Introduction
Neogastropods known as “olive shells” and their relatives (Superfamily Olivoidea, sensu Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017) have been common components in many shallow marine communities for much of the past 50 million years. They include the families Olividae Latreille, Reference Latreille1825 (including the subfamilies Olivinae Latreille, Reference Latreille1825, Olivellinae Troschel, Reference Troschel1869, and Agaroniinae Olsson, Reference Olsson1956), Pseudolividae de Gregorio, Reference de Gregorio1890, Ancillariidae Swainson, Reference Swainson1840, Bellolividae Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, and Benthobiidae Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017 (Fig. 1). Olivoidea includes ~460 extant species (WoRMS, 2021). Ancillariidae, which is of particular interest in this paper, includes at least 100 extant species and subspecies (Kilburn, Reference Kilburn1981).

Figure 1. Phylogenetic relationships among living families of olivoid gastropods (based on Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017).
The earliest known members of Olivoidea appear to have been ancillariids, which may include the stem group of the larger clade (Riedel, Reference Riedel2000; Vermeij, Reference Vermeij2001, p. 507). The oldest ancillariids date to no later than the Late Cretaceous (Maastrichtian) (Sohl, Reference Sohl1964, p. 247–248; Kilburn, Reference Kilburn1981; Tracey et al., Reference Tracey, Todd, Erwin and Benton1993, p. 152). Kilburn (Reference Kilburn1981, p. 356) suggested that, based on poorly preserved material from the Cretaceous of Burma, Ancilla (Sparellina) poenitens Vredenburg (Reference Vredenburg1923, p. 251, pl. 14, figs 5a, b) “was either an Ancilla or an Ancillarina”. Voskuil et al. (Reference Voskuil2011) mentioned four other species of likely Cretaceous Ancillariidae: Tanimasanoria japonica (Kase, Reference Kase1990), Upper Cretaceous (lower Maastrichtian), Azenotani Mudstone Member, near Osaka, Japan; Eoancilla acutula Stephenson, Reference Stephenson1941, Upper Cretaceous (Maastrichtian), Owl Creek Formation, Mississippi and Kemp Clay, Texas; Tanimasanoria sp. (Basse, Reference Basse1932), Upper Cretaceous, Manja, Madagascar; and Oliva vetusta Forbes, Reference Forbes1846, Arriyalur Group, Upper Cretaceous (Maastrichtian), Pondicherry, India. Garvie (Reference Garvie2013, p. 61) indicated that a Lower Cretaceous (Albian) fauna from Texas described by McCall et al. (Reference McCall, Sprinkle and Molineaux2008) contains “a species that appears to be an ancestral Ancilla,” potentially extending the history of the group still further.
Numerous ancillariid species have been reported from the Paleocene and Eocene of Europe. Schnetler and Nielsen (Reference Schnetler and Nielsen2018, pl. 7, fig. 2) reported Ancilla from the Selandian of Denmark, and other European Paleogene species are discussed by Lozouet (Reference Lozouet1992), Pacaud et al. (Reference Pacaud, Merle and Pons2013), and Pacaud (Reference Pacaud2014). Eocene species from New Zealand are discussed by Olson (Reference Olson1956), Michaux (Reference Michaux1987, Reference Michaux1991), and Beu and Maxwell (Reference Beu and Maxwell1990). Kilburn (Reference Kilburn1981, p. 356) suggested that the “earliest-known true Ancilla is probably A. boettgeri Martin (Reference Martin1914, p. 133, pl. 2, fig. 67) of the upper Eocene Nanggoelan beds of Java.” The genus Ancillarina Bellardi, Reference Bellardi1882 (Selandian–Bartonian; type species Ancilla canalifera Lamarck, Reference Lamarck1803) is also present in these beds; it includes “Ancilla-like species with a similarly divided fasciolar band but a total lack of callus on the spire whorls and sutures” (Kilburn, Reference Kilburn1981, p. 356).
