to sustain functional macromolecules. Exporting the excess of protons requires cellular ATP and may result in depletion of cellular energy leading to cellular damage and/or death. The accumulation of anions would be the primary toxic effect of organic acids(Reference Davidson, Doyle, Beuchat and Montville206). Some organisms allow a decline of their internal pH and are therefore more resistant to organic acids than others. Also inherent resistance via exclusion of the antimicrobial chemical, secretion of the toxic compound or metabolic detoxification can influence the efficacy of the organic acids(Reference Davidson, Doyle, Beuchat and Montville206). SCFA are proposed to interfere with cytoplasmic membrane structures and membrane proteins, uncoupling electron transport and therefore reducing ATP production. This interference can also lead to damage in the cytoplasmic membrane, resulting in leakage or disruption of outer membrane permeability. Less direct antibacterial action is the influence of organic acids on macromolecular synthesis or nutrient transport(Reference Davidson, Doyle, Beuchat and Montville206, Reference Cherrington, Hinton and Mead208).
SCFA are used in pathogen control in the field, especially against Salmonella in poultry and pigs. In chickens, during the early stages of life the multiplication of Salmonella is difficult to prevent by endogenously produced SCFA. As it is of great importance to prevent initial colonisation of Salmonella, and because food is a major source for Salmonella introduction to the farm, drinking water and food are commonly acidified. By creating a barrier at the level of drinking water and food, recontamination with pathogens is believed to be prevented. In food, the antibacterial activity of SCFA depends on the temperature and moisture. Since minimal inhibitory concentration values increase at increasing pH values, quite high concentrations of SCFA are needed in the gut to achieve a direct antimicrobial effect. The powder-form acids used as a feed additive exert their effects in the upper GIT. When using acids coated on a carrier or in encapsulated form, the acids are thought to be protected from the intestinal environment which allows the control of their site of action as well as the velocity of release and dissociation(Reference Gauthier134). This is of importance, as the main colonisation places for Salmonella in chickens are the caeca(Reference Desmidt, Ducatelle and Haesebrouck209). In epithelial cell lines and primary chicken caecal epithelial cells, the invasion of Salmonella typhimurium and S. enteritidis is suppressed when the strains are pre-incubated in growth media supplemented with various concentrations of butyrate, while pre-incubation in acetate-supplemented medium results in an increased invasion(Reference Van Immerseel, De Buck and De Smet210). The effect of SCFA on Salmonella invasion is explained by changes in expression patterns of genes of Salmonella pathogenicity island (SPI)-1, which encodes for regulatory and effector proteins and structural components of a needle complex, necessary for invasion in epithelial cells. DNA microarrays of both S. typhimurium and S. enteritidis grown in media supplemented with low concentrations of butyrate show specific down-regulation of SPI-1 while no alterations in metabolic gene expression are seen when compared with control samples(Reference Gantois, Ducatelle and Pasmans211). An opposite effect is observed in enterohaemorrhagic Escherichia coli, a human pathogen causing severe diarrhoea. Nakanishi et al. (Reference Nakanishi, Tashiro and Kuhara212) showed that butyrate enhances the expression of virulence-associated genes in a concentration-dependent manner. Even at low concentrations, butyrate triggers the adherence-related genes, leading to efficient colonisation of the target niche(Reference Nakanishi, Tashiro and Kuhara212).
Besides altering the pathogenicity of bacteria by antimicrobial effects or by an effect on virulence gene expression, butyrate has some effects on the host that can significantly contribute to protection against pathogens. Via in vivo experiments, it was shown that butyrate prevents Clostridium perfringens-induced necrotic enteritis lesions in broiler chickens(Reference Timbermont, Lanckriet and Dewulf213). Because butyrate has no significant antimicrobial effect against Clostridium perfringens, it can be concluded that the observed results are due to effects of butyrate on the host. A possible explanation could be a faster replacement of epithelial cells in the necrotic foci by the trophic effects of butyrate. Similar results can be concluded for cell invasion assays using Campylobacter jejuni. Butyrate-treated Caco2-cells were protected from Campylobacter jejuni invasion in a concentration-dependent manner(Reference Van Deun, Pasmans and Van Immerseel214). This effect was explained by the butyrate-induced differentiation of the Caco-2 cells, as differentiated monolayers cells are less susceptible to Campylobacter jejuni invasion than undifferentiated cells(Reference Siavoshian, Blottière and Le Foll215).
Concluding remarks and perspectives
The present paper underlines that butyrate is a natural component of the biological liquids and tissues as well as of digestive contents and that butyrate has particular properties among the SCFA. It is produced in small quantities in neonates, but its production greatly increases during the development of the hindgut and/or forestomachs. In omnivorous animals and in man, RS and fructo-oligosaccharides are essential precursors for butyrate production in the hindgut.
The present paper aims to highlight the biological role of butyrate, whether it is naturally produced by the GI microbial ecosystem or orally ingested as a feed additive. Since in most circumstances butyrate seems to act in a similar way when it is in the acid or salt form it appears that only the radical [CH3-CH2-CH2-COO-] is of major importance. Under physiological conditions, the increase in performance could be explained, at least in part, by the increased nutrient digestibility thanks to a delayed gastric emptying and optimised pH of gastric digesta. This first phase of digestion leads to a more efficient degradation of dietary components in the gut, which are further processed by pancreatic and intestinal enzymes stimulated by butyrate entering the small intestine. Moreover, a decrease in diarrhoea was observed perhaps in relation to modification of ileal microbiota as well as that of large bowel content and also to an improvement of epithelial integrity and defence systems. The key consequences are beneficial systemic effects on feed efficiency, growth rate and adiposity despite the fact that butyrate remains undetectable in the peripheral blood. Thus, butyrate could have an indirect effect on the upper GIT or a direct effect on the lower GIT.
Numerous mechanisms are believed to contribute to the beneficial effects of butyrate; however, many remain unclear. In the digestive tract and in both situations (low and high doses of butyrate in the lumen), butyrate is able to modify development or rebuilding of the tissues. Thus, trophic effects are on cell proliferation, resulting in a faster repair of necrotic tissues. There is also an indirect way of butyrate action since this molecule is not found in blood. This effect probably involves substances produced by the hormono–neuro–immuno system. Butyrate could also act on the intestinal microbiota composition. Thus, the common mechanism of butyrate's indirect action on host GIT tissue cells and on micro-organisms is at the molecular level, as butyrate was shown to operate at the first stages of DNA damage in cell lines.
In animal production, studies showed that ingested butyrate is a helpful feed additive as it allows pathogen control, increases ration digestibility and growth rate, and decreases oxidative stress and cytokine synthesis, even at very low doses. The effects are predominantly noteworthy in neonates and before weaning in piglets and calves. We suggest that such effects could be developed to extend the use of butyrate in the field of animal production and also considered for new applications in human nutrition.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors greatly acknowledge Mrs Victoria Frances O'Gorman for the English revision of the manuscript.
P. G. was the initiator of the review and all authors contributed equally to the preparation of the paper.
There are no conflicts of interest.






