1. Introduction
With nearly 6000 extant species, Neuroptera is the largest and best-known order of Neuropterida (Vasilikopoulos et al. Reference Vasilikopoulos, Misof, Meusemann, Lieberz, Flouri, Beutel, Niehuis, Wappler, Rust and Peters2020; Lu & Liu, Reference Lu and Liu2021; Oswald, Reference Oswald2021), its adults characterized by gracile bodies and membranous, many-veined wings (Lu & Liu, Reference Lu and Liu2021), many of them resembling insects of other groups, such as the extinct butterfly-like adults of Kalligrammatidae (Labandeira et al. Reference Labandeira, Yang, Santiago-Blay, Hotton, Monteiro, Wang, Goreva, Shih, Siljeström, Rose, Dilcher and Ren2016; Liu et al. Reference Liu, Lu, Zhang, Chen, Zheng, Zhang, Liu and Wang2018 a), extant dragonfly-like adults of Ascalaphidae (Gao et al. Reference Gao, Cai, Yu, Storey and Zhang2018), and mantis or wasp-like adults of Mantispidae (Snyman et al. Reference Snyman, Ohl, Pirk and Sole2020). Compared with adults, neuropteran larvae are less prominent and have received less attention (Tauber et al. Reference Tauber, Tauber, Albuquerque, Resh and Cardé2009); however, they play an important role in the study of lacewing phylogeny (Aspöck & Aspöck, Reference Aspöck and Aspöck2007). They display bizarre and specialized morphological and behavioural traits, mostly associated with diverse predatory habits (Lu & Liu, Reference Lu and Liu2021). Lacewing larvae are generally fierce ambush predators (Myrmeleontidae and related families) or active predators (Chrysopidae and Hemerobiidae), while several other lacewings are highly specialized predators of particular prey items (from sponges (e.g. Sisyridae) to spider eggs (e.g. Mantispidae)) (Brown, Reference Brown1952; Haug et al. Reference Haug, Müller and Haug2018; Pérez-de la Fuente et al. Reference Pérez-de la Fuente, Engel, Delclòs and Peñalver2020), their larval mandibles and maxillae interlocking to form stylets, which are used to seize and impale the prey, deliver venom to subdue it, and subsequently to suck out the liquid contents of the victim (Liu et al. Reference Liu, Zhang, Winterton Shaun, Breitkreuz Laura and Engel Michael2016; Engel et al. Reference Engel, Winterton and Breitkreuz2018; Winterton et al. Reference Winterton, Lemmon, Gillung, Garzon, Badano, Bakkes, Breitkreuz, Engel, Lemmon, Liu, Machado, Skevington and Oswald2018).
Phylogenomics analysis indicates that the origin of Neuropterida may date back to the Late Carboniferous or even earlier, and the origin of Neuroptera back to the Early Permian (Misof et al. Reference Misof, Liu, Meusemann, Peters, Donath, Mayer, Frandsen, Ware, Flouri, Beutel, Niehuis, Petersen, Izquierdo-Carrasco, Wappler, Rust, Aberer, Aspöck, Aspöck, Bartel, Blanke, Berger, Böhm, Buckley, Calcott, Chen, Friedrich, Fukui, Fujita, Greve, Grobe, Gu, Huang, Jermiin, Kawahara, Krogmann, Kubiak, Lanfear, Letsch, Li, Li, Li, Lu, Machida, Mashimo, Kapli, McKenna, Meng, Nakagaki, Navarrete-Heredia, Ott, Ou, Pass, Podsiadlowski, Pohl, von Reumont, Schütte, Sekiya, Shimizu, Slipinski, Stamatakis, Song, Su, Szucsich, Tan, Tan, Tang, Tang, Timelthaler, Tomizuka, Trautwein, Tong, Uchifune, Walzl, Wiegmann, Wilbrandt, Wipfler, Wong, Wu, Wu, Xie, Yang, Yang, Yeates, Yoshizawa, Zhang, Zhang, Zhang, Zhang, Zhao, Zhou, Zhou, Ziesmann, Zou, Li, Xu, Zhang, Yang, Wang, Wang, Kjer and Zhou2014; Wang et al. Reference Wang, Liu, Garzón-Orduña, Winterton, Yan, Aspöck, Aspöck and Yang2017; Engel et al. Reference Engel, Winterton and Breitkreuz2018; Winterton et al. Reference Winterton, Lemmon, Gillung, Garzon, Badano, Bakkes, Breitkreuz, Engel, Lemmon, Liu, Machado, Skevington and Oswald2018; Vasilikopoulos et al. Reference Vasilikopoulos, Misof, Meusemann, Lieberz, Flouri, Beutel, Niehuis, Wappler, Rust and Peters2020; Lu & Liu, Reference Lu and Liu2021); the earliest definite fossil record of Neuroptera is Late Permian (Tauber et al. Reference Tauber, Tauber, Albuquerque, Resh and Cardé2009). The fossil record of adult Neuroptera is relatively rich, with worldwide distribution during the Mesozoic, but almost half of these lacewing groups are now extinct (Engel et al. Reference Engel, Winterton and Breitkreuz2018; Lu & Liu, Reference Lu and Liu2021). Therefore, the Mesozoic is considered to be the ‘golden age’ for the diversification of Neuroptera, and their modern counterparts are a residual shadow of the rich Mesozoic palaeodiversity (Aspöck et al. Reference Aspöck, Plant and Nemeschkal2001; Wang et al. Reference Wang, Liu, Garzón-Orduña, Winterton, Yan, Aspöck, Aspöck and Yang2017; Engel et al. Reference Engel, Winterton and Breitkreuz2018; Winterton et al. Reference Winterton, Lemmon, Gillung, Garzon, Badano, Bakkes, Breitkreuz, Engel, Lemmon, Liu, Machado, Skevington and Oswald2018; Lu & Liu, Reference Lu and Liu2021). The life habits of lacewing larvae have remained almost unchanged since the Cretaceous, many extant behaviours, such as debris-carrying camouflage (Pérez-de la Fuente et al. Reference Pérez-de la Fuente, Delclòs, Peñalver, Speranza, Wierzchos, Ascaso and Engel2012, Reference Pérez-de la Fuente, Delclòs, Peñalver and Engel2016; Wang et al. Reference Wang, Xia, Engel, Perrichot, Shi, Zhang, Chen, Jarzembowski, Wappler and Rust2016; Badano et al. Reference Badano, Engel, Basso, Wang and Cerretti2018; Pérez-de la Fuente et al. Reference Pérez-de la Fuente, Peñalver, Azar and Engel2018), fossoriality (soil digging) (Badano