Numerous species of “oliviform” gastropods (sensu Kantor, Reference Kantor1991) have been recognized in the Paleogene of the U.S. Gulf Coastal Plain over almost 200 years, many of which previously have been allied to the “Bullia group” in the family Nassariidae, and placed in a variety of poorly defined genera. Previous work (Allmon, Reference Allmon1990) argued that these forms were not, in fact, related to Bullia s. s., but did not assign them to any other group. Here we review these forms and revise their generic and familial placement (Table 1). We place most of them in Olivoidea and present a phylogenetic analysis. Figure 2 shows the geological context and stratigraphic ranges of the species discussed here. We also discuss one species from the Eocene of France and the U.K., which we conclude is closely related to Coastal Plain species previously assigned to “Bullia.” The expanded calluses on the shells of some of the species discussed here make them almost spherical, and recently have been analyzed as examples of homoplasy (convergence and parallelism); the phylogenetic analysis presented here supports those conclusions (Pietsch et al., Reference Pietsch, Anderson, Maistros, Padalino and Allmon2021).
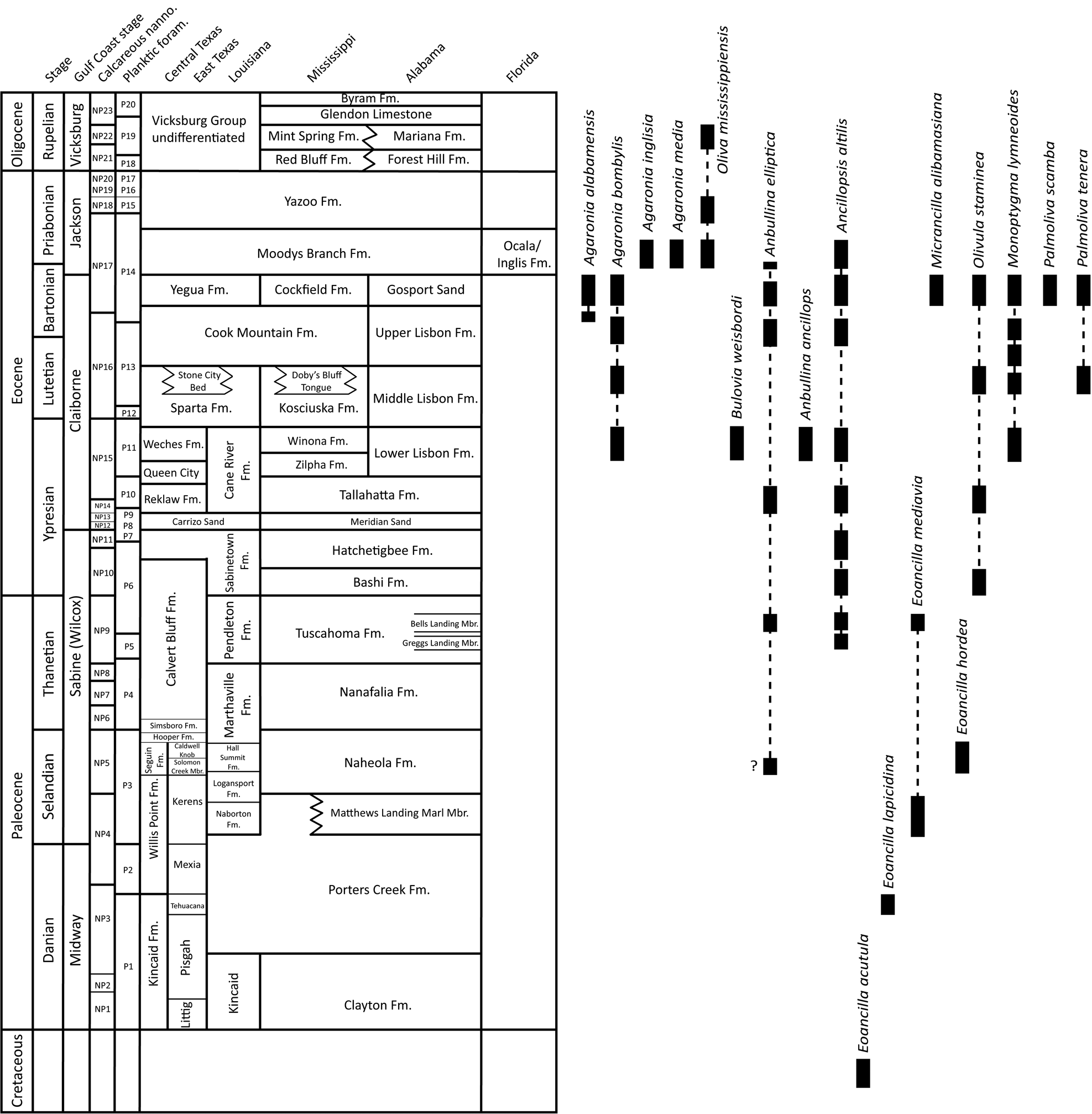
Figure 2. Paleocene and Eocene stratigraphic units in the U.S. Gulf Coastal Plain (based on Garvie, Reference Garvie2013; Dockery and Thompson, Reference Dockery and Thompson2016; Garvie et al., Reference Garvie, Goedert and Janssen2020) and stratigraphic ranges of the species discussed in this paper.
