Introduction
Cystic echinococcosis (CE) is caused by infection with the metacestode stage of Echinococcus granulosus sensu lato (s.l), a cryptic species complex, which based on mitochondrial and nuclear analysis, has been shown to include E. granulosus sensu stricto (s.s) (genotypes G1–G3), Echinococcus equinus (G4), Echinococcus ortleppi (G5), Echinococcus canadensis (G6/G7, G8 and G10) and Echinococcus felidis (Nakao et al., Reference Nakao, McManus, Schantz, Craig and Ito2007; Huttner et al., Reference Huttner, Nakao, Wassermann, Siefert, Boomker, Dinkel, Sako, Mackenstedt, Romig and Ito2008; Thompson, Reference Thompson2008; Romig et al., Reference Romig, Ebi and Wassermann2015). Human and animal CE has been known to be endemic in Great Britain since the early 20th century (Howell, Reference Howell1940; Craig et al., Reference Craig, Rogan and Allan1996) and has historically been recorded from sheep, cattle, horses and humans (Williams and Sweatman, Reference Williams and Sweatman1963). Both E. granulosus and E. equinus are known to occur in the UK. However, the molecular confirmation of E. granulosus s.s infections in humans, and E. granulosus s.s and E. equinus in livestock and canid hosts as well as captive mammals, has only been recently reported (Craig et al., Reference Craig, Woods, Boufana, O'Loughlin, Gimpel, Lett and McManus2012; Boufana et al., Reference Boufana, Stidworthy, Bell, Chantrey, Masters, Unwin, Wood, Lawrence, Potter, McGarry, Redrobe, Killick, Foster, Mitchell, Greenwood, Sako, Nakao, Ito, Wyatt, Lord and Craig2012, Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015).
Domestic cycles of E. granulosus s.s are globally maintained in all types of pastoral regions, where predominantly sheep and other livestock occur (Craig et al., Reference Craig, McManus, Lightowlers, Chabalgoity, Garcia, Gavidia, Gilman, Gonzalez, Lorca, Naquira, Nieto and Schantz2007). In the UK, the distribution of E. granulosus and human CE cases was thought to be restricted to certain sheep farming areas such as mid and south Wales and northwest Scotland (Craig et al., Reference Craig, Rogan and Allan1996; Torgerson and Budke, Reference Torgerson and Budke2003). However, the molecular confirmation of E. granulosus s.s from a farmer in Wales and an engineer from Cumbria (northwest England, considered a low or non-endemic area) whose clinical data showed that they had probably sustained CE infection in their respective areas, indicated a possible wider transmission for CE in the UK than previously considered (Boufana et al., Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015). This observation is consistent with a slaughter trace-back study (A. Brouwer, personal communication; data held by the Welsh Government and published in the Proceedings of the Society for Veterinary Epidemiology and Preventive Medicine, 2013; ISBN: 978-0-948073-20-5) that supports a geographically wider distribution for this parasite in Great Britain.
Echinococcus equinus appears to occur sympatrically in areas of Great Britain where E. granulosus s.s is found (e.g. Wales) (Boufana et al., Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015); however, the analysis of slaughter records for horses carried out in the 1970s indicated that E. equinus had a much wider distribution (Thompson, Reference Thompson1976). Echinococcus equinus from the UK has recently been molecularly confirmed from hydatid cysts retrieved from horses, a zebra born and bred in captivity (England) and from coproDNA (DNA extracted from feces) of dogs and foxhounds in Wales (Boufana et al., Reference Boufana, Stidworthy, Bell, Chantrey, Masters, Unwin, Wood, Lawrence, Potter, McGarry, Redrobe, Killick, Foster, Mitchell, Greenwood, Sako, Nakao, Ito, Wyatt, Lord and Craig2012, Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015). To date however, no human CE cases have been molecularly confirmed to have been caused by E. equinus which is considered to have no zoonotic potential (Eckert and Thompson, Reference Eckert and Thompson1988) although viable E. equinus infections were recently reported from non-human primates (lemurs) born and bred in the UK (Boufana et al., Reference Boufana, Stidworthy, Bell, Chantrey, Masters, Unwin, Wood, Lawrence, Potter, McGarry, Redrobe, Killick, Foster, Mitchell, Greenwood, Sako, Nakao, Ito, Wyatt, Lord and Craig2012, Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015).