et al. Reference Badano, Engel, Basso, Wang and Cerretti2018) and spider parasitism (Haug et al. Reference Haug, Müller and Haug2018), having been discovered in Cretaceous amber. Furthermore, some Cretaceous lacewing larvae display unique morphological modifications and/or evidence of specialized behaviours that have never been found among living Neuroptera, e.g. predation or kleptoparasitism of web-spinning spiders (Liu et al. Reference Liu, Zhang, Winterton Shaun, Breitkreuz Laura and Engel Michael2016), liverwort mimesis (Liu et al. Reference Liu, Shi, Xia, Lu, Wang and Engel2018 b) and ‘tail-like’ terminal segments (Badano et al. Reference Badano, Fratini, Maugeri, Palermo, Pieroni, Cedola, Haug, Weiterschan, Velten, Mei, Di Giulio and Cerretti2021). Moreover, many fossil lacewing larvae possess a combination of characters different from extant families, making it hard to determine their exact systematic position (Wang et al. Reference Wang, Xia, Engel, Perrichot, Shi, Zhang, Chen, Jarzembowski, Wappler and Rust2016; Badano et al. Reference Badano, Engel, Basso, Wang and Cerretti2018; Haug et al. Reference Haug, Herrera-Flórez, Müller and Haug2019 a, b, c, Reference Haug, Pazinato, Haug and Haug2020 b; Herrera-Flórez et al. Reference Herrera-Flórez, Braig, Haug, Neumann, Wunderlich, Hörnig and Haug2020; Hörnig et al. Reference Hörnig, Kiesmüller, Müller, Haug and Haug2020; Badano et al. Reference Badano, Fratini, Maugeri, Palermo, Pieroni, Cedola, Haug, Weiterschan, Velten, Mei, Di Giulio and Cerretti2021).
Neuroptera used to be divided into three suborders: Nevrorthiformia (comprising Nevrorthidae), Hemerobiiformia (comprising Berothidae, Chrysopidae, Coniopterygidae, Dilaridae, Hemerobiidae, Ithonidae (including Polystoechotidae), Mantispidae, Osmylidae, Rhachiberothidae and Sisyridae) and Myrmeleontiformia (comprising Ascalaphidae, Myrmeleontidae, Nemopteridae, Nymphidae and Psychopsidae). However, this classification system is largely outdated because phylogenomic and morphological studies clearly indicate that Hemerobiiformia is paraphyletic and Nevrorthiformia is superfluous (Aspöck et al. Reference Aspöck, Haring and Aspöck2012; Wang et al. Reference Wang, Liu, Garzón-Orduña, Winterton, Yan, Aspöck, Aspöck and Yang2017; Engel et al. Reference Engel, Winterton and Breitkreuz2018; Winterton et al. Reference Winterton, Lemmon, Gillung, Garzon, Badano, Bakkes, Breitkreuz, Engel, Lemmon, Liu, Machado, Skevington and Oswald2018; Vasilikopoulos et al. Reference Vasilikopoulos, Misof, Meusemann, Lieberz, Flouri, Beutel, Niehuis, Wappler, Rust and Peters2020), and Winterton et al. (Reference Winterton, Lemmon, Gillung, Garzon, Badano, Bakkes, Breitkreuz, Engel, Lemmon, Liu, Machado, Skevington and Oswald2018) proposed a new classification that recognizes seven superfamilies (see fig. 1 of their paper).
Myrmeleontiformia is characterized by the larvae having a robust head capsule, strong jaws and an associated series of adaptations allowing them to cope with large prey (Badano et al. Reference Badano, Aspöck, Aspöck and Cerretti2017). As mentioned above, Myrmeleontiformia includes five major lineages, often considered as families: Psychopsidae (silky lacewings), Nemopteridae (thread-winged lacewings), Nymphidae (split-footed lacewings), Ascalaphidae (owlflies) and Myrmeleontidae (antlions). The larvae of Psychopsidae, Nemopteridae and Nymphidae are usually easily recognized, while it is often difficult to distinguish between Ascalaphidae and Myrmeleontidae, especially in the fossil state because of their similar appearance. Moreover, although adults of Myrmeleontiformia (or its stem group) are relatively abundant in the fossil record (Grimaldi & Engel, Reference Grimaldi and Engel2005), fossil larvae are rare and so far only reported from Mesozoic and Cenozoic amber inclusions (Badano et al. Reference Badano, Aspöck, Aspöck and Cerretti2017; Pérez-de la Fuente et al. Reference Pérez-de la Fuente, Engel, Delclòs and Peñalver2020; Haug et al. Reference Haug, Haug, Baranov, Solórzano-Kraemer and Haug2021 a).
Here we describe an extremely large inclusion as a new genus and species, Kuafupolydentes hui gen. et sp. nov., which can be attributed to the early representative of Myrmeleontiformia, from mid-Cretaceous Kachin amber.
2. Materials and methods
The unique specimen is from the Cretaceous deposits in the Hukawng Valley of Myanmar, from a former amber mine located near Danai (Tanai) town (26° 21″ 33.41″ N, 96° 43′ 11.88″ E; palaeolatitude 5.0 ± 4.7° S) (Kania et al. Reference Kania, Wang and Szwedo2015; Thu & Zaw, Reference Thu and Zaw2017; Westerweel et al. Reference Westerweel, Roperch, Licht, Dupont-Nivet, Win, Poblete, Ruffet, Swe, Thi and Aung2019; see the locality in fig. 1 of Jiang et al. Reference Jiang, Szwedo and Wang2019). More than 600 families of invertebrates, vertebrates, protists, plants and fungi have been reported from Kachin amber (Yu et al. Reference Yu, Kelly, Mu, Ross, Kennedy, Broly, Xia, Zhang, Wang and Dilcher2019; Ross, Reference Ross2021). Radiometric U–Pb zircon dating of the volcaniclastic matrix of the amber constrained a refined age of 98.79 ± 0.62 Ma (earliest Cenomanian) (Shi et al. Reference Shi, Grimaldi, Harlow, Wang, Wang, Yang, Lei, Li and Li2012), which is also supported by an ammonite trapped in the amber (Yu et al. Reference Yu, Kelly, Mu, Ross, Kennedy, Broly, Xia, Zhang, Wang and Dilcher2019).