Table 1. Species of olivoid gastropods from the Paleocene and Eocene of the Gulf Coastal Plain (and U.K. and France) discussed in this paper.

Biology, shell morphology, and systematic characters
Living olivoids in general, and ancillariids in particular, are burrowing, sand-dwelling carnivores and scavengers (Kilburn, Reference Kilburn1981; Cyrus et al., Reference Cyrus, Rupert, Silva, Graf, Rappaport, Paladino and Peters2012; Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, p. 495; Robinson and Peters, Reference Robinson and Peters2018). The animal usually has a large foot with multiple folds that frequently extend far outside of, and may completely cover, the shell (Fig. 3) (Kilburn, Reference Kilburn1981; Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, p. 519–522). Some species use the foot to swim or “surf” in turbulent water (Wilson, Reference Wilson1969).

Figure 3. Live ancillariid gastropod showing large foot covering the entire shell. Amalda australis collected from New Zealand (illustration from https://en.wikipedia.org/wiki/Amalda_australis#/media/File:Amalda_australis1.jpg).
The shell of Olivoidea (Fig. 4) is callused to different degrees, the functional significance and mode of formation of which remain poorly understood (Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, p. 519; Pietsch et al., Reference Pietsch, Anderson, Maistros, Padalino and Allmon2021), and this has been described in numerous ways. Sometimes the callus is limited to the inner (parietal) wall of the aperture, but often it extends adapically, sometimes reaching or covering most or all of the spire, leaving only the protoconch and a part of the body whorl exposed. In many cases, the callus overlays or is associated with the sutures, which therefore may not be clearly visible externally. The callus may be uniform or consist of multiple layers, and these may vary throughout ontogeny. Kilburn (Reference Kilburn1977) and Kantor et al. (Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017) have distinguished “primary” from “secondary” callus, with the primary usually forming a band around the anterior portion of each spire whorl, parallel to the suture, and the secondary callus located on the parietal wall of the aperture (ventral side of the shell), sometimes extending onto the spire, where it can cover primary callus. The primary callus, in this terminology, is therefore the secondary callus of earlier ontogenetic stages. Here we use a slightly different terminology, distinguishing spire callus from body whorl callus (Fig. 4.2), with the former forming a band on the anterior (abapical) part of each spire whorl, causing callusing associated with the sutures. For the body whorl callus, we distinguish the lateral extent (over the body whorl) from the posterior extent (extending posteriorly from the aperture toward the spire, sometimes covering the suture). Posterior body whorl callus will become spire callus as a subsequent whorl is added. Extensive posterior body whorl callus on subsequent whorls may then overlie spire callus of previous whorls. “Extreme parietal callus” (EPC) refers to the condition in which callus covers >50% of the ventral surface of the body whorl, which occurs on both olivoid and non-olivoid gastropods (Pietsch et al., Reference Pietsch, Anderson, Maistros, Padalino and Allmon2021).
