Introduction
Scabies is a contagious skin disease caused by the pathogen Sarcoptes scabiei var. hominis (S. scabiei) and is estimated to affect approximately 200 million people globally (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016; Leung et al., Reference Leung, Lam and Leong2020). Although the earliest written reference to scabies appeared in Leviticus in the Bible (1200 BCE) (Roncalli, Reference Roncalli1987), the lack of standardized diagnostic methods has persisted. Clinically, common scabies is characterized by pruritus and multiple skin lesion morphologies, whereas crusted scabies most frequently occurs in immunocompromised patients and presents with hyperkeratosis with or without pruritus (Thomas et al., Reference Thomas, Coates, Engelman, Chosidow and Chang2020). However, these manifestations are non-specific and mimic those of other skin diseases such as atopic dermatitis, eczema, psoriasis, diaper rash, insect bites and poison ivy (Arlian and Morgan, Reference Arlian and Morgan2017). A presumptive diagnosis of scabies may be made based on clinical manifestations; however, scabies is commonly misdiagnosed. A single-centre retrospective study in the United States reported that 45.3% of patients with scabies were previously misdiagnosed (Anderson and Strowd, Reference Anderson and Strowd2017).
To address this issue, the International Alliance for the Control of Scabies (IACS) led a project to develop consensus criteria for the diagnosis of common scabies in 2018 (Engelman et al., Reference Engelman, Fuller and Steer2018), and released the latest consensus standards in 2020 (Engelman et al., Reference Engelman, Yoshizumi, Hay, Osti, Micali, Norton, Walton, Boralevi, Bernigaud, Bowen, Chang, Chosidow, Estrada-Chavez, Feldmeier, Ishii, Lacarrubba, Mahé, Maurer, Mahdi, Murdoch, Pariser, Nair, Rehmus, Romani, Tilakaratne, Tuicakau, Walker, Wanat, Whitfeld, Yotsu, Steer and Fuller2020). The 2020 IACS Criteria comprise 3 levels: confirmed, clinical and suspected scabies. Optical (light) microscopy of skin scrapings is considered the gold standard technique because it can reveal multiple diagnostic features of S. scabiei to support the diagnosis of confirmed scabies. However, it has several disadvantages such as insensitivity, operator dependence, time consumption and invasive operation, and its accuracy depends on the expertise of the operator (Engelman et al., Reference Engelman, Yoshizumi, Hay, Osti, Micali, Norton, Walton, Boralevi, Bernigaud, Bowen, Chang, Chosidow, Estrada-Chavez, Feldmeier, Ishii, Lacarrubba, Mahé, Maurer, Mahdi, Murdoch, Pariser, Nair, Rehmus, Romani, Tilakaratne, Tuicakau, Walker, Wanat, Whitfeld, Yotsu, Steer and Fuller2020).
As described in the 2020 IACS Criteria and in most papers (Nonaka et al., Reference Nonaka, Abe and Sasai1985; Walton and Currie, Reference Walton and Currie2007; Ghosh et al., Reference Ghosh, Jandhyala, Bhatti and Pritt2017; Lydeamore et al., Reference Lydeamore, Campbell, Regan, Tong, Andrews, Steer, Romani, Kaldor, McVernon and McCaw2019; Nelson et al., Reference Nelson, Song and Ng2019; Bernigaud et al., Reference Bernigaud, Fischer and Chosidow2020; Engelman et al., Reference Engelman, Yoshizumi, Hay, Osti, Micali, Norton, Walton, Boralevi, Bernigaud, Bowen, Chang, Chosidow, Estrada-Chavez, Feldmeier, Ishii, Lacarrubba, Mahé, Maurer, Mahdi, Murdoch, Pariser, Nair, Rehmus, Romani, Tilakaratne, Tuicakau, Walker, Wanat, Whitfeld, Yotsu, Steer and Fuller2020), diagnostic features are classified into 3 categories: mites (adults and immature forms), eggs and fecal pellets (scybala). However, a complete mite may be cut into fragments by the sterile blade in the process of extracting materials. Eggshell fragments can also be observed, some of which are easily ignored or confused with contaminating artefacts. However, the important diagnostic values of mite and eggshell fragments have been ignored by the 2020 IACS and most researchers.
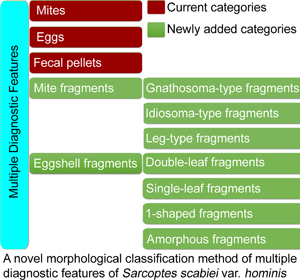
Therefore, a novel morphological classification method is proposed, in which multiple diagnostic features of S. scabiei are classified into 5 categories and 7 subcategories. The method was discussed in detail. Here, the important diagnostic values of mite and eggshell fragments were emphasized. We believe that this novel morphological classification method will be beneficial for operator training in interpreting slides and in improving the 2020 IACS Criteria.
Materials and methods
Data collection
This study was conducted at the Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, in Tianjin, China. Tianjin is the second largest city in northern China. Most of the subjects in this study come from Tianjin, with a small portion coming from other regions of China. Microscopic images and the associated results from subjects who underwent optical microscopy examination of skin scrapings for the diagnosis of scabies between 2018 and 2022 were retrieved. This study did not require personal information from patients. It was approved and monitored by the hospital ethics committee, which approved an exemption from obtaining patient consent.