Observations were performed using a Zeiss Stemi 508 microscope. The photographs were taken with a Zeiss Stereo Discovery V16 microscope system in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, and measurements were taken using Zen software. Photomicrographic composites of 10–100 individual focal planes were digitally stacked using the software Helicon Focus 6.7.1 for a better illustration of 3D structures. Photographs were adjusted using Adobe Lightroom Classic, and line drawings were prepared using CorelDraw 2019 graphic software.
3. Systematic palaeontology
Order Neuroptera Linnaeus, Reference Linnaeus1758
Myrmeleontiformia
Genus Kuafupolydentes gen. nov. (Figs 1–5)
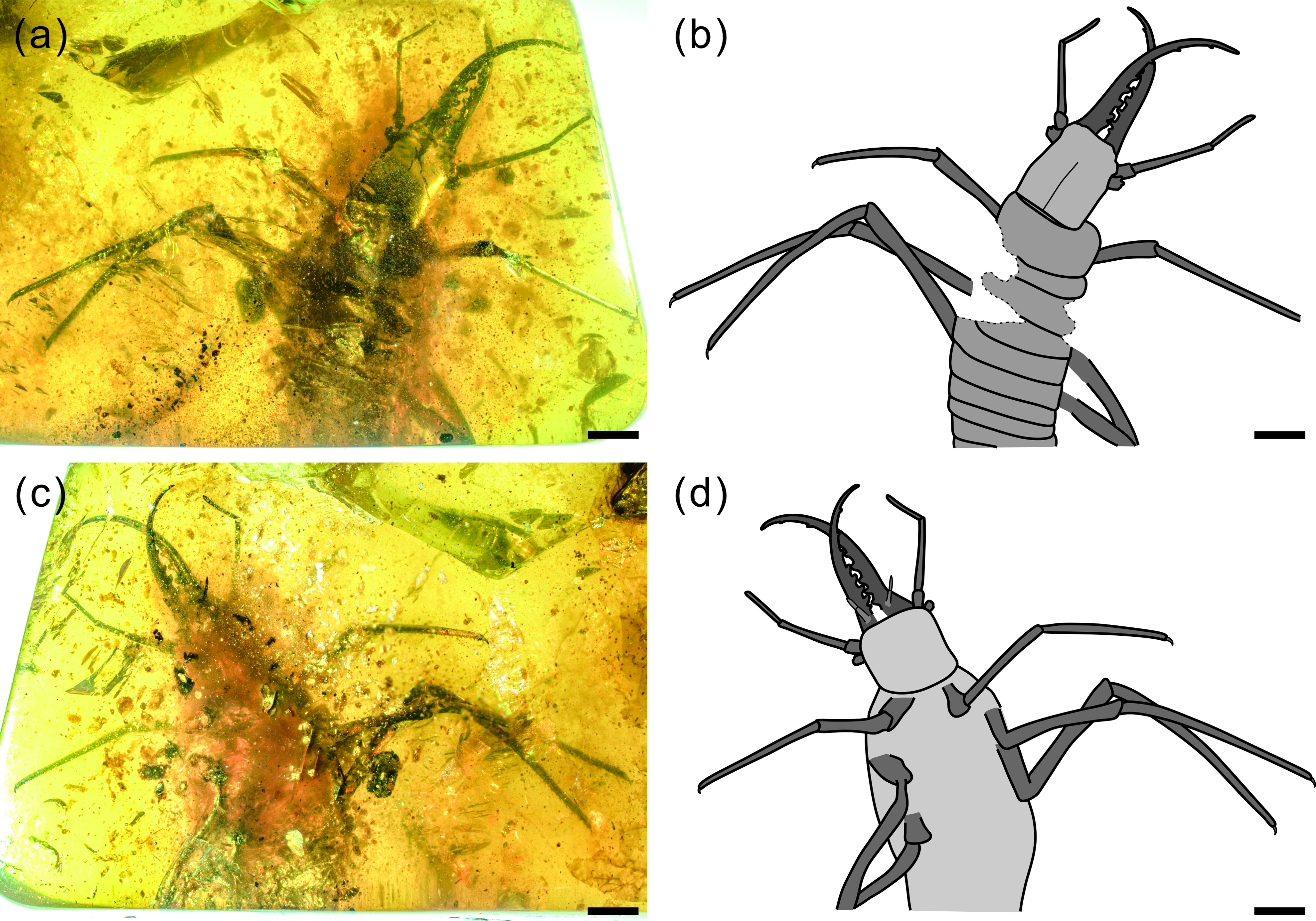
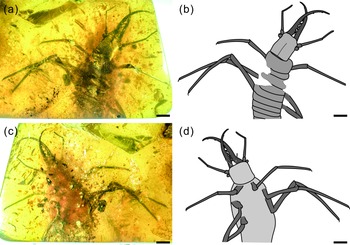
Fig. 1. Photographs of Kuafupolydentes hui Luo, Liu et Jarzembowski, gen. et sp. nov., holotype, SNHM7277. (a) Dorsal view. (b) Ventral view. Scale bars = 2 mm.