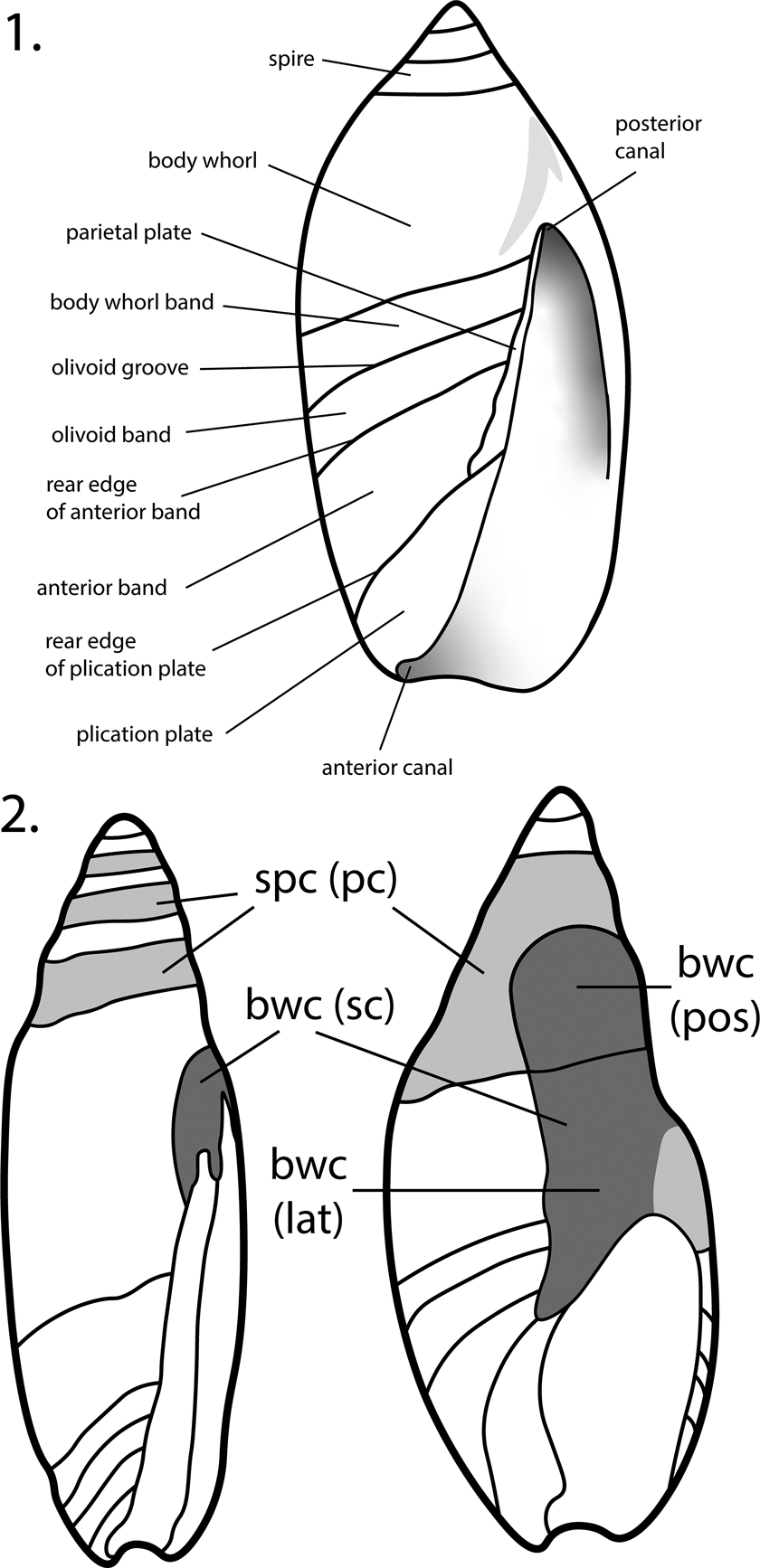
Figure 4. Shell morphological terminology used in this paper. (1) Modified from Kilburn (Reference Kilburn1981). (2) Terminology of the callus; lighter shading is spire callus (spc); darker shading is body whorl callus (bwc); bwc (lat) = body whorl callus, lateral; bwc (pos) = body whorl callus, posterior; (sc) = secondary callus; (pc) = primary callus; sc and pc are the terminology of Kantor et al. (Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017); bwc (sc) means that the terms “body whorl callus” and “spire callus” are synonymous; spc (pc) means that the terms “spire callus” and “primary callus” are synonymous. See text for further discussion.