Ovine hydatidosis does not seem to historically occur in Northern Ireland nor in the Irish Republic (Hatch, Reference Hatch1970; Logan, Reference Logan1971) and no CE autochthonous cases have been reported, although equine echinococcosis has been regularly observed in Ireland (Hatch, Reference Hatch1970; Logan, Reference Logan1971) and is still not uncommonly reported during routine horse necropsies (P.R. Torgerson, personal communication). There are, however, no known molecularly confirmed reports of equine echinococcosis from the island of Ireland.
Following World War II, equine echinococcosis levels in the UK reached high prevalence (>60%), suggested in part to be due to the practice of feeding hunting dogs raw offal from livestock and horses (Dixon et al., Reference Dixon, Baker-Smith and Greatorex1973; Thompson and Smyth, Reference Thompson and Smyth1974). The UK 2004 Hunting Act (https://www.legislation.gov.uk/ukpga/2004/37/pdfs/ukpga_20040037_en.pdf) made it illegal to use hounds to hunt foxes, hares and deer; however, drag and trail hunting is still permitted. According to the Director of the Masters of Fox Hounds Association (MFHA) list of recognized hunts for 2008–2009, there were 174 foxhound packs registered in the UK with between 20 and >100 foxhounds per pack. This population of domestic hunting dogs constitutes a potentially significant group of canids that may be involved in synanthropic transmission cycles of both E. granulosus and E. equinus in the UK.
Since the studies of Thompson and Smyth (Reference Thompson and Smyth1974, Reference Thompson and Smyth1975) when E. granulosus and E. equinus were morphologically identified from purged foxhound packs, no comprehensive investigations have been conducted on the prevalence of canine echinococcosis in foxhound packs in the UK. The current study was undertaken using a genus-specific coproantigen ELISA and coproPCR to investigate the occurrence of Echinococcus spp. in foxhound packs/hunts in England and Wales. In addition, a questionnaire designed to investigate current foxhound kennel practices was distributed by mail to UK registered foxhound packs to ascertain potential risk association for transmission of Echinococcus spp.
Materials and methods
Study design and protocol
This foxhound study was carried out over the autumn/winter period between 2010 and 2011. With the support of the MFHA, every foxhound kennel in the UK registered on the MFHA list was sent a letter requesting permission from the kennelmen/huntsmen to sample their foxhound packs. Consequently, the study was conducted in different hunts that were determined by the responses received from individual kennelmen (Fig. 1). The number of foxhounds per pack as reported by the kennelmen and the actual numbers of retrieved foxhound fecal samples are presented in Table 1. Out of the foxhound packs that were contacted in 2010, eight (Powys, Wales, n = 4; Monmouthshire, Wales, n = 1; Northumberland, England, n = 2; Cheshire, England, n = 1) agreed to participate in the sampling. For the purpose of this study, the names of all the hunts were anonymized and will therefore be designated as follows: Powys, Wales (Hunts#1–4); Monmouthshire, Wales (Hunt#5); Northumberland, England (Hunts#6 and 7); and Cheshire, England (Hunt#8). Hunt#1 was previously sampled by us in 1996, 1997 and 1998. Several kennelmen returned the completed questionnaires but declined for their foxhounds to be sampled (n = 9). These were hunts from Cornwall, England (Hunt#9); Devon, England (Hunt#10); North Yorkshire, England (Hunts#11 and 12); Lincolnshire, England (Hunt#13); Shropshire, England (Hunt#14); Cheshire, England (Hunt#15); North Wales (Hunts#16 and 17) (Table 1).
