Introduction
Cleavers (Galium spp.) are considered weedy plants globally and are found in many different types of ecosystems (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1977; Malik and Vanden Born Reference Malik and Vanden Born1984). Two species of cleavers, catchweed bedstraw (Galium aparine L.) and false cleavers (Galium spurium L.) are found in cropped fields across North America and Europe. North American populations of G. aparine require nutrient-rich, shaded, and moist environments to complete their life cycle (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1977; Malik and Vanden Born Reference Malik and Vanden Born1988). In contrast, G. spurium is commonly found in open, brighter habitats with high moisture and fertility, including cropped fields (Malik and Vanden Born Reference Malik and Vanden Born1984; Moore Reference Moore1975). In most cases, both Galium species are commonly identified as “cleavers” (Hübner et al. Reference Hübner, Fykse, Hurle and Klemsdal2003; Reid and Van Acker Reference Reid and Van Acker2005), because they have common growth patterns and are difficult to distinguish visually. However, De Roo et al. (Reference De Roo, Eckstein, Benaragama, Beattie and Willenborg2019) sampled several Galium spp. populations from across western Canada and based on molecular and morphological analyses, determined all populations were G. spurium.
Galium spp. can be problematic in spring- and winter-sown crops (Aziz et al. Reference Aziz, Tanveer, Ali and Yaseen2009; Froud-Williams Reference Froud-Williams1985). They exhibit high relative abundance on the Canadian Prairies, particularly in the province of Saskatchewan, where this species is now ranked seventh in abundance across all types of annual crops (Leeson et al. Reference Leeson, Thomas, Hall, Brenzil, Andrews, Brown and Van Acker2005). Within canola crops (Brassica napus L.), Galium spp. were ranked 6th in relative abundance, up from 31st in the 1970s (Leeson Reference Leeson2012). The increasing relative abundance of Galium spp. in field crops impacts crop production, because Galium spp. reduce yield, increase crop lodging, interfere with harvest operations, and reduce crop grades. For example, canola yield losses varied between 4% and 28% where competition from Galium spp. was documented (Malik and Vanden Born Reference Malik and Vanden Born1987). However, Galium spp. have been reported to reduce spring wheat (Triticum aestivum L.) yields by up to 60% (Rola Reference Rola1971) and winter wheat yields by 57% (Wright and Wilson Reference Wright and Wilson1987). One hypothesis that may explain the increased relative abundance of Galium spp. across western Canada is the development of resistance to acetolactate synthase (ALS) inhibitors and auxin-mimicking herbicides (Hall et al. Reference Hall, Stomme, Horsman and Devine1998). Approximately 20% of Galium spp. populations surveyed in Saskatchewan have evolved resistance to ALS-inhibiting herbicides, and Galium spp. are considered at high risk for evolving glyphosate resistance (Beckie et al. Reference Beckie, Harker, Hall, Holm and Gulden2011; Beckie et al. Reference Beckie, Shirriff, Leeson, Hall, Harker, Dokken-Bouchard and Brenzil2020). A second hypothesis involves the ability of Galium spp. to evade weed management tactics due to their plasticity. Galium aparine ecotypes, in particular, have high phenotypic plasticity and genetic diversity, enabling them to adapt to changing environmental conditions (Taylor Reference Taylor1999). Other than G. aparine’s own biological and physiological adaptations, changes in crop production practices, notably the adoption of no-till practices in the Canadian Prairies and changes in climatic conditions, may have contributed to its increasing abundance in the past decade. Furthermore, different G. aparine ecotypes are markedly different in seed germination ecology (Auge and Mahn Reference Auge and Mahn1988; Bain and Attridge Reference Bain and Attridge1988). Masuda and Washitani (Reference Masuda and Washitani1992) also identified differences in germination characteristics among different local ecotypes of G. spurium. Differences in emergence periodicity of Galium species could lead to cohorts of populations escaping either preemergence or in-crop herbicide applications. However, this is speculative, as no literature has characterized the germination characteristics or emergence periodicity of Galium spp. populations, particularly of G. spurium in western Canada.
Emergence timing contributes substantially to weeds’ potential success (Forcella et al. Reference Forcella, Benech, Arnold and Sanchez Ghersa2000). It is thus considered an essential adaptive strategy for plants under natural selection (Fenner Reference Fenner1985, Reference Fenner1987). For example, if weed control measures are implemented prematurely, growers will fail to control late-emerging cohorts, which can cause yield losses (Harker et al. Reference Harker, Blackshaw and Clayton2001) and contribute seeds to the weed seedbank. Annual weeds are often more susceptible to herbicides at early growth stages, so understanding their germination requirements and emergence patterns is essential for weed management (Kusdk and Streibig Reference Kusdk and Streibig2003), particularly for herbicide-resistance management.
Recent European studies have shown that G. aparine has two major emergence cohorts in spring and fall (Royo-Esnal et al. Reference Royo-Esnal, Torra, Conesa and Recasens2010b). The early-fall cohort emerges just after seeding the winter crop, and the emergence period is often more prolonged than for other broadleaved weed species. Spring-emerging European Galium spp. populations have emergence percentages ranging from 2% to18% (Cussans and Ingle Reference Cussans and Ingle1999). The cause behind the variability of these populations is unknown, although environmental variation among sites and years likely contributed to the variable emergence patterns. The seasonal emergence patterns of Galium spp. populations in western Canada have not been studied but could help to explain some of the reasons for the difficulty in controlling Galium spp. in crop fields.