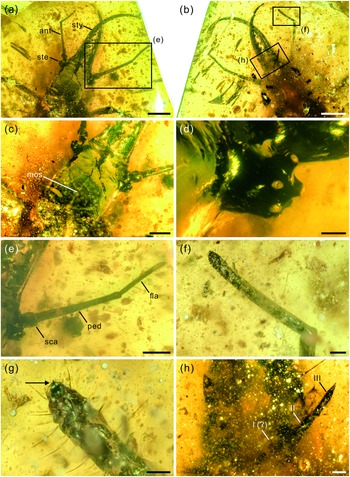
Fig. 2. Photographs of structural details of head capsule of Kuafupolydentes hui Luo, Liu et Jarzembowski, gen. et sp. nov., holotype, SNHM7277. (a) Head and mandibular–maxillary stylets in dorsal view. (b) Head and mandibular–maxillary stylets in ventral view. (c) Head capsule. (d) Right stemmata. (e) Right antenna in dorsal view. (f) Flagellum of the left antenna in ventral view. (g) The apex of the flagellum of the left antenna in ventral view, arrow showing the small teeth (or short apical setae). (h) Labial palpus (segments numbered). Abbreviations: ant, antenna; fla, flagellum; mos, moulting suture; ped, pedicel; sca, scape; ste, stemmata; sty, style. Scale bars for (a), (b) = 2.0 mm; (c), (e) = 1.0 mm; (d), (f), (h) = 0.2 mm; (g) = 0.1 mm.
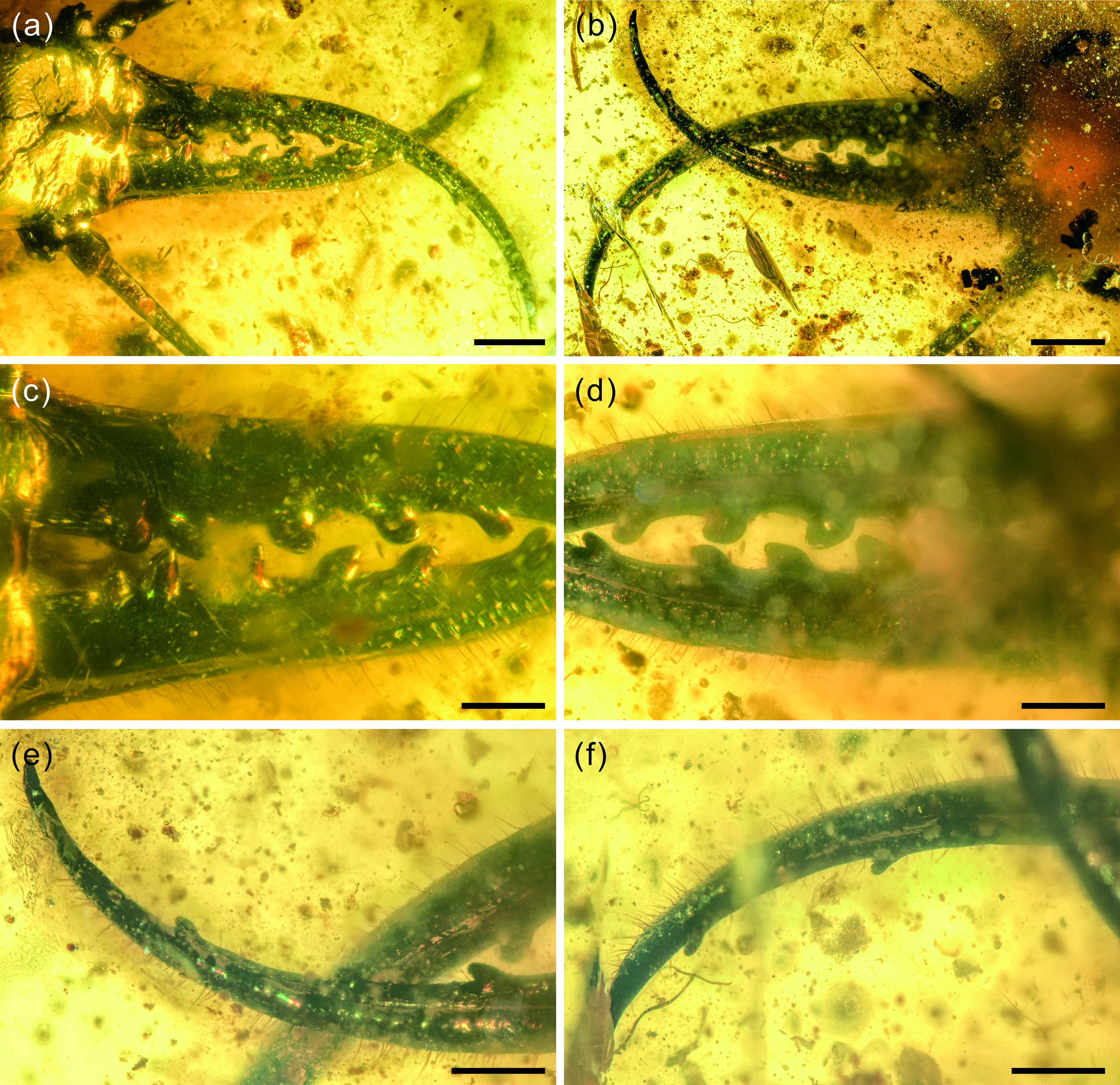
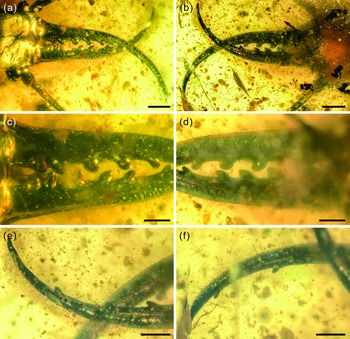
Fig. 3. Photographs of structural details of mandibular–maxillary stylets of Kuafupolydentes hui Luo, Liu et Jarzembowski, gen. et sp. nov., holotype, SNHM7277. (a) Mandibular–maxillary stylets in dorsal view. (b) Mandibular–maxillary stylets in ventral view. (c) Basal part of stylets in dorsal view. (d) Basal part of stylets in ventral view. (e) The apex of the left stylet in ventral view. (f) The apex of the right stylet in ventral view. Scale bars for (a), (b) = 1.0 mm; (c)–(f) = 0.5 mm.