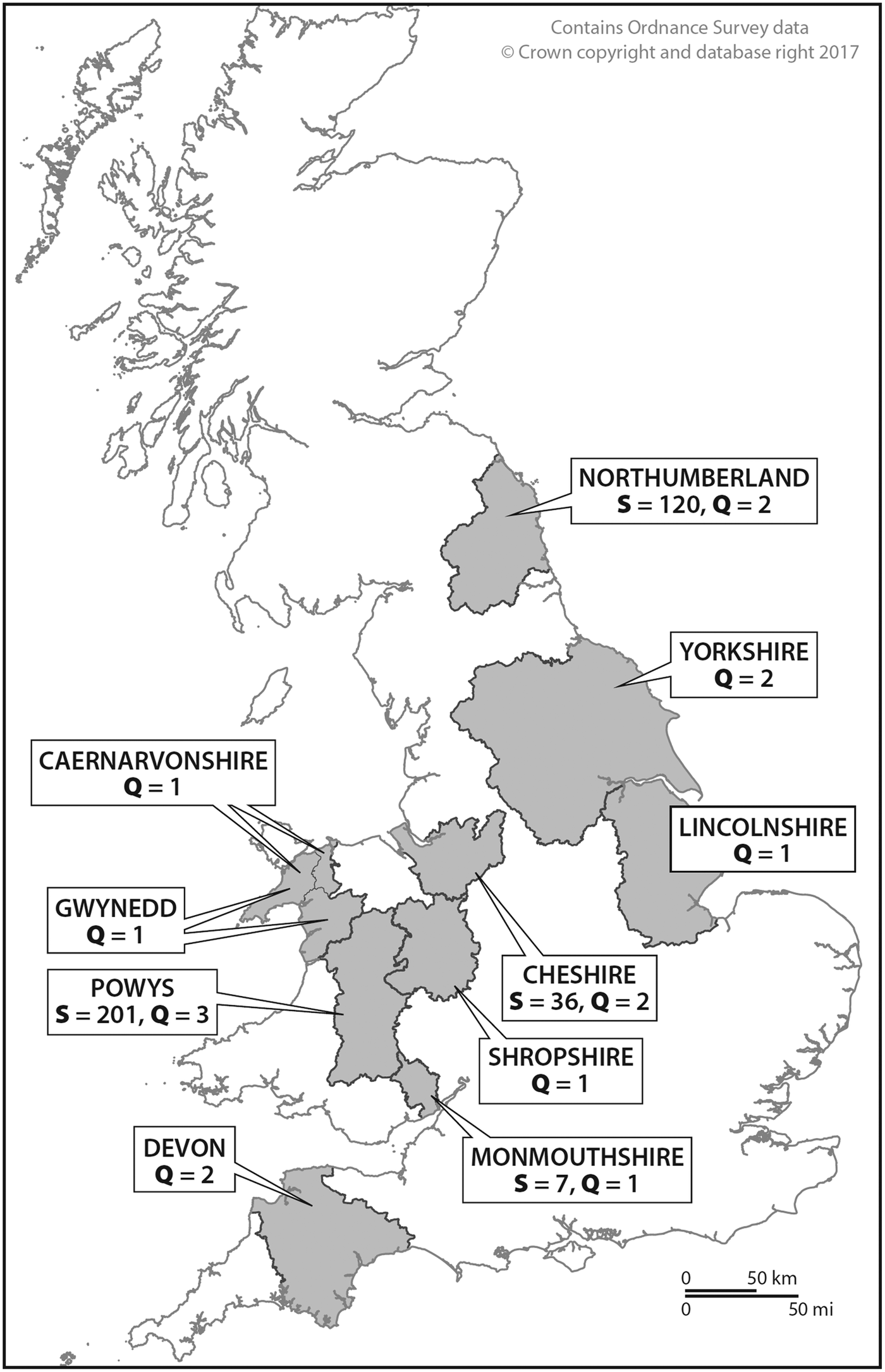
Fig. 1. Map of the UK showing the origin of foxhound packs included in this study. S, number of fecal samples; Q, number of questionnaires.
Table 1. Foxhound pack coproantigen and coproDNA results and corresponding questionnaire data

NS, not sampled.
a Foxhound pack size not known.
Fecal samples: collection and processing
On an agreed date, kennelmen were asked to segregate foxhounds away from ground fecal deposits, in order to facilitate the collection of samples that had been deposited <24 h earlier. Foxhound fecal samples (~2–4 gm) were collected from the ground (usually concrete floor with raised sleeping areas) where the foxhounds were kept, consequently each sample could not be uniquely identified to an individual foxhound. The samples were collected from the enclosed areas of the pens using pre-labelled 50 mL universal tubes and assigned a unique laboratory and hunt ID number. Samples were transported to the Cestode Zoonoses Research Group (CZRG) laboratory at the University of Salford and were placed in a −80 °C freezer for at least 3 days to inactivate Echinococcus eggs.