Subjects
The subjects who underwent optical microscopy examination were selected by clinicians based on clinical evaluation, including the following individuals:
(1) Individuals with confirmed scabies, who required an efficacy evaluation after treatment;
(2) Individuals with clinical or suspected scabies, who required a diagnosis of confirmed scabies;
(3) Individuals with other skin diseases, who required a differential diagnosis from scabies;
(4) Individuals with a history of contact with other individuals with confirmed scabies, who required to determine whether they were infected with scabies.
Morphological classification method of multiple diagnostic features of S. scabiei
The diagnostic features of S. scabiei observed under an optical microscope were classified into 5 categories and 7 subcategories.
(I) Mites. The developmental stages of S. scabiei include eggs, larvae, nymphs (protonymphs and tritonymphs) and adults (Lydeamore et al., Reference Lydeamore, Campbell, Regan, Tong, Andrews, Steer, Romani, Kaldor, McVernon and McCaw2019). In this study, mites refer to immature (larvae and nymphs) and mature (adult) forms (Fig. 1A–E). The anatomical structures of a mite include a gnathosoma and an idiosoma; the idiosoma bears setae, cuticular spines, cuticular striations, long bristles and 3 (larvae) or 4 (nymphs and adults) pairs of legs (Nonaka et al., Reference Nonaka, Abe and Sasai1985; Walton and Currie, Reference Walton and Currie2007; Nelson et al., Reference Nelson, Song and Ng2019) (Fig. 1A–E). Most of the anatomical structures of a mite should be complete. In some cases, a mite can be cut into several parts by a sterile blade in the process of extracting materials; however, the gnathosoma and most idiosoma structures should be observed in the same field under an optical microscope (Fig. 1F). Note that the eggshells of larvae should be either absent or incomplete (Fig. 1G–J). Examples and the anatomical structures are shown in Fig. 1.