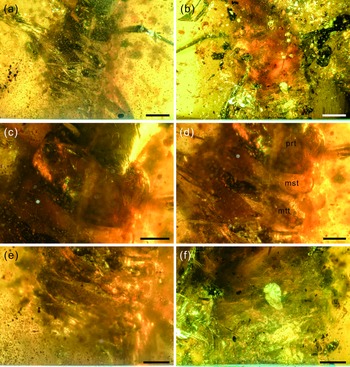
Fig. 4. Photographs of structural details of the trunk of Kuafupolydentes hui Luo, Liu et Jarzembowski, gen. et sp. nov., holotype, SNHM7277. (a) Trunk in dorsal view. (b) Trunk in ventral view. (c) Prothorax. (d) Mesothorax and metathorax. (e) Abdomen in dorsal view. (f) Abdomen in ventral view. Abbreviations: mst, mesothorax; mtt, metathorax; prt, prothorax. Scale bars for (a), (b) = 2.0 mm; (c)–(f) = 1.0 mm.
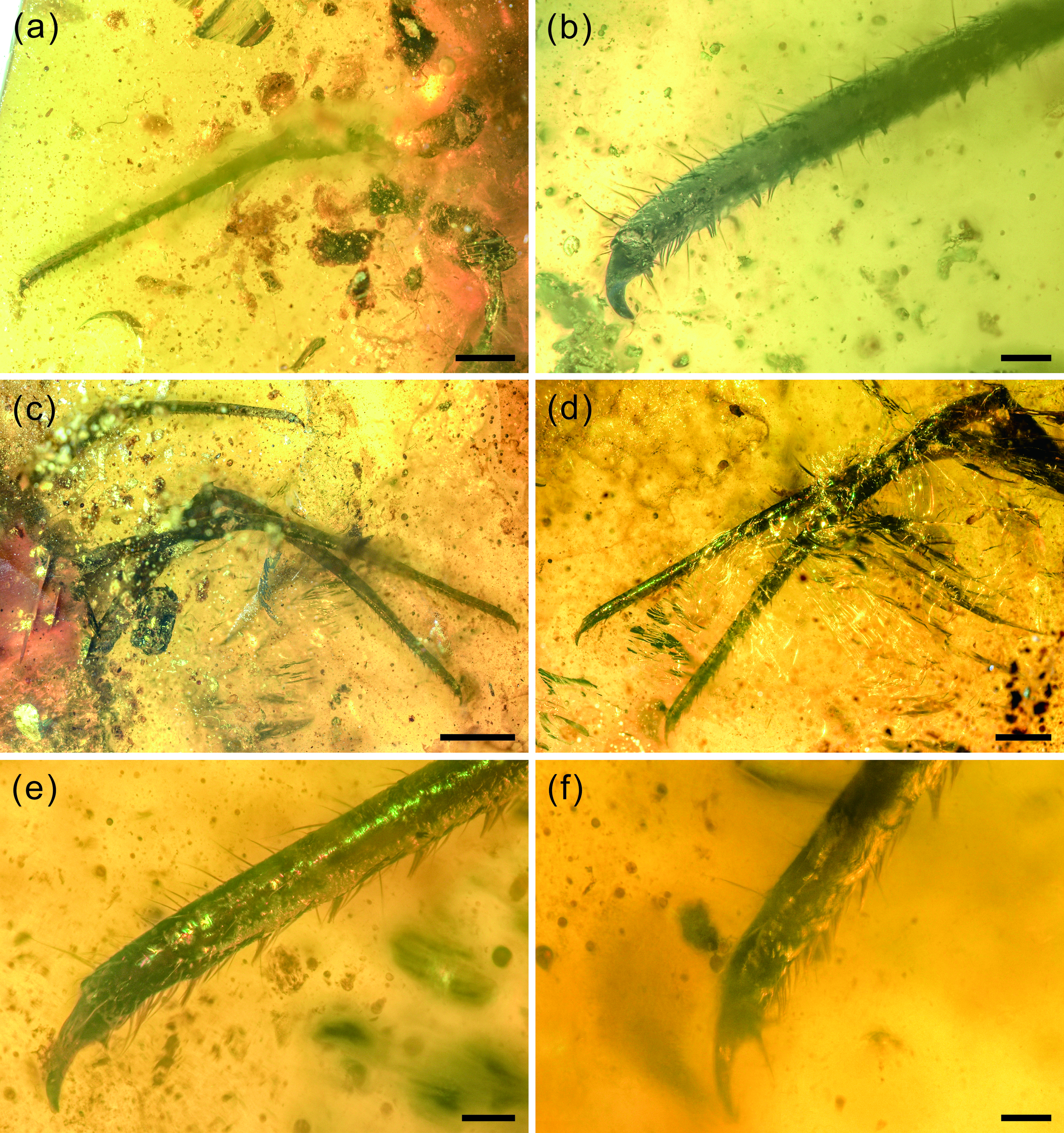
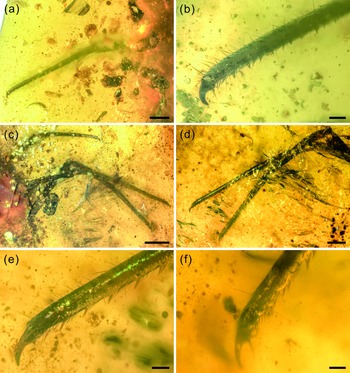
Fig. 5. Photographs of structural details of legs of Kuafupolydentes hui Luo, Liu et Jarzembowski, gen. et sp. nov., holotype, SNHM7277. (a) Right foreleg in ventral view. (b) The apex of the right foreleg in ventral view. (c) Left midleg and hindleg in ventral view. (d) Tarsus and tibia of left midleg and hindleg in dorsal view. (e) The apex of the left midleg in dorsal view. (f) The apex of the left hindleg in dorsal view. Scale bars for (c) = 2.0 mm; (a), (d) = 1.0 mm; (b), (e), (f) = 0.2 mm.
LSID: urn:lsid:zoobank.org:act:97F351E0-0ADA-4453-9535-61C8250B7F51
Etymology. The generic name is derived from a combination of Chinese names and latinized words: ‘Kua Fu’ is a famous giant in Chinese mythology, referring to the large size of this genus; ‘poly’ meaning ‘many’, ‘dentes’ meaning ‘teeth’, refer to the large number of teeth in this genus. The gender of the name is masculine.
Type species. Kuafupolydentes hui Luo, Liu et Jarzembowski, sp. nov.; by original designation and monotypy.
Included species. Type species only.
Diagnosis (larva). As for species.
Age and occurrence. Mid-Cretaceous (late Albian/early Cenomanian); amber from Kachin State, northern Myanmar.