Coproantigen ELISA
Between 0.5 and 1 g of thawed feces was removed from the original tube and placed into a 5 mL Bijou tube and topped with 0.3% phosphate-buffered saline with Tween 20. The tube was sealed, vortexed and then centrifuged at about 750 × g for 5 min. The resultant supernatant was transferred into a labelled 2 mL Eppendorf tube and frozen at −20 °C until required. Genus-specific Echinococcus coproantigens in foxhound supernatants were detected using the coproELISA developed by Allan et al. (Reference Allan, Craig, Garcia-Noval, Mencos, Liu, Wang, Wen, Zhou, Stringer, Rogan and Zeyhle1992) with minor modifications described by Lahmar et al. (Reference Lahmar, Lahmar, Boufana, Bradshaw and Craig2007). This coproELISA was previously shown to detect coproantigens in the feces of dogs experimentally infected with E. equinus which were confirmed by sequencing (unpublished observations). Therefore, control fecal samples collected from two dogs, at 7, 10, 14, 21, 28, 34 and 50 days after experimental infection (using protoscoleces from UK horse hydatid cysts that exhibited E. equinus worm burdens of 11 300 and 25 at necropsy) were available to this study. Echinococcus granulosus s.s controls (individual and pooled supernatants) from coproantigen-positive dogs originating from confirmed naturally infected dogs (Australia) were also included. Copro-negative controls were collected from pet dogs from Manchester, UK. The cut-off value was calculated as the mean optical density (OD) of dog fecal supernatants (n = 20) from uninfected dogs plus three standard deviations.
CoproDNA detection
CoproDNA was extracted from foxhound fecal samples using the QIAamp DNA Mini Stool Kit (Qiagen, Hilden, Germany) as recommended by the manufacturer. The presence of E. granulosus s.l coproDNA was investigated using the protocol described by Abbasi et al. (Reference Abbasi, Branzburg, Campos-Ponce, Abdel Hafez, Raoul, Craig and Hamburger2003). Echinococcus equinus-specific in-house probes (Forward, 5′-GGT TTT GAG ATA CAT AAT AAT GTC CGG AC-3′ and Reverse, 3′-CTC ACA CCA AGC ACC TAC ACA TAA ATA TAG TT-5′) designed to amplify a fragment within the mitochondrial NADH dehydrogenase subunit 2 (ND2) gene of E. equinus (GenBank accession no. AF346403) were used to screen for coproDNA of this parasite. Primers were validated using BLAST biosoftware (www.ncbi.nlm.nih.gov/BLAST/). Amplified products were run on a 1.5% gel red stained agarose gel (Cambridge Biosciences, Cambridge UK), at 110 V and visualized using Syngene G:Box gel documentation system (Cambridge Biosciences) to ascertain the amplification of diagnostic bands. PCR products were commercially sequenced in both directions using the PCR primers (Source Bioscience, Nottingham, UK) (Boufana et al., Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015).
Questionnaire
A questionnaire designed to determine whether foxhound husbandry influenced the occurrence of Echinococcus spp. infection in UK foxhound packs was sent to all 174 foxhound hunts identified in the UK. The questionnaire comprised of 23 questions about foxhound husbandry practices and knowledge/perception of echinococcosis/hydatid disease, specifically, questions related to what was fed to the hounds, i.e. bagged food, fallen stock, cooked or raw offal. Another question related to deworming practices in order to establish whether effective tapeworm drugs containing praziquantel (PZQ), which has high efficacy against canine echinococcosis (Eckert et al., Reference Eckert, Gemmell, Meslin and Pawlowski2001), were used to treat the hounds. Another question queried whether kennelmen were aware of echinococcosis or hydatid disease and if so were they aware of how humans became infected, i.e. was the infection derived from dogs, sheep or other.