Soil temperature is a major determinant of germination for most temperate weed species. The effect of temperature on weed emergence has been well documented, and thermal time (growing degree days [GDD]) can be used directly to predict plant emergence (Angus et al. Reference Angus, Cunningham, Moncur and Mackenzie1981). Currently, the literature reports wide variability in the minimum and optimum temperatures for germination of Galium spp., with estimates ranging between 2 and 20 C (Arai et al. Reference Arai, Chisaka and Ueki1961; Malik and Vanden Born Reference Malik and Vanden Born1988). Further, many previous germination studies used G. aparine from European locations, but most Galium spp. populations in western Canada are G. spurium (De Roo et al. Reference De Roo, Eckstein, Benaragama, Beattie and Willenborg2019). It cannot be assumed that the base temperature reported in previous studies with G. aparine would be the same as that for G. spurium. Also, the ecotypic difference in seed germination ecology was mainly known for G. aparine populations but not for G. spurium. Given these limitations, the objectives of this study were to determine the base temperature and emergence periodicity of G. spurium populations from across western Canada. We hypothesized that if natural selection were operating locally, base germination temperature and emergence timing would vary between Galium spp. populations. Still, populations nearest each other would have similar germination and emergence characteristics.
Materials and Methods
Determination of Base Temperature
Experimental Procedure
Germination of Galium populations was characterized using a thermogradient plate (TGP) custom built at the Agriculture and Agri-Food Canada Saskatoon location. The TGP contains more than 200 individually controlled cells, each varying in temperature. The temperature of each cell is controlled with a thermoelectric pump, and each cell can deliver temperatures accurately to within 0.1 C (Tozzi et al. Reference Tozzi, Beckie, Weiss, Gonzalez-Andujar, Storkey, Zhcici and Van Acker2014). The methodology used for the TGP experiment was similar to that in Tozzi et al. (Reference Tozzi, Beckie, Weiss, Gonzalez-Andujar, Storkey, Zhcici and Van Acker2014). Four populations from Saskatchewan termed “Clancy,” “Heavin,” “Travin,” “SPG” (Saskatchewan Pulse Growers), and two from Alberta termed “Vegreville” and “Lacombe” (Table 1), were included in the study. Seeds of each population were obtained from a seed plant at each location (Table 1; Figure 1). Germination of these populations was compared with germination of two known populations of G. aparine and G. spurium (De Roo et al. Reference De Roo, Eckstein, Benaragama, Beattie and Willenborg2019).
Table 1. Approximate GPS coordinates of collection sites of Galium spp. seed for emergence timing and plant characteristic measurements.
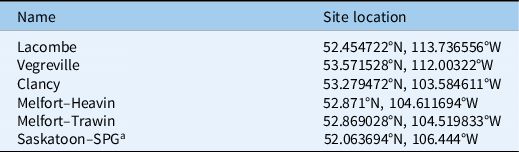
a SPG, Saskatchewan Pulse Growers

Figure 1. Google map showing Saskatchewan and Alberta locations where Galium spp. populations were collected.
One hundred seeds from each population were counted, placed in small paper envelopes, and stored at −4 C. Any seed dormancy induced by storage was broken by placing the seeds in a freezer for 1 mo at −18 C, followed by storage in a refrigerator (2 C) for 2 wk. Further to this, each seed was scratched with abrasive sandpaper to scarify the testa. For experimentation, 25 Galium seeds were placed in a petri dish lined with filter paper, dampened using de-ionized water, and placed in a TGP cell. The filter paper was kept moist throughout the experiment. Seeds were then subjected to 10 different constant temperatures ranging from 1 to 10 C, at 1.0 C increments. Temperature treatments were arranged in a completely randomized design. Because of the perceived range of germination temperatures for Galium species, this experiment focused on identifying the lowest threshold for germination in these populations. Germination times were recorded daily for 21 d to determine the lowest temperature at which seeds germinated. Seeds were deemed to have germinated when the radicle broke through the seed coat and was at least 1 mm in length. For each Galium spp. population, there were two replicates of each treatment (temperature), and the experiment was repeated three times.
Determination of Emergence Timing
A common garden field experiment was conducted at two sites near Saskatoon, SK, in 2013 and 2014; the Kernen Crop Research Farm (52.152861°N 106.544861°W) on a Black Chernozemic loam (pH 7.7, organic matter 2.9%), and the Goodale Research Farm (52.0635°N, 106.499917°W) on a Dark Brown Chernozem (pH 7.0, organic matter 1.9%). Germination tests were initially conducted to determine the approximate germination percentages of each sample. The methodology for this test was similar to what was described earlier for the base temperature experiment. Seed samples were also sent to Discovery Seed Labs (Saskatoon, SK) for viability testing using tetrazolium chloride.
The background Galium spp. population was quantified at each experimental site before sowing, using the methodology described by Forcella (Reference Forcella1992). Briefly, two 10-cm-diameter soil cores were taken from each replicate at each experimental site and placed in individual containers. These containers were then placed in a growth chamber for a 16-h daylight cycle with temperatures of 18/12 C day/night. Galium spp. were counted and removed as they emerged. Once emergence stopped, the containers were dried, stirred, and rewatered. That cycle was repeated three times before the trays were chilled at 4 C for 14 d and then re-exposed to the growing light.