The anterior end of the olivoid shell bears a complex structure commonly referred to as the fasciole, formed by successive accretions of the anterior siphonal notch, which surrounds the anterior canal and its associated callus (Tursch and Greifeneder, Reference Tursch and Greifeneder2001, p. 114–115; Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, p. 513–519). In all olivoids, the fasciole includes several more or less discrete zones or bands, which have been variously named in the literature (e.g., Kilburn, Reference Kilburn1981; López et al., Reference López, Montoya and López1988; Tursch and Greifeneder, Reference Tursch and Greifeneder2001; Pacaud et al., Reference Pacaud, Merle and Pons2013). Here we use the terminology proposed by Kantor et al. (Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017) (Fig. 4.1). The structure of the fasciole is important in discriminating olivoid shells from those of other neogastropods. For example, all representatives of the family Nassariidae lack the olivoid and anterior bands and show at least a slight terminal fold on the end of the fasciole (Allmon, Reference Allmon1990; Galindo et al., Reference Galindo, Puillandre, Utge, Lozouet and Bouchet2016).
Species of Ancillariidae can be distinguished conchologically from other olivoids by characters of callusing on the shell (Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, p. 535). Ancillariids are generally more strongly callused than other Olivoidea (but see Tursch and Greifeneder, Reference Tursch and Greifeneder2001, p. 107–110), especially on the body whorl, and the suture between the spire and body whorl is usually overlaid with callus to varying degrees.
In this paper, we use the conception of fossil species advocated by Allmon (Reference Allmon, Allmon and Yacobucci2016), which includes reference to morphological differences between extant species of a clade. The value of shell characters for recognition of species and genera in living olivoids remains unclear and is likely variable across the group. A number of modern olivid genera are distinguished only by non-shell characters. For example, some species of Oliva can be distinguished from species of Agaronia and Ancilla only by the radula (Zeigler and Porreca, Reference Zeigler and Porreca1969, p. 21). Kantor and Bouchet (Reference Kantor and Bouchet2007, p. 27) described a new genus of Recent olivids, Calyptoliva, noting that it differs from the very similar Belloliva mainly “by the absence of a mantle filament and the presence of a mantle lobe.” Tursch and Greifeneder (Reference Tursch and Greifeneder2001) argued that morphospecies of Oliva are highly variable but frequently recognizable. Michaux (Reference Michaux1987) showed that species of Amalda distinguished by electrophoresis also were distinguishable morphologically, but Kantor et al. (Reference Kantor, Fedosov, Puillandre and Bouchet2016) found that several molecularly distinct species of Ancilla were morphologically cryptic. Thus, it is possible that morphospecies recognized here based solely on fossils include more than a single biological species.
Phylogenetic analysis
Methods
Our preliminary phylogenetic analysis included 19 Paleocene–Eocene species representing three genera of Olividae and seven genera of Ancillariidae. We also included the Recent species Agaronia testacea (Lamarck, Reference Lamarck1811) and Oliva sericea (Röding, Reference Röding1798) for comparison. We used type and figured material to code each species for the following discrete character suites: (1) suture; (2) callus; (3) bands (including the olivoid, anterior, subsutural, and body whorl bands); (4) columella and plications; (5) ornamentation and texture; and (6) shell shape. In instances where museum specimens were unavailable, taxa were coded using primary taxonomic figures and literature. Species were coded for 27 discrete characters (10 binary and 17 multistate) (Table 2) that were selected to capture morphological variation among the clades and are inferred to represent homologous structures among sampled taxa. Eoancilla was designated as the outgroup because the genus is a putative ancestor of the other ancillariids (Garvie, Reference Garvie2013).
Table 2. Characters scored for phylogenetic analysis (see Figure 4 for shell terminology).