Results
Coproantigen ELISA
In total, 364 foxhounds fecal samples from five Welsh (Hunts#1–5) and three English (Hunts#6–8) hunts were tested. Although for Hunt#1, the kennelman reported (in 2010) that the pack consisted of 58 foxhounds, in total 71 foxhound fecal samples were collected from the ground in the penned areas. For Hunts#3–8, the number of foxhound fecal samples collected from the ground was lower than the total number of foxhounds reported for each pack. Additionally, the foxhound pack size for Hunt#2 was unknown. Table 1 shows the coproantigen prevalence for each hunt with reference to the total number of foxhound feces tested. Echinococcus coproantigen ELISA was positive in 25.6% (93/364) of tested foxhound fecal samples. Positive coproantigen foxhounds were present in five out of the eight sampled packs, three Welsh (Hunts#1, 2 and 4) and two English foxhound packs (Hunts#6 and 7) (Table 1). The coproantigen prevalence varied from pack to pack from 0 to 61.2% with high coproantigen prevalence of 30.9 and 61.2% reported from Hunts#1 and 4 (Powys, Wales) and 17.5 and 44.4% in Hunts#7 and 6 (Northumberland, England) (Table 1, Fig. 2). Hunt#1 (Powys) was previously sampled in 1996, 1997 and 1998 (rectal loop sample prior to arecoline purgation) when the Echinococcus coproantigen ELISA (Allan et al., Reference Allan, Craig, Garcia-Noval, Mencos, Liu, Wang, Wen, Zhou, Stringer, Rogan and Zeyhle1992) results indicated positive rates of 41.6, 26.3 and 6.9%, respectively. In addition, purge analysis in the field (1996–98) revealed the presence of other tapeworm species including Taenia hydatigena (1.4–2.6%), Taenia ovis (1.3–1.4%), Taenia pisiformis (2.8–5.3%) and Dipylidium caninum (5.3–11.1%) (ARF & PSC, unpublished observations).
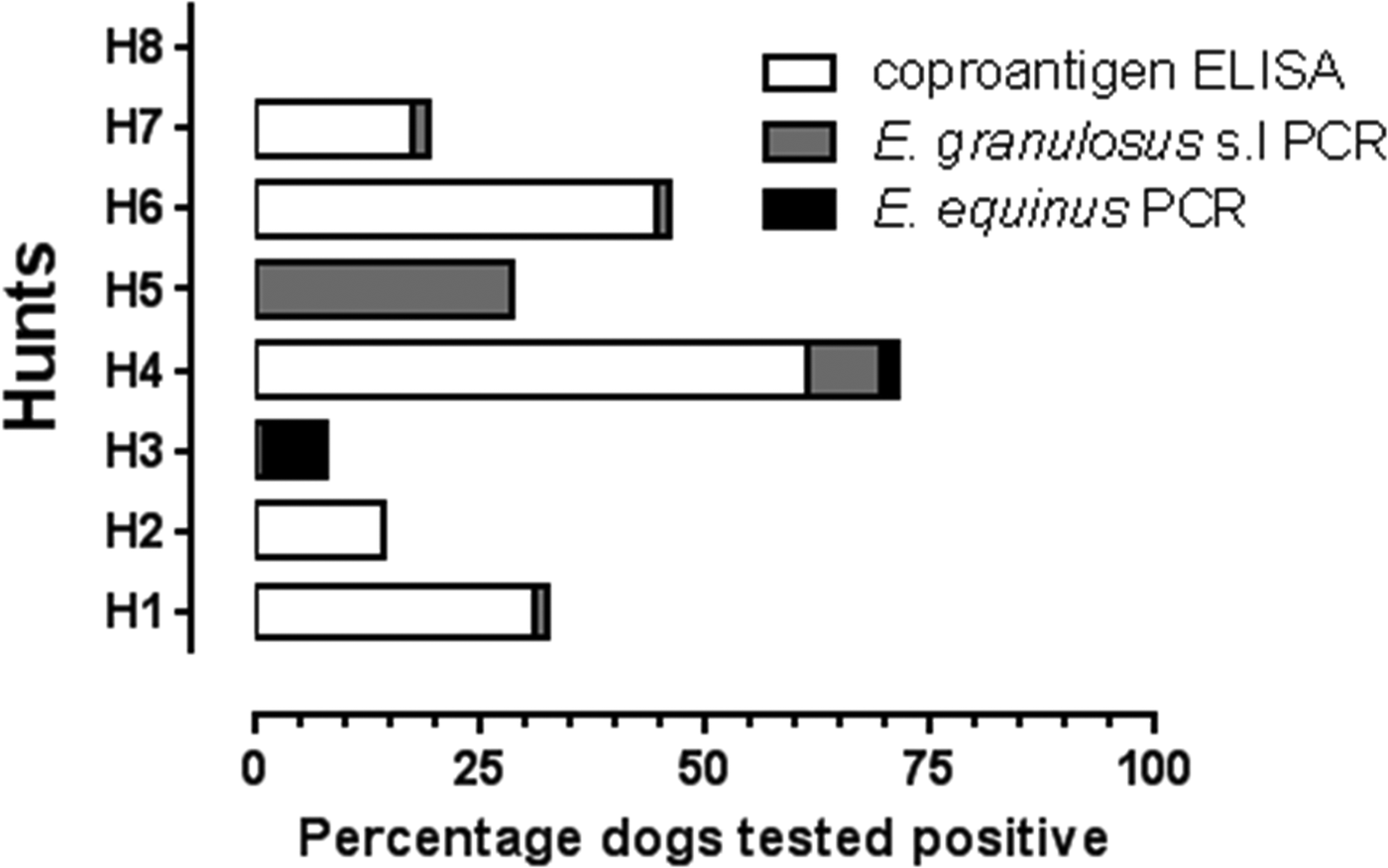
Fig. 2. Foxhound fecal samples from eight UK hunts (H1–H8) tested using coproantigen ELISA and coproPCR.