(II) Eggs. Eggs refer to ova or eggs with complete eggshells. A complete eggshell is the key characteristic of this category (Fig. 2).
(III) Mite fragments. Mite fragments are incomplete structures of mites cut using a sterile blade (Fig. 3). This category includes 3 subcategories: gnathosoma-type, idiosoma-type and leg-type fragments. Gnathosoma-type fragments refer to incomplete structures with gnathosoma (Fig. 3 A1–A4); whereas idiosoma-type fragments refer to incomplete structures without gnathosoma (Fig. 3 B1–B4); and leg-type structures are incomplete structures with only legs (Fig. 3 C1–C4). In contrast to mites, the gnathosoma and most body structures of idiosoma should not be observed in the same field under an optical microscope.
(IV) Eggshell fragments. Eggshell fragments are incomplete eggshells left by eggs after hatching into larvae. As shown in Fig. 4, the eggshell fragments exhibited different shapes. Based on the differences in size and shape, this category can be classified into 4 subcategories: double-leaf, single-leaf, 1-shaped and amorphous fragments.
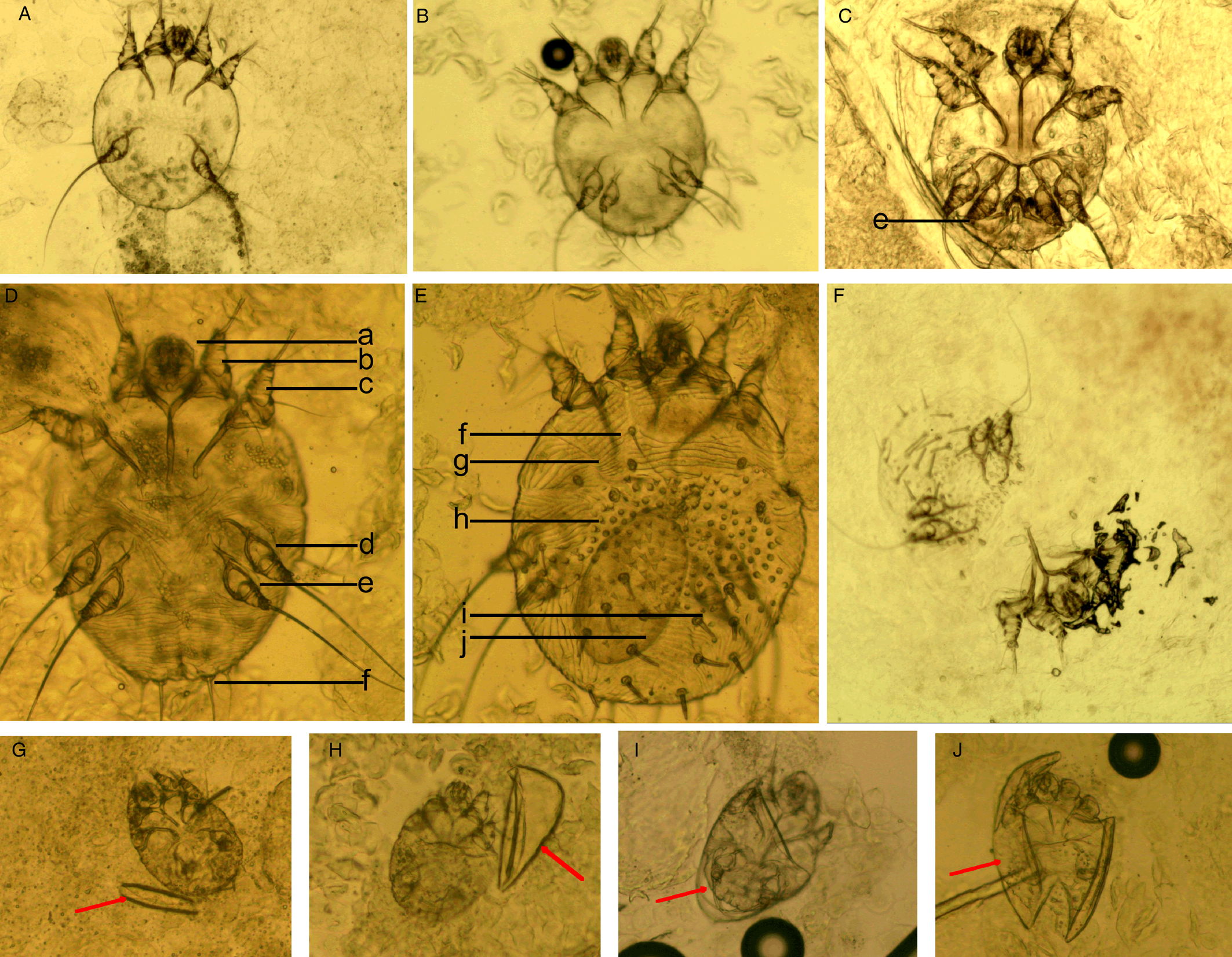
Figure 1. Examples of mites and the anatomic structures. (A) Larvae; (B) nymphs; (C) adult male mites; (D) adult female mites (ventral aspect); (E) adult female mites (dorsal aspect); (F) a mite was cut into 2 parts; however, the gnathosoma and most idiosoma structures can be observed in a field; (G-J) larva with incomplete eggshells (red arrow). Complete body structures: a, gnathosoma; b-d, legs Ⅰ, Ⅱ, Ⅲ, and Ⅳ; f, long bristles; g, cuticular striations; h, cuticular spines; i, setae; j, ovum (magnification: 100).
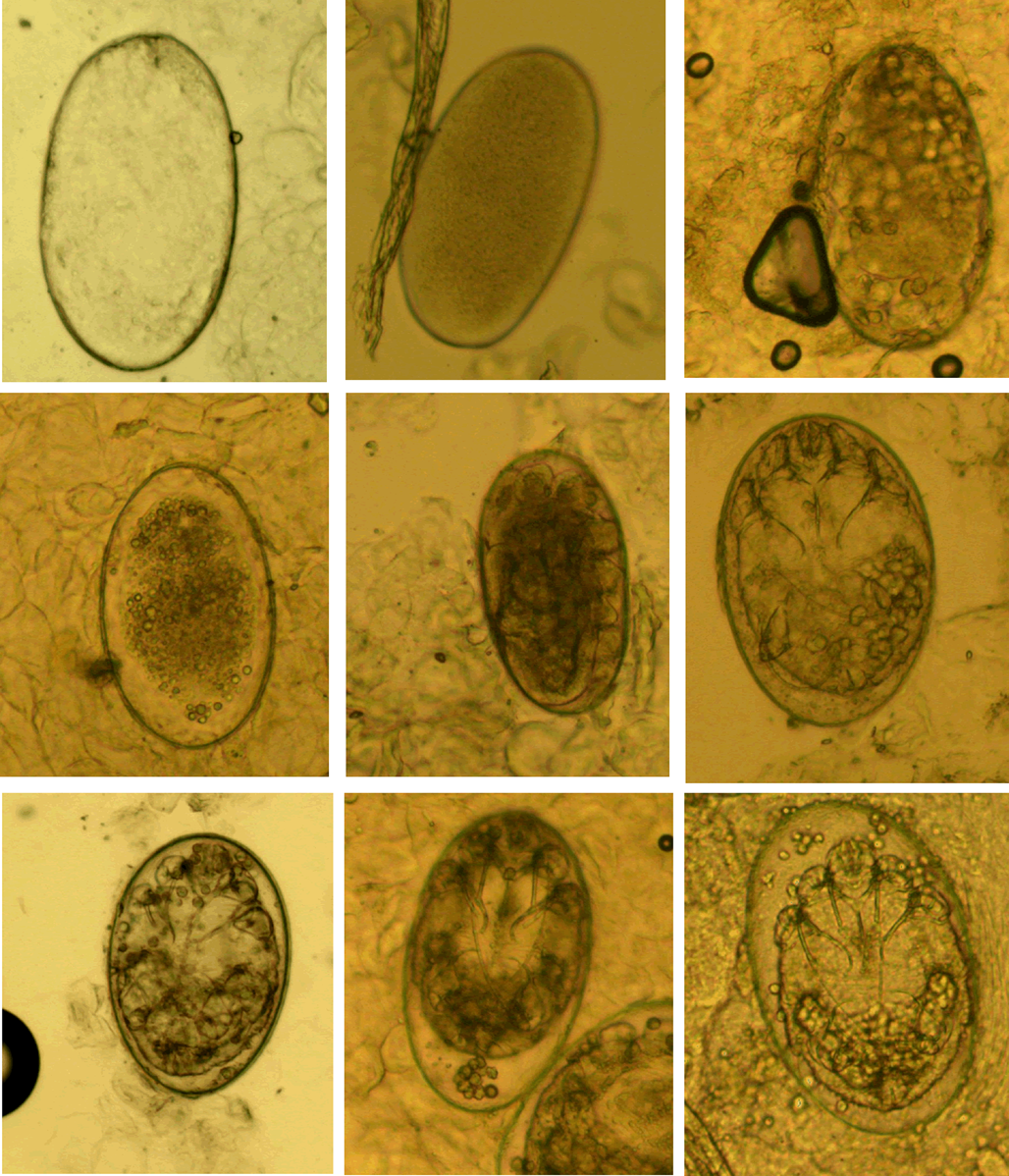
Figure 2. Eggs at different developmental levels (magnification: 100).