Kuafupolydentes hui sp. nov. (Figs 1–5)
LSID: urn:lsid:zoobank.org:act:67EEFA7E-3811-47BC-93BC-B387780DF20C
Etymology. The specific name is dedicated to the collector, Mr Jiang HU.
Material. Holotype. Burmese amber cabochon, 31 × 20 × 6 mm, weight 3.6 g, specimen number SNHM7277, the amber is permanently deposited in Shanghai Natural History Museum. Holotype inclusion incomplete: trunk partly destroyed.
Locality and horizon. Burmese amber, from deposits near Tanai village in the Hukawng Valley of northern Myanmar, upper Albian / lower Cenomanian (mid-Cretaceous).
Diagnosis (larva). Body large with sparse setae, more than 15 mm long; head rectangular, antennae extremely robust, slightly shorter than mandible, pedicel extremely elongate, more than half of antennal length, flagellum without apparent subdivisions, apex with few small teeth; mandibular–maxillary stylets nearly twice as long as head capsule, setae of external margin denser than on internal margin, each stylet with eight blunt and short teeth, almost as long as wide, most basal one smaller than second one, second tooth largest, then teeth becoming smaller consecutively; legs long and robust compared with body, hindlegs longest, forelegs shortest, midlegs intermediate; all legs with elongate femur, tarsus and tibia, tarsus and tibia continuous with relatively densely arrayed setae in line on internal margin; claw single, hook-shaped, enlarged on all legs, pretarsal empodium lacking.
Description (larva). Body robust, very large, only with 13.1 mm preserved (excluding mandible–maxilla complex), separated distinctly into head and trunk (Fig. 1). Head capsule rectangular with smooth margins, without distinct setae, dorso-ventrally flattened, longer than wide, 3.75 mm long and 3.02 mm wide in dorsal view; head capsule with slightly elevated, moulting suture present, straight in posterior half of head capsule; hypostomal bridge distinct, anterior margin of cranium straight between mandibles and maxillae, posterior margin of head capsule also straight (Fig. 2a–c). Ocular region raised on prominent tubercle with a subcircular cross-section, c. 0.54 mm long and 0.53 mm wide, with at least six stemmata (Fig. 2d). Antennae situated just below base of forward-oriented mouthparts, extremely robust, slightly shorter than mandible, 5.65 mm long; scape wider than following antennomeres, subconical in shape, 0.66 mm long and 0.55 mm wide; pedicel extremely elongate, more than half of antennal length, slightly tapering forward, with several setae, 3.30 mm long and c. 0.25 mm wide; flagellum without apparent subdivisions, with several setae, not tapered until apex, apex with three(?) small teeth (or short apical setae), 1.69 mm long and 0.16 mm wide (Fig. 2e–g). Mandibles and maxillae (upper and lower jaws) forming a pair of mandibular–maxillary stylets; mandibular–maxillary stylets large and prominent, arising anteriorly (prognathous), curved inward, covered with setae and setae of external margin denser than on internal margin, mandible nearly twice as long as head capsule, c. 7.39 mm in length; each stylet with eight blunt and short teeth, almost as long as wide, most basal one smaller than second (distal) one; second tooth largest, then teeth becoming progressively smaller (Fig. 3). Labial palpus situated just below inner margin of stylets, short and thin compared to antennae and stylets, tapering toward apex, probably with three palpomeres (but only two clearly visible), covered with few setae, first (I) unrecognizable, second (II) palpomere 0.67 mm long and 0.15 mm wide (at middle region), third (III) palpomere 0.57 mm long and 0.10 mm wide (at middle region) (Fig. 2h).
Trunk region not clearly preserved, blurred by large amounts of small debris (Fig. 4). Cervix (neck region) very short, membranous, collar-like, no setae discernible. Prothorax tubular, long and wide, wider than head capsule, covered with few short setae, 2.04 mm long and 4.34 mm wide (Fig. 4c). Mesothorax partly destroyed, transversely broad, much shorter but slightly wider than prothorax, covered with few short setae, gently bulging laterally, 0.71 mm long and at least 4.40 mm wide. Metathorax indistinct, partly destroyed, transversely broad, shorter than prothorax, but wider than mesothorax, gently bulging laterally, 1.68 mm long, width indeterminate (Fig. 4d).
Legs extremely long compared with body. Foreleg coxa robust, covered with few short setae, 0.69 mm wide; trochanter unrecognizable; femur elongate, cylindrical, almost parallel-sided, covered with few short setae, 4.01 mm long and 0.50 mm wide; tarsus and tibia continuous, elongate, cylindrical, slightly tapering toward apex, covered with few short setae, with relatively densely arrayed setae in line on internal margin, 4.96 mm long and 0.23–0.42 mm wide; claw single, hook-shaped, enlarged, pretarsal empodium lacking (Fig. 5a, b). Midleg coxa robust, covered with few short setae, tapering toward apex, 1.27 mm long and 0.81 mm wide; trochanter unrecognizable; femur elongate, cylindrical, almost keeping same width, covered with few short setae, 5.06 mm long and 0.55 mm wide; tarsus and tibia continuous, elongate, cylindrical, slightly tapering toward apex, covered with few short setae, with relatively densely arrayed setae in line on internal margin, 8.13 mm long and 0.24–0.43 mm wide; claw single, hook-shaped, enlarged, pretarsal empodium lacking. Hindleg coxa robust, covered with few short setae, 2.10 mm long and 0.84 mm wide; trochanter unrecognizable; femur elongate, cylindrical, almost keeping same width, covered with few short setae, 5.70 mm long and 0.62 mm wide; tarsus and tibia continuous, elongate, cylindrical, slightly tapering toward apex, covered with few short setae, with relatively densely arrayed setae in line on internal margin, 8.40 mm long and 0.24–0.51 mm wide; claw single, hook-shaped, enlarged, pretarsal empodium lacking (Fig. 5c–f).
Abdominal segments indistinct, tubular, without distinct tubercles (?), slightly tapering posteriorly, spiracles unrecognizable (Fig. 4e, f).