In the current study, three hunts (Hunts#3, 5, 8) with a total of 103 tested foxhound fecal samples were coproantigen-negative, although Hunts#3 and 5 (Powys, Wales) were both positive by coproPCR for E. granulosus s.l, and E. equinus was additionally confirmed from foxhounds in Hunt#3. Foxhounds from Hunt#8 (Cheshire, England) were negative by both coproantigen and coproPCR (for E. granulosus s.l and E. equinus). Unfortunately, since data were not attributable to individual hounds and the number of hunts providing data was limited to eight, it was not possible to conduct inferential statistical analyses on the present dataset because of low statistical power.
CoproPCR
All 364 foxhound samples collected from the eight foxhound packs were tested for the presence of E. granulosus s.l (Abbasi et al., Reference Abbasi, Branzburg, Campos-Ponce, Abdel Hafez, Raoul, Craig and Hamburger2003) and for E. equinus coproDNA using in-house probes. Positive signals with the amplification of a diagnostic product within the tandem repeat unit of E. granulosus s.l (133 bp) was obtained for dogs from four Welsh (Hunts#1, 3, 4, 5) and two English foxhound packs (Hunts#6 and 7). Echinococcus granulosus s.l coproPCR was positive in 2.8% (10/364) of tested foxhounds (Table 1, Fig. 2). In addition, these 10 samples tested negative with the E. equinus probes used in this study. Sequencing of coproDNA from one foxhound (Hunt#4) confirmed that the infection was caused by E. granulosus s.s as reported by Boufana et al. (Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015). Echinococcus equinus was detected in 1.4% (5/364) of tested foxhounds. An E. equinus 299 bp diagnostic band was produced for foxhound packs from two Welsh hunts (Hunt#3; 4/5 and Hunt#4; 1/5). Sequencing of the five amplified products confirmed that these dogs were infected with E. equinus (GenBank accession no. AB786665) (Boufana et al., Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015).
Congruence of coproantigen and coproPCR results for tested hunts
Hunts#1, 4, 6 and 7 had congruent coproantigen and coproPCR results. However, discrepancies were observed for results seen for Hunts#2, 3 and 5 (Table 1, Fig. 2). Of the 21 fecal samples tested from Hunt#2, only three were coproantigen-positive but coproPCR results for both E. granulosus s.l and E. equinus were negative. The genus specificity of the coproELISA used in this study is around 95–98% (Allan et al., Reference Allan, Craig, Garcia-Noval, Mencos, Liu, Wang, Wen, Zhou, Stringer, Rogan and Zeyhle1992) and false positives of around 5% are thought to occur due to cross-reactions with Taenia species. All 60 foxhound fecal samples from Hunt#3 (Welsh, Powys) were coproantigen-negative but 8.3% (5/60) of samples from this hunt were coproPCR-positive for E. granulosus s.l (1.7%) and E. equinus (6.7%). This in part may be related to the reduced sensitivity of the coproELISA in detecting coproantigens in canids with low worm burdens (<100) (Deplazes et al., Reference Deplazes, Jimenez-Palacious, Gottstein, Skaggs and Eckert1994; Craig, Reference Craig, Anderson, Ouhelli, Kachani and Provo1997; Buishi et al., Reference Buishi, Walters, Guildea, Craig and Palmer2005). A similar situation was observed for Hunt#5, where of the three utilized assays, only the coproPCR for E. granulosus s.l was positive (2/7; 28.6%).