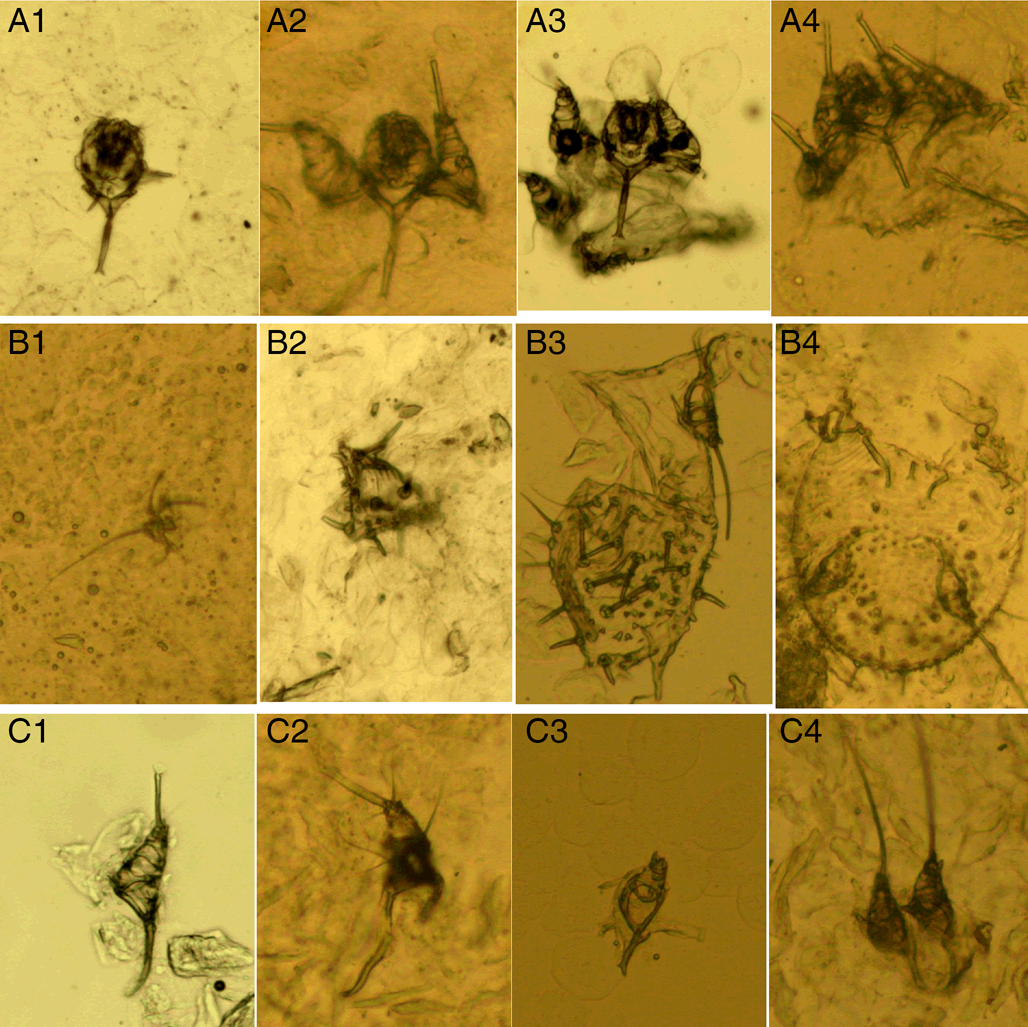
Figure 3. Mite fragments. (A1–A4) Gnathosoma-type fragments; (B1–B4) idiosoma-type fragments; (C1–C4) leg-type fragments (magnification: 100).