4. Discussion
Kuafupolydentes hui gen. et sp. nov. can be readily attributed to Myrmeleontiformia due to its robust head capsule and strong jaws (stylets), and it can be easily excluded from Psychopsidae, Nemopteridae and Nymphidae due to the lack of a projecting labrum, presence of two or more mandibular teeth, and short cervix (Badano et al. Reference Badano, Aspöck, Aspöck and Cerretti2017, Reference Badano, Engel, Basso, Wang and Cerretti2018; Haug et al., Reference Haug, Haug, van der Wal, Müller and Haug2021 d). However, this specimen also lacks several apomorphies of Myrmeleontidae and Ascalaphidae, such as the setiferous processes on the postcephalic body (Badano et al., Reference Badano, Aspöck, Aspöck and Cerretti2017, Reference Badano, Engel, Basso, Wang and Cerretti2018). The continuous hindleg tarsus and tibia is one of the main representative characters of Myrmeleontidae + Ascalaphidae, but the continuity of tibia and tarsus in all legs of K. hui also occurs in the larvae of Ithonidae and Mantispoidea (Grebennikov, Reference Grebennikov2004; Badano et al. Reference Badano, Engel, Basso, Wang and Cerretti2018). In Myrmeleontidae + Ascalaphidae, the continuity is instead limited to the hindlegs and it is an adaptation to burrowing, while the tarsal continuity of K. hui has probably a completely different origin because this larva lacks any obvious adaptation to a fossorial life, so this character very likely evolved independently, and it does not support a close affinity to Myrmeleontidae + Ascalaphidae (Badano et al. Reference Badano, Engel, Basso, Wang and Cerretti2018). Some other morphological characters, such as the robust and long antennae and the multi-toothed stylet, suggest that this larva was likely an early representative of Myrmeleontiformia.
One of the most conspicuous features of K. hui is its large size. We can estimate that its body length is probably greater than 15 mm since the apex of the abdomen is not preserved and the overall length, including mandible–maxilla complex, clearly exceeds 20 mm. Although it falls within the size range of extant antlions (e.g. Palpares libelluloides with body length 21.89 mm, Acanthaclisis occitanica with body length 23.37 mm, Synclisis baetica with body length 19.60 mm) (Badano & Pantaleoni, Reference Badano and Pantaleoni2014), K. hui is much larger than most extinct lacewing larvae known from Burmese amber (e.g. table S1 in Wang et al. Reference Wang, Xia, Engel, Perrichot, Shi, Zhang, Chen, Jarzembowski, Wappler and Rust2016; supplementary table 1 in Badano et al. Reference Badano, Engel, Basso, Wang and Cerretti2018). In fact, K. hui is one of the biggest lacewing larvae preserved in amber known so far. However, such an extremely large lacewing larva is not alone in Burmese amber: specimen 35 (Psychopsidae) in Haug et al. (Reference Haug, Haug, Pazinato, Braig, Perrichot, Gröhn, Müller and Haug2020 a) has a length of c. 17.8 mm, and specimen 52 (Psychopsidae) in Haug et al. (Reference Haug, Haug, Pazinato, Braig, Perrichot, Gröhn, Müller and Haug2020 a) has a length estimated at c. 30 mm (but unfortunately only with its head preserved, so its length is just a conjecture). Considering that large species are usually more difficult to preserve in amber, the proportion of these large species must have been higher in the mid-Cretaceous Kachin rainforest. Interestingly, the oldest confirmed myrmeleontiformian lacewing larva from the Lower Cretaceous Crato Formation also has a large size, 15.3 mm in total length including stylets. It can be considered that large size is a distinctive plesiomorphy among some Mesozoic myrmeleontiformian lacewing larvae.
Each stylet with eight short and blunt teeth is another interesting feature. Extant Myrmeleontidae and Ascalaphidae usually have three teeth, Nymphidae have one, and Psychopsidae and Nemopteridae lack teeth (but a few nemopterid larvae have tooth-like protuberances, e.g. Monserrat, Reference Monserrat1996, Reference Monserrat2008; see also discussion and summary in Haug et al. Reference Haug, Herrera-Flórez, Müller and Haug2019 a, Reference Haug, Baranov, Wizen, Pazinato, Müller, Haug and Haug2021 b, c). The character ‘stylet with more than three teeth’ has been abundantly reported from the Cretaceous amber, e.g. undescribed specimens of upper figure on page 99 (five teeth), lower figure on page 99 (four teeth), upper figure on page 100 (ten teeth) and lower figure on page 99 (four teeth) in Xia et al. (Reference Xia, Yang, Zhang, Shi and Wang2015); morphotype MVI (four teeth; fig. 3E in their paper) from mid-Cretaceous French amber in Wang et al. (Reference Wang, Xia, Engel, Perrichot, Shi, Zhang, Chen, Jarzembowski, Wappler and Rust2016); undescribed specimens of the figure on page 100 (nine teeth), the upper figure on page 402 (four teeth) and another specimen of the upper figure on page 402 (four teeth) in Zhang (Reference Zhang2017); Cladofer huangi Badano, Engel et Wang, Reference Badano, Engel, Basso, Wang and Cerretti2018 (seven teeth; supplementary fig. 1d, e in their paper) in Badano et al. (Reference Badano, Engel, Basso, Wang and Cerretti2018); ‘Superfang’ (at least eleven teeth) in Haug et al. (Reference Haug, Müller and Haug2019 c); SNSB-BSPG 2020 XCIII 19 (seven or six teeth) in Hörnig et al. (Reference Hörnig, Kiesmüller, Müller, Haug and Haug2020); an undescribed myrmeleontoid larva (nine teeth; fig. 3I in their paper) in Lu & Liu (Reference Lu and Liu2021); specimen 66 (four teeth), specimen 67 (BUB 1803) (ten teeth), specimen 68 (BUB 1804) (eight teeth), specimen 69 (PED 0085) (four teeth) and specimen 70 (PED 0250) (eight teeth) in Haug et al. (Reference Haug, Baranov, Wizen, Pazinato, Müller, Haug and Haug2021 b); PED 0361, PED 0088 and PED 0112 (all bearing six teeth) in Haug et al. (Reference Haug, Baranov, Müller and Haug2021 e). All these specimens come from mid-Cretaceous Kachin amber expect the French one. The large number of stylet teeth might be another plesiomorphy among some Mesozoic myrmeleontiformian lacewing larvae and it may have helped to hold the prey. However, K. hui is different from the above multi-toothed specimens in the shape of its teeth: those specimens’ teeth are relatively thin and long, but in K. hui they are blunt and short, and this kind of teeth is extremely rare among extant and extinct Neuroptera, only Electrocaptivus xui Badano, Engel et Wang, 2018 having similarly shaped teeth. It is probable that the prey of K. hui was also very large, possibly even bigger than K. hui, so the gripping jaws might be more helpful than piercing jaws. This is another character lacking in the morphological diversity of living Neuroptera larvae.