Questionnaire data
In total, 16 questionnaires were completed and returned in which eight kennelmen (50%) from different hunts reported that they fed the hounds raw offal from fallen stock including sheep, lamb, calves, cattle and horses (Table 1). At least seven types of anthelminthic treatments were reported by the kennelmen to be used for foxhound deworming. These included Cyclactin®, Drontal®, Drontal® Plus, Equitape®, Ivomec®, Milbemax® and Panacur®. Only four of these products, namely Drontal®, Drontal® Plus, Equitape® and Milbemax®, are known to contain PZQ as an active ingredient. Cyclactin® is a probiotic feed additive that limits the growth of bacteria; Ivomec® is a 1% sterile solution of ivermectin whereas fenbendazole, a broad-spectrum anthelmintic, is the active ingredient in Panacur®. Only eight out of the 16 compliant hunts (i.e. Hunts#3, 4, 6, 8, 12, 14, 15, 17) reported treating hounds using a PZQ-based dewormer, while six of the remaining eight hunts used Panacur as the main dewormer. Of the four hunts that used a PZQ-based dewormer (Hunts#3, 4, 6, 8), for which we have coproantigen and coproPCR results, three hunts had coproantigen and coproPCR Echinococcus-positive foxhounds (Table 1).
Of the 16 completed questionnaires, five kennelmen (31.3%) reported that they did not know about echinococcosis or hydatid disease; one kennelman reported that ‘humans became infected through association with both sheep and dogs’. The remaining 10 (62.5%) reported that they knew what echinococcosis/hydatid disease was and that it was transmitted from infected dogs. Of the eight hunts that used PZQ-based dewormer, six were aware of echinococcosis.
Discussion
The current study utilized coproantigen ELISA and coproPCR to investigate the occurrence of echinococcosis in foxhound packs in the UK. A total of 364 fecal samples originating from eight hunts in England and Wales were screened and coproELISA results showed that a quarter (25.6%) of foxhound fecal samples were Echinococcus coproantigen-positive, with five of eight hunts (62.5%) having test-positive hounds. Coproantigen prevalence levels were particularly high for Hunt#4 (61.2%) and Hunt#1 (30.9%) in Wales; and Hunt#6 (44.4%) and Hunt#7 (17.5%) in England. The high percentage of coproantigen-positive foxhounds observed in this study for Hunt#1 was not surprising as previous annual sampling (1996–1998) had shown high Echinococcus coproantigen prevalence (6.9–41.6%). Also, oral arecoline purgation from this same hunt in the 1990s had indicated that foxhounds had access to sheep carcasses or offal due to the recovery of other taeniid species (T. ovis, T. hydatigena).
In this study, both E. granulosus s.s and E. equinus were confirmed by sequence analysis to occur in foxhounds in the UK. A total of 10 out of 364 foxhound fecal samples (2.8%) were positive for E. granulosus s.l (from four Welsh and two English hunts) with molecular sequence confirmation of E. granulosus s.s achieved for one hound from Hunt#4. Echinococcus equinus DNA was detected in fecal samples from 1.4% (5/364) of foxhounds from two Welsh hunts (Hunts#3 and 4). Our findings indicate that both E. granulosus s.s and E. equinus are transmitted in foxhound hunts in both England and Wales, and suggest that canine echinococcosis still retains broad geographical distribution in foxhound packs in the UK as originally described by morphological analyses in the 1970s (Thompson and Smyth, Reference Thompson and Smyth1975). Recently, E. granulosus s.s and E. equinus were molecularly confirmed from 17 and three farm dogs in Powys and Gwent (Wales), respectively (Boufana et al., Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015). That same publication confirmed an Echinococcus infection due to E. granulosus s.s and E. equinus in one and five foxhounds, respectively, using coproDNA from the current survey.
Of the eight foxhound packs that were sampled, only seven had corresponding survey questionnaire data that could be associated with the laboratory results. The data indicated that where foxhounds were fed raw offal, there was at least one Echinococcus coproantigen or coproDNA positive dog. In contrast, the kennelman from Hunt#8 reported that raw liver or lungs were never fed to hounds, which was supported by laboratory findings that showed a 0% (0/36) coproantigen prevalence and the absence of Echinococcus spp. coproDNA.