A parsimony analysis was conducted in PAUP* v. 4.0a147 (Swofford, Reference Swofford2003) using a heuristic search with 10,000 random addition sequences. TBR (tree bisection reconnection) was used for the branch-swapping algorithm with no reconnection limit and collapsing all branches with a maximum branch length of zero. All characters were left unordered and equally weighted. Nexus files utilized are provided as Supplement 1. Values for consistency index (CI) and retention index (RI) were recorded for recovered trees, and bootstrap values and Bremer support were calculated using PAUP*.
Results
The parsimony analysis recovered 82 most parsimonious trees with tree lengths of 111 steps (CI 0.485, RI 0.541). Strict and semi-strict consensus of the most parsimonious trees resulted in a tree topology with poor resolution (Fig. 5.1). The 50% majority rule consensus tree (Fig. 5.2) gives better resolution and was plotted against the observed stratigraphic ranges of sampled genera to produce a time-scaled phylogeny (Fig. 20).

Figure 5. Phylogenetic relationships among the fossil species discussed in this paper. Numbers on branches are the number of trees with that arrangement. (1) Strict consensus of 82 equally parsimonious trees. (2) 50% majority-rule consensus of 82 equally parsimonious trees. Sister taxa are relatively well supported with four of the six pairs appearing in all of the most parsimonious trees, although support was lowest for the Palmoliva n. gen. pair. As the only representatives of their genera, M. alibamasiana and B. weisbordi support their genus’ distinction from the other genera (Agaronia, Oliva, Anbullina, Monoptygma, and Palmoliva n. gen.) in their larger clade. See text for further discussion.
Material
Repositories and institutional abbreviations
Academy of Natural Sciences of Drexel University, Philadelphia, PA, USA (ANSP); Alabama Museum of Natural History, Tuscaloosa, AL, USA (ALMNH); Bureau of Economic Geology, Austin, TX, USA (BEG; collections now referred to as NPL); Florida Geological Survey, Tallahassee, FL, USA (FGS; collection now at Florida Museum of Natural History, Gainesville); Field Museum, Chicago, IL, USA (FMNH); Geological Survey of Alabama (Type Cabinet), Tuscaloosa, AL, USA (GSA (GSATC)); Museum of Geosciences, Louisiana State University, Baton Rouge, LA, USA (LSU); Department of Invertebrate Paleontology, Museum of Comparative Zoology, Harvard University, Cambridge, MA, USA (MCZIP); Mississippi Geological Survey collection, Jackson, MS, USA (MGS); Muséum National d'Histoire Naturelle, collection de Paléontologie, Paris, France (MNHN); Paleontological Research Institution, Ithaca, NY, USA (PRI); Non-Vertebrate Paleontological Laboratory, University of Texas, Austin, TX, USA (NPL = NVPL of some previous authors); Texas Memorial Museum, Austin, TX, USA (TMM; collections now referred to as NPL); Université Claude Bernard, Lyon, France (UCBL); Florida Museum of Natural History, University of Florida, Gainesville, FL, USA (UF); National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (USNM).
Systematic paleontology
In the species accounts below, morphological terminology follows Figure 4. Specimen measurements for all species are given in Table 3.
Table 3. Measurements for representative specimens.

Phylum Mollusca Linnaeus, Reference Linnaeaus1758
Class Gastropoda Cuvier, Reference Cuvier1797
Family Olividae Latreille, Reference Latreille1825
Subfamily Olivinae Latreille, Reference Latreille1825
Genus Oliva Bruguière, Reference Bruguière1789
Type species
Voluta oliva Linnaeus, Reference Linnaeaus1758; subsequent monotypy by Lamarck, Reference Lamarck1799.
Remarks
Conchologically, the genus Oliva is distinguished by having a “[p]lication plate subdivided into parietal plate, shoe and belt. Filament channel well defined, eventually overlaid by primary spire callus on upper spire whorls, but free at least on last whorl” (Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, p. 526). Tursch and Griefender (Reference Tursch and Greifeneder2001) recognized 74 extant morphospecies.