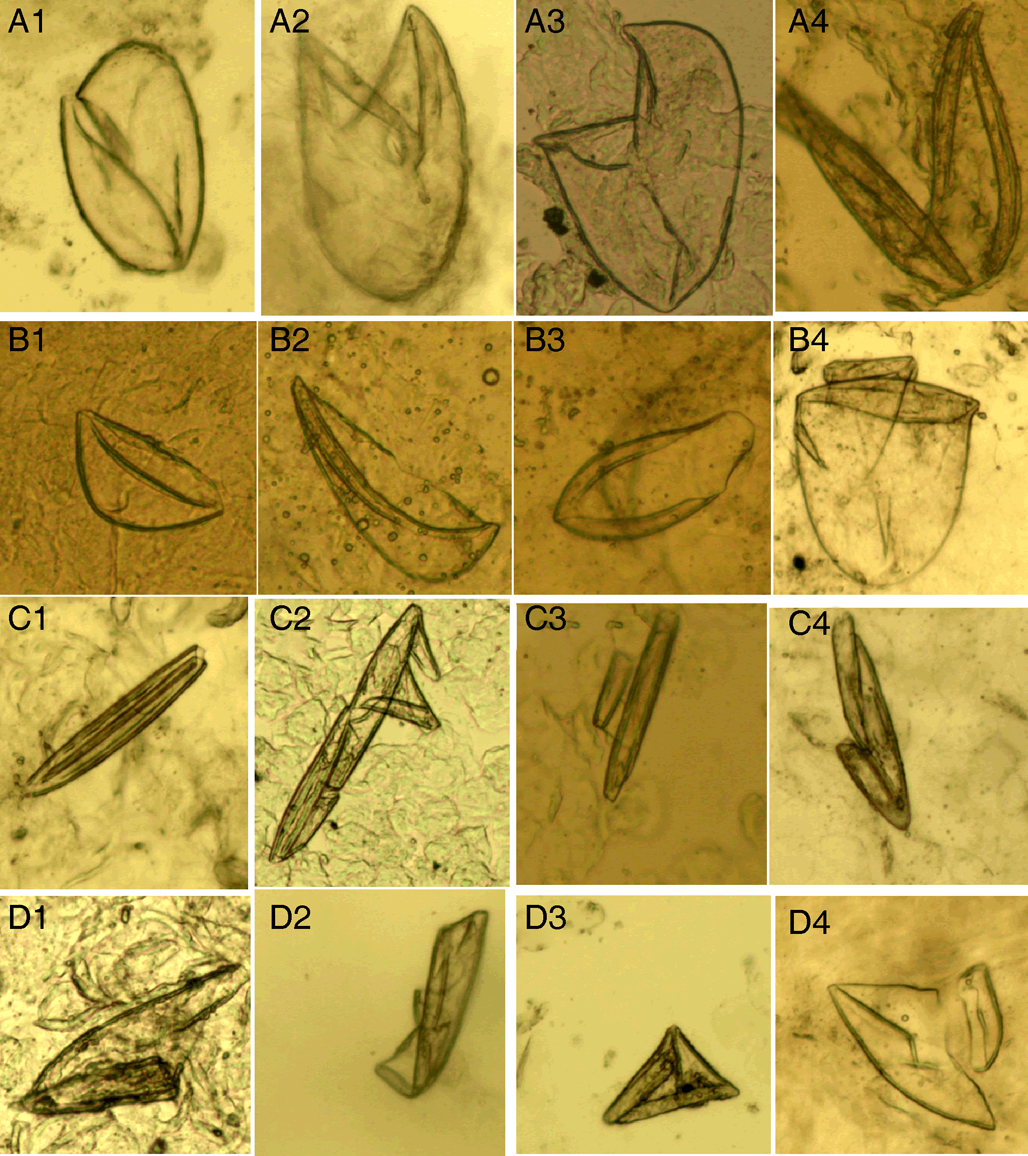
Figure 4. Eggshell fragments. (A1–A4) Double-leaf fragments; (B1–B4) single-leaf fragments; (C1–C4) 1-shaped fragments; (D1–D4) amorphous fragments (magnification: 100).
Double-leaf fragments are relatively large. In some cases, the eggshell is slightly damaged with only 1 chink (Fig. 4 A1); in most cases, the eggshell is broken into 2 connected fragments, shaped like the letter ‘V’ (Fig. 4 A2–A4).
Single-leaf fragments are smaller than the double-leaf fragments. The eggshell is broken into 2 unconnected fragments shaped like a boat, helmet or pan (Fig. 4 B1–B4).
If an eggshell is severely damaged, several small fragments will remain. Some small fragments are shaped like the Arabic numeral ‘1’, which are referred to as 1-shaped fragments (Fig. 4 C1–C4); some irregular small fragments are referred to as amorphous fragments (Fig. 4 D1–D4). Note that the 1-shaped and amorphous fragments are easily confused with contaminating artefacts.
(A) Fecal pellets (scybala). Fecal pellets are the products of mites and are seen as small, oval structures that are easily confused with contaminating artefacts. Therefore, fecal pellets must appear in piles (Fig. 5).