The experimental design was a randomized complete block with four replicates. Each replicate had a factorial treatment design (two factors), with the first factor being spring or fall sowing and the second factor being the Galium spp. population. The same six Galium spp. populations that were included in the base temperature study (four Saskatchewan and two Alberta populations) were also included in this experiment. Galium spp. populations were assessed in 1 by 2 m microplots. Glyphosate (Roundup WeatherMax®, Bayer Crop Science, 160 Quarry Park Boulevard, SE Calgary, AB T2C 3G3, Canada) was applied to the site at a rate of 900 g ae ha−1 before sowing. Galium spp. seeds were blended with sand and then broadcasted at a rate of 400 seeds m−2, as described in Reid and Van Acker (Reference Reid and Van Acker2005). Plots were raked individually to cover the seeds with a shallow layer of soil. Sowing dates for the spring treatment in 2013 were May 21 and 23 at the Kernen and Goodale sites, respectively. Fall treatments were sown on August 21 at both sites in 2013. In 2014, the spring treatment was sown on May 13 at both sites, while the fall treatment was sown on August 14 and 15 at the Kernen and Goodale sites, respectively. To maintain weed-free plots, broadleaf weeds were hand weeded as required, and grassy weeds were managed by applying clethodim (Select®, Arysta LifeSciences, 15401 Weston Parkway, Suite 150, Cary, NC 27513, USA) at 45 g ai ha−1 in 2013 and quizalofop (Assure II®, DuPont Canada, P.O. Box 2300, Streetsville, Mississauga, ON L5M 2J4, Canada) at 47 g ai ha−1in 2014.
Galium spp. emergence was recorded daily in three randomly placed 0.15-m2 quadrats in each microplot. Emergence was monitored from the time the first plant emerged until emergence was complete and no further plants emerged over a 2-wk period. Newly emerged seedlings were recorded and marked with colored toothpicks to ensure they were not counted twice. Cumulative (final) emergence percentages were obtained by dividing the final number of emerged plants by the number of seeds planted.
Data Analysis
Data from the TGP germination study were analyzed using nonlinear regression analysis with the drc package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015) in R software (R Core Team 2022). Data from the two runs were combined and used to fit a nonlinear three-parameter Gompertz sigmoidal model representing the relationship between temperature and germination (Equation 1).
where y is percentage germination, t is temperature, a is the asymptote, b is the rate of change in germination, and c is the inflection point. Model selection was based on the extra sum-of-squares lack-of-fit test using the drc package in R, as well as on examination of model residuals and Akaike’s information criterion (AIC) values. The extra sum-of-squares lack-of-fit test was used to determine whether populations could be combined for analyses. Based on the sum-of-squares lack-of-fit test, models were fit for each population separately, and parameters were compared by ANOVA. The inflection points of the modeled germination curve were compared with the average inflection point for all populations. Means for parameter values were separated using the compParm function in the drc package in R at P < 0.05.
Emergence curves were developed for the Goodale site only, as a sizable background population of Galium spp. was found at Kernen in 2013. The Kernen site in 2014 had no background Galium spp. population, but the emergence was too low to properly establish a good model fit for predicting emergence timing. Cumulative GDD were calculated for emergence curve fitting using Equations 2 and 3:
 $${\rm{Daily \;growing \;degree \;day:}}\,{\rm{GD}}{{\rm{D}}_{{\rm{daily}}}} = \left[ {{{\left( {{T_{{\rm{max}}}} + \;{T_{{\rm{min}}}}} \right)} \over 2}} \right] - \;{T_{{\rm{base}}}}$$
$${\rm{Daily \;growing \;degree \;day:}}\,{\rm{GD}}{{\rm{D}}_{{\rm{daily}}}} = \left[ {{{\left( {{T_{{\rm{max}}}} + \;{T_{{\rm{min}}}}} \right)} \over 2}} \right] - \;{T_{{\rm{base}}}}$$
where T max is the maximum daily air temperature, T min is the minimum daily air temperature, T base is the base temperature (2 C) at which no biological activity occurs, and n is the number of elapsed days from seeding to the end of the emergence period. The base temperature of 2 C was used, as it was the lowest recorded germination temperature of Galium spp. at the time of study initiation (Malik and Vanden Born Reference Malik and Vanden Born1988). Maximum and minimum air temperatures were obtained from an Environment Canada weather station located at the Kernen Research Farm. Air temperatures were used as a proxy for soil temperature, as the Galium spp. were seeded at a shallow depth in this experiment.
Emergence periodicity was presented as a proportion of the total emergence. Cumulative daily emergence values over each plot were recorded and used for statistical analysis. Nonlinear models were fit to the emergence data using the drc statistical package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015) in R (R Core Team 2022). The three-parameter Weibull model (Equation 4) was utilized to model the emergence of Galium spp. populations in both spring and fall of 2014, and a two-parameter model was used for the fall 2013 emergence data (Equation 5):
where Y represents the proportion of Galium spp. emergence, the d parameter is the upper limit or maximum emergence, the e parameter is the TE50 or the time to 50% emergence, and the b parameter is the slope around TE50. The lower limit, typically parameter c, was fixed at 0, because all samples exhibited a delayed period where germination was zero, and germination percentages cannot be negative. Model selection was based on the extra sum-of-squares lack-of-fit test in the drc package of R and examination of model residuals and AIC values. The extra sum-of-squares lack-of-fit test was also used to determine whether years and populations could be combined for analyses.
Final cumulative emergence percentages were analyzed with ANOVA using a mixed model (PROC MIXED) in SAS v. 9.2 (SAS Institute Inc. 2011). PROC UNIVARIATE and Bartlett’s test were used to examine normality and homogeneity of variance, respectively. In the mixed model, the population was treated as a fixed effect, while year, replicate within the year, and year by population interactions were considered random effects. Means were separated using Tukey’s honestly significant difference at P < 0.05.