Oliva mississippiensis Conrad, Reference Conrad1848
Figure 6.1, 6.2
- Reference Conrad1848a
Oliva mississippiensis Conrad, p. 289.
- Reference Conrad1848b
Oliva mississippiensis Conrad, p. 119, pl. 3, figs. 6, 38.
- Reference Conrad1865a
Lamprodoma Mississippiensis; Conrad, p. 22.
- Reference Conrad1866
Lamprodoma Mississippiensis; Conrad, p. 30.
- Reference Casey1903
Oliva mississippiensis; Casey, p. 281.
- Reference Gardner1945
Oliva mississippiensis; Gardner, p. 216.
- Reference Harris and Palmer1947
Agaronia mississippiensis; Harris and Palmer, p. 410, pl. 63, figs. 17–19.
- Reference Palmer and Brann1966
Agaronia mississippiensis; Palmer and Brann, p. 487.
- Reference Dockery1977
Agaronia mississippiensis; Dockery, p. 79, pl. 11, fig. 3A, B.
- Reference Drez1981
Strephonella mississippiensis; Drez, p. 105.
- Reference MacNeil and Dockery1984
Oliva (Strephonella) mississippiensis; MacNeil and Dockery, p. 157, pl. 33, figs. 17, 18, pl. 56, figs. 13, 14.
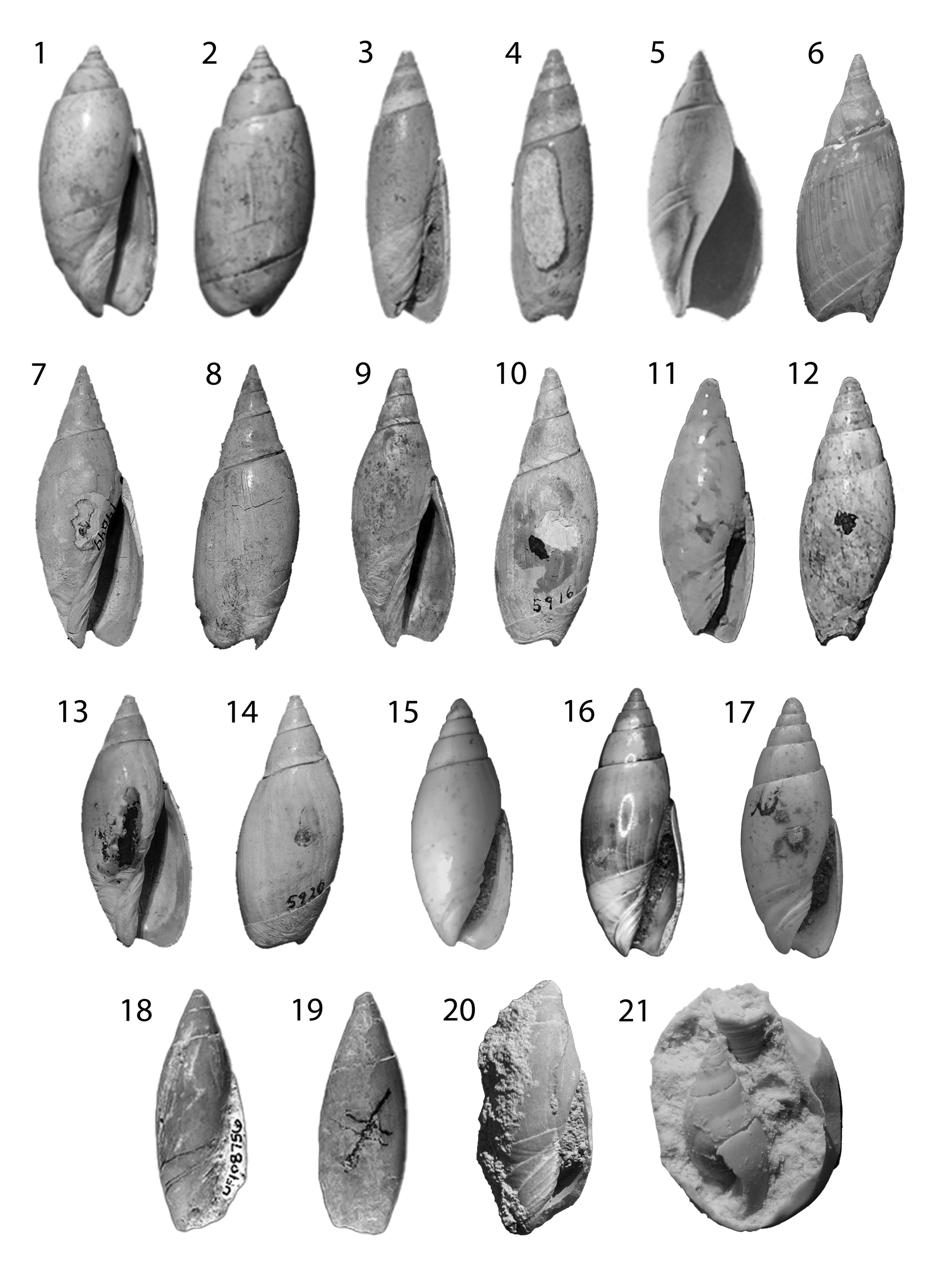
Figure 6. Oliva, Bulovia, and Agaronia. (1, 2) Oliva mississippiensis lectotype ANSP 13450; height 27.1 mm. (3, 4) Agaronia bombylis (Oliva bombylis lectotype ANSP 14627); height 22.3 mm. (5, 6) Bulovia weisbordi holotype PRI 3048; height 22.2 mm. (7–14) Agaronia alabamensis: (7, 8) Oliva alabamensis lectotype ANSP 14649; height 41 mm. (9, 10) Oliva greenoughi holotype ANSP 5916; height 42 mm. (11, 12) Oliva gracilis holotype ANSP 5914. (13, 14) Oliva dubia holotype ANSP 5920; height 39 mm. (15–17) Agaronia media: (15) lectotype GSA-I17375; height 7 mm. (16) hypotype MGS 2074; height 19.5 mm. (17) hypotype (Harris and Palmer, Reference Harris and Palmer1947) PRI 20009; height 9 mm. (18–21) Agaronia inglisia: (18, 19) holotype UF 108756; height 29.4 mm. (20) UF 5455; height 38 mm. (21) UF 66680 silicone cast of mold in limestone. Cast measures 40 × 50 mm.