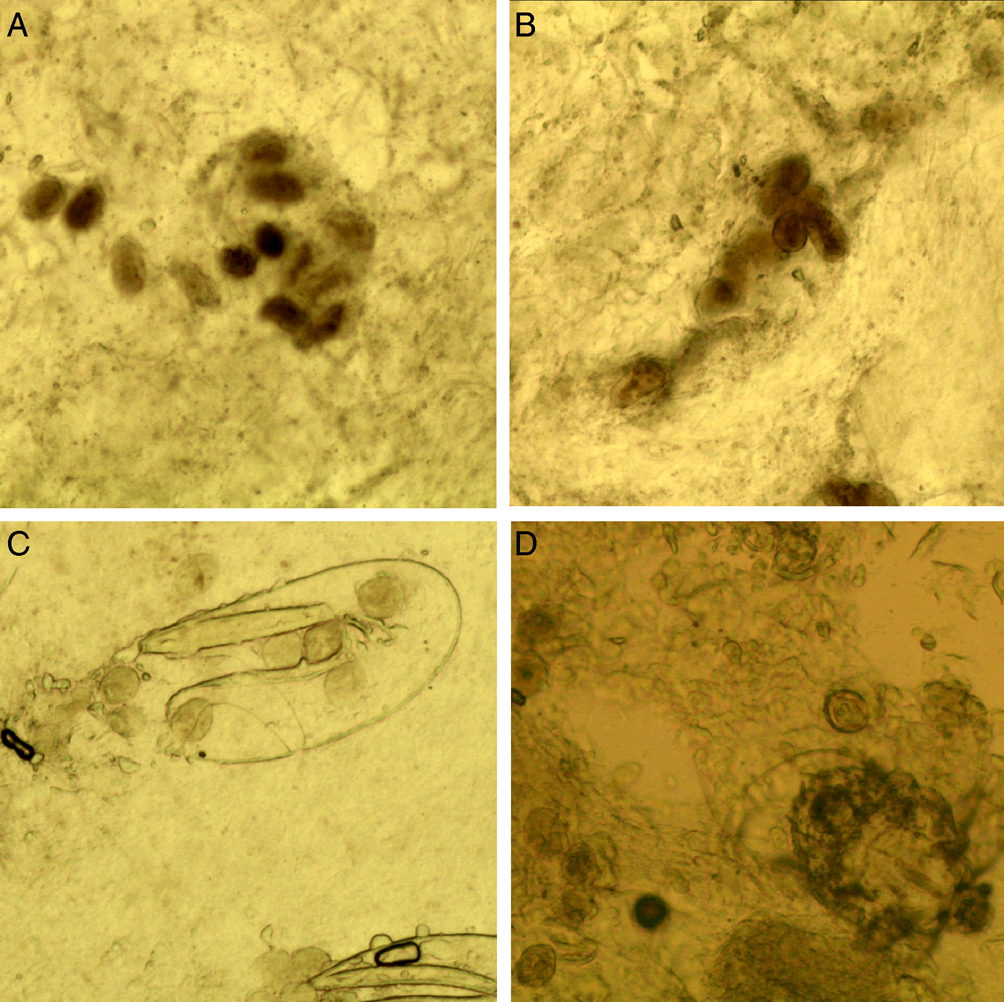
Figure 5. Fecal pellets. (A and B) Fecal pellets in piles; (C) fecal pellets and a double-leaf fragment; (D) fecal pellets and a mite (magnification: 100).
Optical microscopy examination
The examination was performed by operators with more than 3 years of clinical experience as described elsewhere (Engelman et al., Reference Engelman, Yoshizumi, Hay, Osti, Micali, Norton, Walton, Boralevi, Bernigaud, Bowen, Chang, Chosidow, Estrada-Chavez, Feldmeier, Ishii, Lacarrubba, Mahé, Maurer, Mahdi, Murdoch, Pariser, Nair, Rehmus, Romani, Tilakaratne, Tuicakau, Walker, Wanat, Whitfeld, Yotsu, Steer and Fuller2020). Firstly, several suspected sites were determined based on multiple skin lesion morphologies involving finger web-spaces, hands, the volar surfaces of the wrists, axillae, buttocks, the areola in women, the toe seams in infants or young children and genitalia in men. Following that, skin scrapings were collected from the suspected sites on the same individual using a sterile blade (#15) after disinfection with 75% alcohol. The scrapings were then carefully placed on a standard glass microscope slide, mixed with 1–2 drops of potassium hydroxide (KOH) at a 15% concentration, and covered with a coverslip. The slide was left to stand for several minutes before being scanned under a microscope (Olympus CX33; Tokyo, Japan) at 100× magnification to search for the diagnostic features of S. scabiei. Finally, a field was selected according to the priority order of diagnostic features stated as follows, and then saved as a microscopic image using a camera (JEDA SmartVF650DO, Jiangsu, China) along with its automated imaging system.
Priority order of diagnostic features of S. scabiei
Some diagnostic features (mites and eggs) are easy to identify, whereas others (mite and eggshell fragments, and fecal pellets) may be difficult to identify or easily confused with contaminating artefacts. Meanwhile, multiple diagnostic features can be observed in a single examination. In this study, the priority order of diagnostic features is as follows: (1) mites; (2) eggs; (3) double-leaf fragments; (4) leg-type fragments; (5) single-leaf fragments; (6) gnathosoma-type fragments; (7) idiosoma-type fragments; (8) 1-shaped fragments; (9) amorphous fragments; (10) fecal pellets.
Positive detection rates
In this study, a positive case refers to an optical microscopy examination in which at least 1 diagnostic feature of S. scabiei was observed, whereas a negative case refers to an optical microscopy examination in which no diagnostic features of S. scabiei were observed. The annual and overall numbers of positive cases and total cases between 2018 and 2022 were counted. The annual and overall positive detection rates were then calculated as shown in equation (1).