Results and Discussion
Germination Characteristics
A single germination response curve common to all populations could not fit into the data, indicating germination response to temperature is not uniform among populations (Figure 2). The emergence model provided a good fit to the data for each population, with coefficients of determination (R2) ranging from 0.92 to 0.97 (Table 2). Few statistical differences were observed between parameter estimates. The inflection point of the curve can be used to estimate the temperature at which 50% of the population has germinated (T 50). Galium aparine required a temperature of 8.3 C to reach 50% germination. In contrast, the T 50 for all other G. spurium populations was similar, with most populations approximating 6.5 C. On average, G. aparine (8.3 C) exhibited a significantly higher T 50 than the Clancy (6.0 C), Canora (6.1 C), Heavin (6.1 C), and Trawin (5.7 C) populations (Table 2). The Lacombe and Saskatoon populations had an intermediate T 50 value (Table 2). Galium populations also exhibited differences in germination rates. The known G. spurium population had a significantly faster germination rate (3.7%) than both the SPG (1.8%) and Trawin (2.0%) populations. However, there were no significant differences in germination rates among the other Galium spp. populations. Furthermore, there were no significant differences between the upper asymptotes (maximum germination percentage) for any of the populations (Table 2).

Figure 2. The effect of temperature on percent germination of Galium spp. populations from different locations on the Canadian Prairies. Data points represent the means of two trial runs conducted using the thermogradient plate. SPG, Saskatchewan Pulse Growers.
Table 2. Base germination (C) and inflection point (C for 50% germination) of each logistic three-parameter model on each population of Galium spurium and Galium aparine across western Canada. a
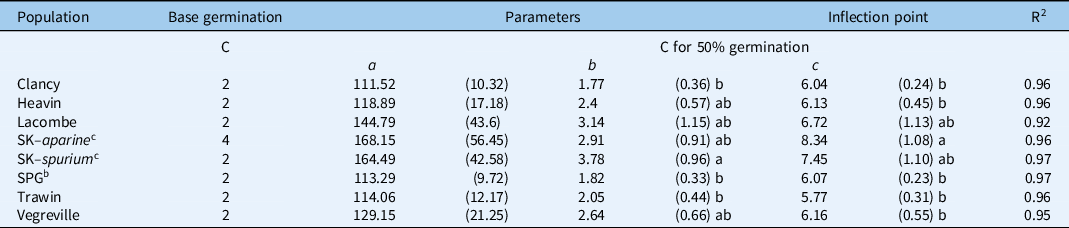
a Values in parentheses are standard errors of each parameter estimate. The similar letters following each parameter estimate indicate they are statistically similar at P < 0.05.
b SPG, Saskatchewan Pulse Growers.
c SK- Saskatoon.
The base germination temperature derived from the TGP experiment was 2 C for all populations except G. aparine, which had a base germination temperature of 4 C (Table 2).
The base temperature and the optimum temperature (T 50) identified in this study for the G. aparine population were in the range reported in other regions (<5 to10 C) with G. aparine populations of Europe and China (Froud-Williams Reference Froud-Williams1985; Taylor Reference Taylor1999; Wang et al. Reference Wang, Zhang, Dong and Lou2016). Differences in germination rates within G. spurium populations in those studies may be due to the location of the source populations for the two studies and suggest local variation may have selected for differences among the biotypes found in North America and Europe. However, it is essential to highlight that this experiment was unable to adjust for maternal effects; thus, any variation among the populations for germination characteristics could be due to a combination of the local environment as well as the legacies of different biotic and abiotic factors. Nevertheless, all populations in our study had base temperatures consistent with the known reference population of G. spurium, which further confirms that all western Canadian populations of Galium spp. are G. spurium (De Roo et al. Reference De Roo, Eckstein, Benaragama, Beattie and Willenborg2019). Further, the results suggest that local selection has not created a divergence in the base temperature of Galium spp. across western Canada, but rather, differences in base temperature and germination characteristics are greater among species than populations. Given the low base temperature identified for both Galium species, the findings indicate that all tested populations have the potential to germinate very early in the spring and late into the fall.
Spring Emergence
Emergence models could not be combined across years or seasons, as years were significantly different from each other within spring and fall sowings. Similarly, a single emergence curve could not be fit for populations originating from the same province based on the extra sum-of-squares lack-of-fit test, indicating that populations differed in their emergence patterns within a season.
Moisture and temperature during the emergence period differed between years and seasons, which likely influenced the emergence periodicity of Galium spp. populations. The temperature was similar between years but was significantly warmer than the 30-yr average (Table 3). Precipitation was 18% below and 11% above the long-term average in 2013 and 2014, respectively. Galium spp. time to emergence in spring was consistent across the years, commencing at 223 GDD on June 3 in 2013 and 214 GDD on June 1 in 2014 (Figure 3). The lack of variation in the base temperature identified among these populations (Table 2) is likely the main reason for the synchronous onset of emergence in spring. Despite the similarity in time to emergence, a cool and wet environment in 2014 was favorable for Galium spp. emergence, producing a longer emergence period in 2014 compared with 2013 (Figure 3). The timing of precipitation events probably impacted Galium spp. emergence patterns (Figure 3). Relatively consistent rainfall events in 2013 following emergence led to a uniform period of gradual emergence of all populations in spring, except for when small rainfall events occurred (8.4 and 47 mm in 2013 at 200 and 350 GDD, respectively) (Figure 3). In 2014, there appeared to be two cohorts of emergence after significant rain events at 200 GDD (51 mm from May 27 to 31) and 400 GDD (63.6 mm from June 19 to 23) (Figure 3).