Despite questionnaire reports of good foxhound husbandry and feeding practices, there was nevertheless evidence to suggest that foxhounds had access to infected livestock material. A hunt in Powys (Hunt#3) reported that it only ever fed foxhounds with commercial bagged meal produced specifically for hounds; however, PCR results confirmed the presence of E. granulosus s.l coproDNA in one dog (1.7%) and the presence of E. equinus coproDNA (including sequence confirmation) in 6.7% of the foxhound fecal samples from this hunt. An epidemiological survey of foxhound packs in 1975 in the UK carried out using arecoline purgation found that 52% harboured E. equinus infected dogs (Thompson and Smyth, Reference Thompson and Smyth1975). Overall, 50% (8/16) of hunts in the current study reported that they fed livestock carcasses/raw offal to hounds. Apart from direct feeding of livestock carcasses and offal to foxhounds by kennelmen, other routes of access to hydatid cysts by hounds could be as a result of scavenging carcasses of fallen stock during hunt outings. In addition, many foxhound packs are disbanded during the spring/summer period with hounds distributed (billeted) to farms where access to carcasses and offal may also occur (Boufana et al., Reference Boufana, Lett, Lahmar, Buishi, Bodell, Varcasia, Casulli, Beeching, Campbell, Terlizzo, McManus and Craig2015).
It has been suggested that the fox hunting ban in England and Wales (passed in 2004) may play a role in reducing equine hydatidosis in the UK. However, since fox hunting has been replaced by drag and trail hunting, and hunts retained, foxhound packs still cover large areas of the countryside and feeding practices may not have changed. Furthermore, while all respondent hunts reported the use of commercial dewormers, only 50% (8/16 hunts) used a PZQ-based anthelmintic with good efficacy against canine echinococcosis. For example, the kennelman for Hunt#6 used a suitable combination of Ivomec® and Drontal® to treat for worms, yet he regularly fed the hounds raw liver from fallen stock such as sheep, lamb, cattle and horse and a high percentage (44.4%) of fecal samples from this hunt tested positive for Echinococcus coproantigens and at least one dog was molecularly confirmed to be infected with E. granulosus s.l. The kennelman for Hunt#7 in Northumberland reported that he used Panacur® (a deworming drug that does not contain PZQ) and fed the foxhound pack raw liver and lungs from fallen stock including sheep and horses. The coproantigen results for this hunt showed that 10/57 (17.5%) of fecal samples tested positive for Echinococcus coproantigens with molecular confirmation of E. granulosus s.l.
Overall, five of 16 (31.3%) foxhound kennelmen reported that they did not know what echinococcosis or hydatid disease was. In a recent case, CE due to E. granulosus s.s (G1 genotype, sheep strain) was molecularly confirmed in a UK foxhound kennel worker from England, which suggested that this occupation may be a risk factor for contracting human CE (Craig et al., Reference Craig, Woods, Boufana, O'Loughlin, Gimpel, Lett and McManus2012). Foxhound workers should be made fully aware of the risks of echinococcosis. It is recommended that a tightening up of guidelines/practices/policies should be made to the MFHA Code of Practice and conveyed to hunt workers. These should include strict recommendations to treat hounds with a PZQ-based dewormer at least four times per year, and the use of only cooked/boiled offal/carcasses or proprietary dog food. In comparison with the last study (1975) carried out on canine echinococcosis on foxhounds in the UK (Thompson and Smyth, Reference Thompson and Smyth1975), our findings show that in 2010/2011, the Echinococcus coproantigen prevalence (with DNA confirmation) remains high in some foxhound packs in both England and Wales. Further studies using species-specific PCR assays should include screening more foxhound hunts, including from other UK regions (such as the southern and eastern counties), and targeted sampling of both sheep dogs and foxhounds on farms based on trace-back of livestock hydatid slaughter data.
Acknowledgements
The support provided by Alistair Jackson (Director of the Masters of Fox Hounds Association, 2010) is gratefully acknowledged. Thanks are also due to David Jenkins (Charles Sturt University, New South Wales, Australia) for providing some of the control fecal samples used in this study and to David Brian Lett for the support provided.
Financial support
This study was financially supported by the University of Salford and in part by the Office of the Chief Veterinary Officer of the Welsh Government, Cardiff, UK.
Conflict of interest
None.
Ethical standards
Not applicable.