Frequency distribution of various diagnostic features of S. scabiei
According to the priority order of diagnostic features, all positive cases between 2018 and 2022 were divided into 10 groups: mites, eggs, double-leaf fragments, leg-type fragments, single-leaf fragments, gnathosoma-type fragments, idiosoma-type fragments, 1-shaped fragments, amorphous fragments, and fecal pellets. The numbers of positive cases in each group ni (i = 1, …, 10) and the total positive cases were counted. The frequency of each group was then calculated as shown in equation (2).
Statistical analysis
All statistical analyses were performed using Microsoft Excel. Positive detection rates and frequencies were used for descriptive analysis, and the statistical results of the descriptive studies were directly compared.
Results
Positive detection rates
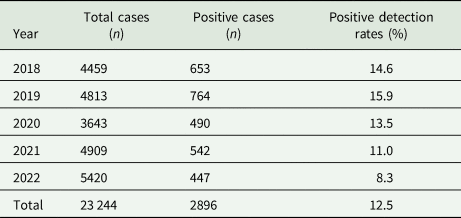
A total of 23 244 cases were retrieved from our database between 2018 and 2022: 4459 cases in 2018, 4813 cases in 2019, 3643 cases in 2020, 4909 cases in 2021 and 5420 cases in 2022. Of these cases, 2896 were confirmed to be positive, with an overall positive detection rate of 12.5% (2018–2022). The positive detection rate in 2019 was the highest (15.9%, 764 of 4813), followed by 2018 (14.6%, 653 of 4459), 2020 (13.5%, 490 of 3643), 2021 (11.0%, 542 of 4909) and 2022 (8.3%, 447 of 5420) (Table 1).
Table 1. Positive detection rates between 2018 and 2022
Frequency distribution of various diagnostic features of S. scabiei
Of the 2896 positive cases, 1193 (41.2%) were confirmed through the identification of mites, followed by eggs (23.9%, 691 cases), eggshell fragments (19.2%, 556 cases), mite fragments (15.4%, 447 cases) and fecal pellets (0.3%, 9 cases). Importantly, 34.6% of the positive cases (1003 of 2896) were confirmed through the identification of eggshell or mite fragments. Specifically, 250 cases (8.6%) were confirmed through the identification of double-leaf fragments, followed by leg-type fragments (6.4%, 185 cases), single-leaf fragments (5.4%, 156 cases), gnathosoma-type structures (4.7%, 136 cases), idiosoma-type structures (4.4%, 126 cases), 1-shaped fragments (3.4%, 99 cases) and amorphous fragments (1.8%, 51 cases). More detailed information is provided in Table 2.
Table 2. Frequency distribution of various diagnostic features of S. scabiei between 2018 and 2022
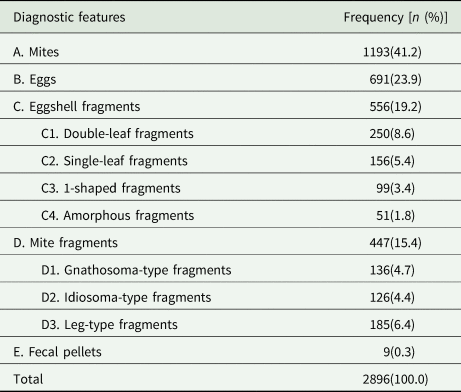
A, B, C, D, and E: 5 categories of diagnostic features; C1, C2, C3, and C4: 4 subcategories of eggshell fragments; D1, D2, and D3: 3 subcategories of mite fragments.
Discussion
Optical microscopy is considered the gold standard for diagnosing scabies (Arora et al., Reference Arora, Rudnicka, Sar-Pomian, Wollina, Jafferany, Lotti, Sadoughifar, Sitkowska and Goldust2020). Multiple diagnostic features of S. scabiei can be identified using microscopy. In this study, the diagnostic features were classified into 5 categories. Our results revealed that 65.2% of the positive cases were determined through the identification of mites, eggs or fecal pellets. Importantly, up to 34.8% of the positive cases were determined through the identification of mite or eggshell fragments. This result proved the important diagnostic values of mite and eggshell fragments.
Conflicting views regarding the diagnostic value of fecal pellets have been reported. Most researchers affirmed the diagnostic value of fecal pellets (Anderson and Strowd, Reference Anderson and Strowd2017; Arlian and Morgan, Reference Arlian and Morgan2017; Engelman et al., Reference Engelman, Fuller and Steer2018; Engelman et al., Reference Engelman, Yoshizumi, Hay, Osti, Micali, Norton, Walton, Boralevi, Bernigaud, Bowen, Chang, Chosidow, Estrada-Chavez, Feldmeier, Ishii, Lacarrubba, Mahé, Maurer, Mahdi, Murdoch, Pariser, Nair, Rehmus, Romani, Tilakaratne, Tuicakau, Walker, Wanat, Whitfeld, Yotsu, Steer and Fuller2020; Thomas et al., Reference Thomas, Coates, Engelman, Chosidow and Chang2020); whereas others considered that the presence of fecal pellets should not be considered diagnostic, as they resemble artefacts or debris (Arora et al., Reference Arora, Rudnicka, Sar-Pomian, Wollina, Jafferany, Lotti, Sadoughifar, Sitkowska and Goldust2020). Our results showed that only 0.3% (9 of 2896) of the positive cases were confirmed only through the identification of fecal pellets. We agree with the diagnostic value of fecal pellets. We suggest that fecal pellets must appear in piles and that a positive conclusion requires the unanimous consent of 2 experienced operators.