Table 3. Monthly rainfall (mm) and mean daily temperature (C) for Saskatoon, Saskatchewan, and climate normals (30-yr average).
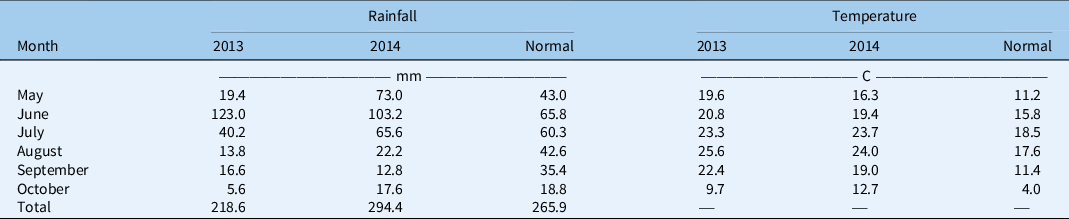
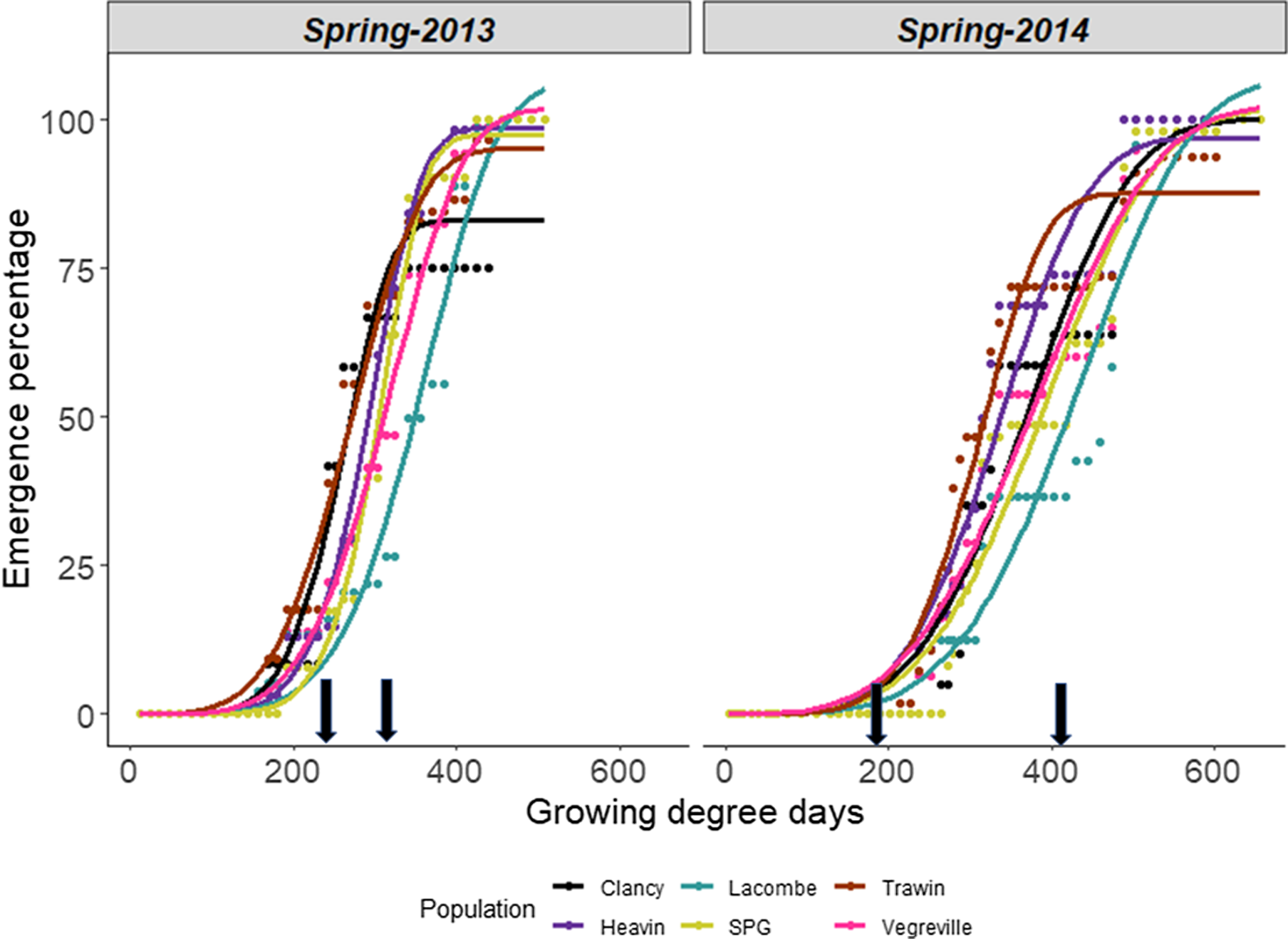
Figure 3. Emergence timing of Galium spp. at Goodale in the spring of 2013 (left) and 2014 (right). Growing degree days (GDD) were determined with a base temperature of 2 C. Arrows indicate significant rainfall events (8.4 and 47 mm in 2013 at 200 and 350 GDD, and 51 and 63 mm in 2014 at 200 and 400 GDD, respectively). SPG, Saskatchewan Pulse Growers.