The antennae of Myrmeleontiformia are usually relatively short and thin, with flagellum subdivided into several segments. Compared with this usual situation, the antennae of K. hui are rather special. However, such robust antennae are also present in some other families of lacewing larvae, e.g. Hemerobiidae and Coniopterygidae (Haug et al. Reference Haug, Müller and Haug2019 b). ‘Superfang’ from mid-Cretaceous Kachin amber in Haug et al. (Reference Haug, Müller and Haug2019 c) and specimen 39 (BUB 0797; Nymphidae) in Haug et al. (Reference Haug, Haug, van der Wal, Müller and Haug2021 d) also have relatively robust antennae. It seems that the structure of the antennae of Mesozoic lacewing larvae is variable, but why this new larva developed such robust antennae remains unknown at present; one possibility is that it was to avoid damage during capture.
Long and robust legs with continuous tarsus and tibia and enlarged claws is also a unique combination among all known extant and extinct lacewing larvae, but enlarged pretarsal claws on all legs are also present in the myrmeleontiformian Mesoptynx unguiculatus Badano, Engel et Wang, Reference Badano, Engel, Basso, Wang and Cerretti2018. Extant antlion larvae are characterized by hindlegs being more robust than other legs, the hindleg tarsus and tibia continuous with claws enlarged, and digging behaviour using a characteristic backward movement, accomplished by the abdomen and hindlegs (Badano et al. Reference Badano, Aspöck, Aspöck and Cerretti2017). Such behaviour probably led to the specialization of the hindleg structure. Considering the large size and special mandibular–maxillary stylets, it is very likely that K. hui needed to battle with some large prey, and continuous tarsus and tibia with enlarged claws only on the hindlegs were not enough. Thus, K. hui might have evolved stronger legs with all tarsi and tibiae continuous plus all claws enlarged to cope with this adversary. It is also possible that all legs are strong because it hunted on the horizontal rather than in a pit, but this remains unknown since the apex of the abdomen has not been preserved.
The Cretaceous has yielded many unusual-looking neuropteran larvae with morphological characters greatly exceeding the known disparity of the group (e.g. Wang et al. Reference Wang, Xia, Engel, Perrichot, Shi, Zhang, Chen, Jarzembowski, Wappler and Rust2016; Badano et al. Reference Badano, Engel, Basso, Wang and Cerretti2018, Reference Badano, Fratini, Maugeri, Palermo, Pieroni, Cedola, Haug, Weiterschan, Velten, Mei, Di Giulio and Cerretti2021; Haug et al. Reference Haug, Herrera-Flórez, Müller and Haug2019 a, b, c, Reference Haug, Pazinato, Haug and Haug2020 b; Herrera-Flórez et al. Reference Herrera-Flórez, Braig, Haug, Neumann, Wunderlich, Hörnig and Haug2020; Hörnig et al. Reference Hörnig, Kiesmüller, Müller, Haug and Haug2020, and K. hui also represents such a case. All the evidence indicates that the new larva was a fierce and voracious predator and hunted using a classic example of a lie-in-wait or ambush tactic. Its prey must have been much larger than the prey of living antlions, and therefore K. hui developed stronger legs, specialized leg structure and more stylet teeth to combat the prey, but differed from extant antlions in that the short and blunt teeth of the new larva probably could not impale. Thus K. hui might have relied on a different killing strategy. The discovery of this new type of lacewing larva further supports the assumption that the Mesozoic was the ‘golden age’ of Neuroptera (Aspöck et al. Reference Aspöck, Plant and Nemeschkal2001; Wang et al. Reference Wang, Liu, Garzón-Orduña, Winterton, Yan, Aspöck, Aspöck and Yang2017; Engel et al. Reference Engel, Winterton and Breitkreuz2018; Winterton et al. Reference Winterton, Lemmon, Gillung, Garzon, Badano, Bakkes, Breitkreuz, Engel, Lemmon, Liu, Machado, Skevington and Oswald2018; Lu & Liu, Reference Lu and Liu2021). However, although Cretaceous lacewing larvae displayed many unique combinations of characters, their predatory strategies did not have any real innovations and only some modifications, so that in time their habitual Coniferopsida-dominated dynasty fell and a brand-new angiosperm-hegemonic kingdom arose; some of their prey also disappeared, which probably led to their decline after the K/Pg boundary (Haug et al. Reference Haug, Haug and Haug2021 c, f).
5. Conclusion
Kuafupolydentes hui gen. et sp. nov. represents a new morphotype among Cretaceous early representatives of Myrmeleontiformia, and can be identified mainly by its large size, mandibular–maxillary stylets, antennae and legs. The morphological characters of K. hui exceed the known diversity of Neuroptera lacewing and imply that the Mesozoic radiation of these insects was much greater than present-day diversity would suggest. However, although the prey of K. hui was probably much bigger than today’s, its predatory strategy was likely similar to extant antlions to a great extent. The conservatism of evolution in Neuroptera may have led to their decline after the Cretaceous.
Acknowledgements
We thank Yong Cai for amber collection. We express our sincere thanks to Joachim T. Haug and another anonymous reviewer for their thoughtful comments on the manuscript. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000), the Second Tibetan Plateau Scientific Expedition and Research (2019QZKK0706), and National Natural Science Foundation of China (42125201, 41688103). E.A.J. thanks the Chinese Academy of Sciences for support under the President’s International Fellowship Initiative (PIFI 2020VCA0020). This is a Leverhulme Emeritus Fellowship Contribution for E.A.J. and a contribution to UNESCO-IUGS IGCP Project 679.
Declaration of Interest
The authors declare no conflict of interest.