One disadvantage of optical microscopy is operator dependence – training and expertise are required to find lesions, extract material and prepare and interpret slides (Engelman et al., Reference Engelman, Yoshizumi, Hay, Osti, Micali, Norton, Walton, Boralevi, Bernigaud, Bowen, Chang, Chosidow, Estrada-Chavez, Feldmeier, Ishii, Lacarrubba, Mahé, Maurer, Mahdi, Murdoch, Pariser, Nair, Rehmus, Romani, Tilakaratne, Tuicakau, Walker, Wanat, Whitfeld, Yotsu, Steer and Fuller2020). Generally, the diagnostic features of S. scabiei can be observed in skin scrapings from lesions around the fingers, hands, wrists, infant toe seams and male genitalia. Some researchers think that lesions on the trunk most commonly develop from hypersensitivity reaction and cannot find mites of S. scabiei (Engelman et al., Reference Engelman, Yoshizumi, Hay, Osti, Micali, Norton, Walton, Boralevi, Bernigaud, Bowen, Chang, Chosidow, Estrada-Chavez, Feldmeier, Ishii, Lacarrubba, Mahé, Maurer, Mahdi, Murdoch, Pariser, Nair, Rehmus, Romani, Tilakaratne, Tuicakau, Walker, Wanat, Whitfeld, Yotsu, Steer and Fuller2020); however, it applies to small lesions such as papules, but not to big lesions such as nodules. In fact, diagnostic features can be observed in scrapings from nodules around the armpit and buttocks (data not shown). It should be noted that nodules (especially those on male genitalia) can be long-term and it is difficult to observe diagnostic features from skin scrapings obtained around the nodules after treatment.
Another disadvantage of microscopy is insensitivity (Engelman et al., Reference Engelman, Yoshizumi, Hay, Osti, Micali, Norton, Walton, Boralevi, Bernigaud, Bowen, Chang, Chosidow, Estrada-Chavez, Feldmeier, Ishii, Lacarrubba, Mahé, Maurer, Mahdi, Murdoch, Pariser, Nair, Rehmus, Romani, Tilakaratne, Tuicakau, Walker, Wanat, Whitfeld, Yotsu, Steer and Fuller2020). One study reported that the sensitivity of optical microscopy was 0.46 (95% CI 0.31–0.62) (Walter et al., Reference Walter, Heukelbach, Fengler, Worth, Hengge and Feldmeier2011). A study in Elazığ reported that the overall positive detection rate was 18.6% (139 of 746) (Yücel and Yılmaz, Reference Yücel and Yılmaz2021). Another study in Accra, Ghana reported that 92 patients were diagnosed with clinical scabies, but all skin scrapings examined with optical microscopy turned out to be negative (Kaburi et al., Reference Kaburi, Ameme, Adu-Asumah, Dadzie, Tender, Addeh, Aryee, Addo-Lartey, Sackey, Wurapa, Afari and Kenu2019). In the current study, the overall positive detection rate over 5 years (2018–2022) was 12.5%; however, it should not be considered to be due to the sensitivity of optical microscopy. In this study, some examinations were conducted on individuals after treatment, or those with other skin diseases. In these cases, the results were usually negative.
There are several reasons why microscopy is insensitive. Firstly, the examination is invasive. Some subjects, especially infants and young children, may be poorly tolerated in the process of extracting materials, resulting in a failed or invalid sample collection. Secondly, the reagent used in sample processing is KOH solution, which can only degrade keratin and cannot increase the contrast between diagnostic features and sample. Recently, it has been reported that S. scabiei can produce fluorescence under the ultraviolet mode of the dermatoscope (Nie et al., Reference Nie, Gou, Xu, Wang, Zhang and Lu2021). This is of great significance for improving the sensitivity of microscopy.
This study has some limitations, as it is the first study to classify the diagnostic features of S. scabiei into 5 categories and 7 subcategories. Therefore, this morphological classification method and the related terminology should be optimized in the future, and we look forward to suggestions and discussions from other researchers.
Although all diagnostic features share the same diagnostic value, some features are difficult to identify or easily confused with contaminating artefacts. In some cases, only a long bristle or several cuticular spines were identified (Fig. 3 B1–B2), which may be ignored by inexperienced operators. To the best of our knowledge, few studies have focused on the morphological classification of multiple diagnostic features of S. scabiei under a microscope. This study may be beneficial for operator training. Importantly, we suggest that the IACS Criteria should be revised in the following version to emphasize the important diagnostic values of mite and eggshell fragments.
Data availability statement
The complete datasets are available upon reasonable request.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
Lin Song conceived and designed the study. Yaliu Wu, Xiaoli Li and Jianjun Li conducted data gathering. Hongfeng Li performed statistical analyses. Wanchen Li and Lin Song wrote the article.
Financial support
This study was supported by the Research Program of Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital (Grant No. 2022001) and the Research Program of Tianjin Science and Technology Service Industry Association (grant No. 23TJKJFWY08001).
Competing interests
None.
Ethical standards
Ethical approval was obtained from the Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Tianjin, China (approval No. LLKY2023-04).