Model parameters for spring emergence varied between Galium spp. populations and years (Table 4; Figure 3). The median time of emergence (TE50) of all populations was longer in 2014 than in 2013. Nevertheless, the TE50 was significantly different between all populations, except for Heavin and SPG in 2013 (Table 4). Lacombe was the slowest population to emerge, taking on average 380 GDD to reach TE50 compared with 274 GDD for Clancy, the most rapidly emerging population (Table 4). The Vegreville, SPG, and Clancy populations did not differ for median emergence time but required 100 GDD (8 to 9 d) longer to emerge than Heavin and Trawin (Table 4). European populations also were shown to differ in emergence timing, which corroborates the results of this study. However, the European populations in Royo-Esnal et al. (Reference Royo-Esnal, Torra, Conesa, Forcella and Recasens2010a) required greater thermal time for germination (∼400 to 500 GDD) than did the Canadian populations tested here.
Table 4. Parameter estimates of spring emergence timing of Galium spp. populations across western Canada in 2013 and 2014. a
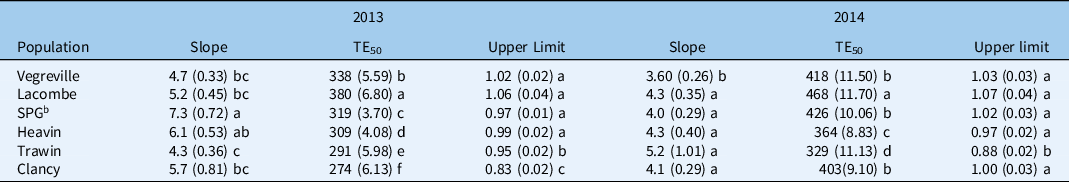
a Values in parentheses are standard errors, and similar letters following each parameter estimate indicate they do not differ statistically at P = 0.05.
b SPG, Saskatchewan Pulse Growers.
The cumulative emergence of Galium spp. in the current study ranged between 2% and 14% in 2013 and 2014, respectively. In 2013, the Clancy population exhibited emergence percentages that were 5- to 8-fold lower (P = 0.022) than those of the other populations (Figure 4). Likewise, the parameter that sets the upper limit of the curve (d) also varied between populations, with the Clancy population having an upper limit 20% lower than the other populations. In 2014, the cumulative emergence of the SPG population was 2- to 4-fold greater (P = 0.001) than cumulative emergence of all other populations, except Trawin. Cumulative emergence percentages significantly differed between populations that were collected in close proximity to each other. The Trawin population was the only population in 2014 with a significantly different upper limit from the other populations (9% to 18% lower) (Table 4). The differences in the d parameter further suggest some regional similarities among the populations within Saskatchewan and those from Alberta. Taken together, both the cumulative emergence percentages and the upper limit of the emergence curve (d) showed that Galium spp. populations closer in origin did not necessarily have similar spring emergence patterns. Differences in cumulative emergence among G. spurium populations were also identified by Royo-Esnal et al. (Reference Royo-Esnal, Torra, Conesa and Recasens2012).

Figure 4. Cumulative emergence percentage of Canadian Galium spp. populations in spring of (A) 2013 and (B) 2014. Error bars represent SE, and similar letters indicate values do not differ statistically at P = 0.05. SPG, Saskatchewan Pulse Growers.
Fall Emergence
The fall emergence of Galium spp. populations in 2013 was fit to a two-parameter Weibull model in which the upper and lower limits were 1 and 0, respectively. Emergence in the fall of 2014 was fit to a three-parameter Weibull model in which the lower limit was equal to 0 (Figure 5). Despite the drought-like conditions in fall 2013, the Vegreville, Heavin, and Trawin populations all began to emerge at approximately 300 GDD. Immediately after emergence began, it ceased in these populations, which then required 21, 31, and 29 d to complete emergence, respectively. The emergence timing of all remaining populations began after a series of short rain events on September 26 to 27 (16.2 mm) and October 2 to 4 (3.2 mm), around 600 GDD. The fall of 2014 had significantly more moisture than in 2013. The consistent rainfall resulted in more uniform emergence among populations, with the exception of the Lacombe and Clancy populations; both populations had a 7- to 9-d pause in emergence at 250 GDD (Figure 5).
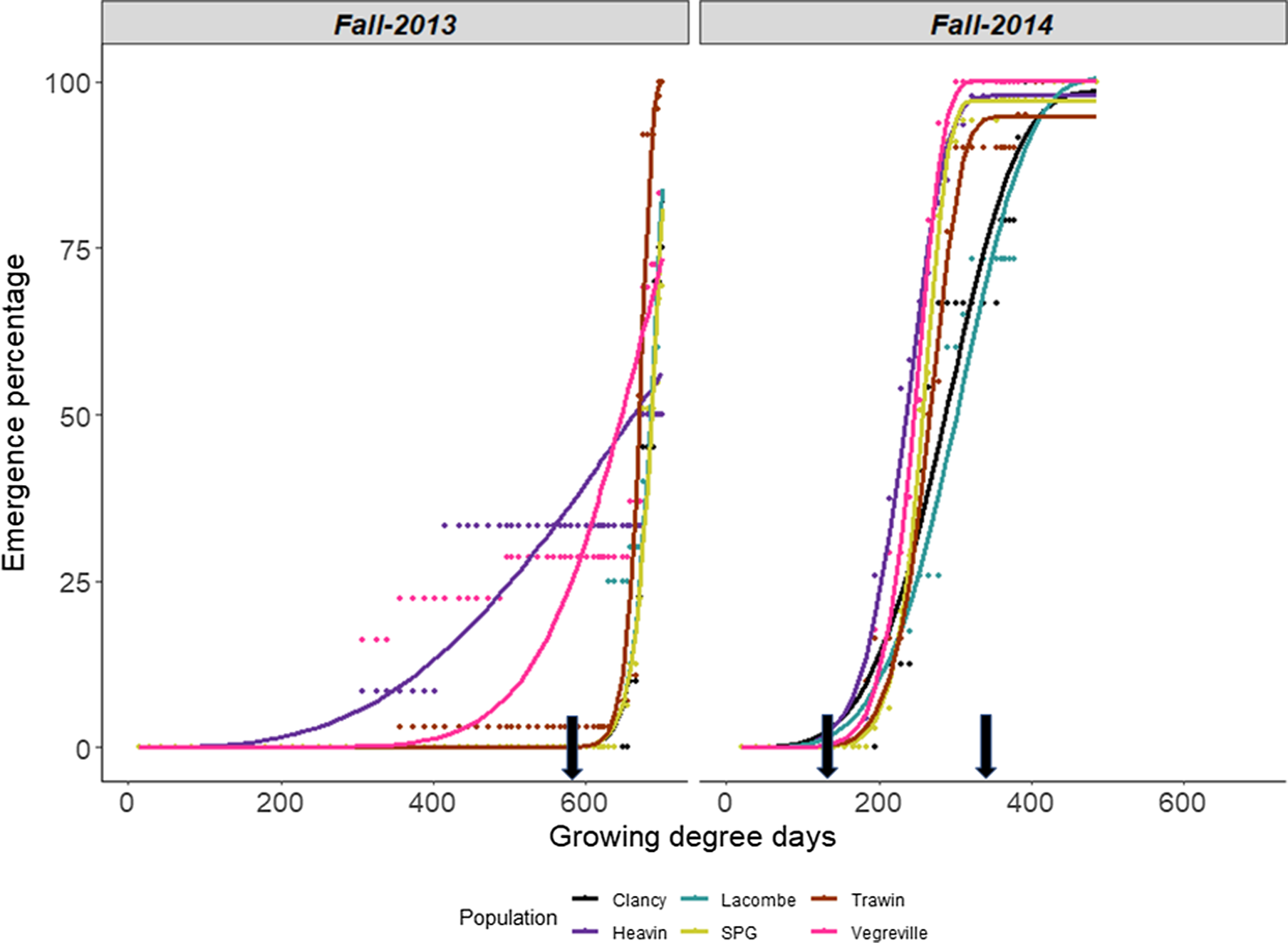
Figure 5. Emergence timing of Galium spp. populations (observed and predicted values, respectively) at Goodale in the fall of (A) 2013 and (B) 2014. Growing degree days (GDD) were determined with a base temperature of 2 C. Arrows indicate significant rainfall events (16.2 mm at 600 GDD in 2013 and 4.8 mm and 7.8 mm at 150 and 360 GDD in 2014). SPG, Saskatchewan Pulse Growers.
The model parameters for fall emergence differed between years and populations (Table 5). The 2013 data showed that the rate of emergence (b) for Heavin was nearly 3-fold lower than that of Vegreville and 13- to 17-fold lower than those of the other populations (Table 5; Figure 5). The median emergence timing (TE50) of populations was characterized by three groupings (Table 5). Heavin had a significantly longer median emergence time than all populations (Table 5). Although the Lacombe, Clancy, and SPG populations did not differ from each other, these populations had greater median emergence times than Trawin and Vegreville (Table 5). The median emergence time in 2014 differed between all populations and ranged from 249 to 328 GDD. In contrast to 2013, Heavin exhibited the lowest median emergence time in 2014. Lacombe again took the greatest time to reach 50% emergence, as it did in 2013. There were no differences among populations for cumulative emergence in the fall (P = 0.08 and P = 0.07 for 2013 and 2014, respectively). However, means separation indicated some differences existed among populations. This was due to the Clancy population having substantially lower cumulative emergence than the other populations in both years (2% to 4% cumulative emergence), while other differences among populations were minor (Figure 6). The cumulative emergence of the other populations varied between 5% and 15% in both site-years (Figure 6). The upper asymptote r of the emergence curves did not differ and was thus not associated with the region of origin.
Table 5. Parameter estimates of fall emergence timing data of Galium spp. populations across western Canada in 2013 and 2014. a

a Values in parentheses are standard errors, and similar letters following each parameter estimate indicate they do not differ statistically at P = 0.05.
b SPG, Saskatchewan Pulse Growers.

Figure 6. Emergence percentage of Canadian Galium spp. populations in fall of (A) 2013 and (B) 2014. Error bars represent SE, and similar letters indicate values do not differ statistically at P = 0.05. SPG, Saskatchewan Pulse Growers.
This study showed that differences in base temperatures were greater between species than populations within species; all G. spurium populations had similar germination characteristics, even though they originated from different geographic regions across a wide area of western Canada. This implies that germination characteristics are genetically determined rather than environmentally selected. Because all western Canadian populations included in this study exhibited similar base temperatures, this study provides further evidence that these populations are composed only of G. spurium, as shown by De Roo et al. (Reference De Roo, Eckstein, Benaragama, Beattie and Willenborg2019). We suggest the low base temperature of G. spurium may, at least in part, be a significant contributor to its predominance in western Canada, where very low spring and fall temperatures are common. On the other hand, G. aparine has a base temperature of 4 C and would be expected to emerge later in the spring and cease emergence early in the fall. Given this, it should be better controlled with herbicides applied in early spring and late fall (postharvest). On the other hand, because G. spurium has a low base temperature, this species may be too large for adequate control with herbicides during pre-seed burnoff, particularly if plants have emerged late in the fall and overwintered into the spring.
Despite the similarity in base temperature, these populations were found to have differences in the rate of germination, which suggests populations would have different emergence characteristics. The field experiment confirmed that the emergence characteristics of western Canadian populations varied between populations within the year as well as between years and among seasons within a year. The variation we observed in emergence periodicity and percentages between populations is not uncommon, as other studies have shown that populations can exhibit plasticity in emergence characteristics (Griffith et al. Reference Griffith, Andonian, Weiss and Loik2014), including Galium spp. (Auge and Mahn Reference Auge and Mahn1988; Bain and Attridge Reference Bain and Attridge1988). This variability in emergence characteristics, combined with a low base temperature, could help explain the increase in the relative abundance of G. spurium in Canada over the past two decades (Leeson Reference Leeson2012; Leeson et al. Reference Leeson, Thomas, Hall, Brenzil, Andrews, Brown and Van Acker2005). Further research should examine whether the efficacy of weed control tactics as a function of emergence timing of Galium spp. explains the increased abundance of this species.
A common garden approach is useful, because it allows one to neutralize the impact of the environment on specific treatments. This study allowed the evaluation of whether genetic differences existed among the populations for emergence timing. Although specific genetic evaluations on emergence were not utilized in this study, it can be presumed that the origin of the Galium spp. seeds influenced genetic variation between populations. Genetic differences between populations often stem from characteristics of the maternal environment, which has been shown to influence seed characteristics such as dormancy in offspring through maternal conditioning (Roach and Wulff Reference Roach and Wulff1987). For example, shorter photoperiods in the maternal environment in Arabidopsis thaliana L. increased the germination response of progeny when cold stratification released the secondary dormancy (Munir et al. Reference Munir, Dorn, Donohue and Schmidt2001). Additionally, different collections of A. thaliana resulted in altered plasticity responses to combinations of variable maternal photoperiod and stratification, further increasing variation. In our study, Galium spp. were collected from as far as 800 km away from each other east to west and 400 km north to south. This could have affected the photoperiod exposure of maternal plants (populations that are apart north–south) and caused microclimatic variation (populations that are apart east–west), thereby selecting for genetic variation between populations. Although the base temperature did not differ among populations, emergence characteristics and periodicity did. This indicates selective forces have acted locally on G. spurium populations with respect to emergence characteristics, as we had originally hypothesized.
The high variability in emergence periodicity and time to median emergence observed in this study was likely due to the availability of moisture. In 2013, populations emerged 300 to 600 GDD after planting and took 670 to 750 GDD to reach 50% emergence, while in 2014, emergence began at 180 to 200 GDD and reached 50% emergence at 250 to 330 GDD. In both years, the majority of fall emergence occurred after significant rainfall events. The experimental sites experienced drought-like conditions in the early fall period of 2013, but rapid Galium emergence after significant rainfall events ensued in both years. Both temperature and moisture are key factors for germination and emergence timing (Bradford Reference Bradford2002; Gummerson Reference Gummerson1986), and a moisture threshold must be achieved for the emergence of Galium populations in the fall. Boyd and Van Acker (Reference Boyd and Van Acker2003) also found that Galium spp. recruitment is significantly reduced when soil moisture is limited. It has been shown that G. spurium (with a −1.2 MPa water potential requirement for germination) is more responsive to soil water potential than G. aparine (−2.5 MPa) and thus has a greater requirement for moisture (Royo-Esnal et al. Reference Royo-Esnal, Torra, Conesa, Forcella and Recasens2010a). Nevertheless, at least a portion of all tested populations in our study acted as winter annuals. Given that we observed both spring and fall emergence of these populations, G. spurium populations from across western Canada appear to be acting as facultative winter annuals and should be classified as such (Cici and Van Acker Reference Cici and Van Acker2009).
In summary, the current study identified a base temperature for germination of 2 C for most populations of G. spurium, while it was 4 C for G. aparine. At the same time, the optimum temperature (temperature for median germination) was greater (8.4 C) for G. aparine than for G. spurium populations (6.4 C). Galium spurium spring emergence was consistent across years, commencing at 223 GDD on June 3 in 2013 and 214 GDD on June 1 in 2014. The spring emergence characteristics differed among G. spurium populations, especially for emergence rate and median emergence time. Cumulative emergence also differed between G. spurium populations within years and between years in both spring and fall. All populations emerged in both the spring and fall, indicating populations of G. spurium in western Canada act as facultative winter annuals. The differences in emergence characteristics and periodicity observed in this study are likely to cause weed escapes that serve to replenish the seedbank and may partly explain the increasing abundance of Galium spp. in western Canada over the past two decades. From a management perspective, growers can expect G. spurium populations to start emerging in early May and well into the fall (unless secondary dormancy comes into play). Therefore, residual herbicides applied in the fall or the incorporation of cover crops would be expected to improve the control of G. spurium populations.
Acknowledgments
We acknowledge the research staff at Kernen, Aaron Gerein, and Gerry Stuber for all the technical assistance provided throughout the study. Further, we extend our gratitude to Saskatchewan Canola Development Commission and the Agriculture Development Fund of the Government of Saskatchewan for funding this project. The authors wish to declare that there is no conflict of interest.