Type material
Lectotype ANSP 13450; hypotypes (Harris and Palmer, Reference Harris and Palmer1947, pl. 63) PRI 20010, 20011, 20012.
Occurrence
Louisiana: upper Eocene (Bartonian–Priabonian), Moodys Branch and Yazoo formations (Loc. LA-GR-1); Mississippi: lower Oligocene (Rupelian), Mint Springs Formation (Loc. MS-WA-23).
Remarks
Drez (Reference Drez1981) and Petuch and Sargeant (Reference Ponder and Warén1986, p. 10–11) identified this species as the earliest olivid; Drez placed it in the genus Strephonella, and Petuch and Sargeant in Oliva. MacNeil and Dockery (Reference MacNeil and Dockery1984, p. 157) placed Strephonella as a subgenus of Oliva, and recognized a second similar species, O. (Strephonella) affluens Casey, Reference Casey1903, in the Moodys Branch Formation. Both of these forms appear to be closer to Oliva than to Agaronia, due to their inflated body whorl, wide and complex plication plate bearing sharp plications, and presence of a parietal plate posterior of the plication plate (see Tursch and Greifeneder, Reference Tursch and Greifeneder2001, p. 112). Given its similarity to Agaronia, it is possible that this species (and therefore the clade Olivinae) is derived from a species of that genus (see further discussion below).
Subfamily Agaroninae Olsson, Reference Olsson1956
Genus Agaronia Gray, Reference Gray and Beechey1839
Type species
Voluta hiatula Gmelin, Reference Gmelin and Gmelin1791, by monotypy.
Remarks
Conchologically, the genus Agaronia is distinguished by having a “[p]lication plate not distinctly subdivided, with distinct spiral plicae. Olivoid groove present, shallow. Olivoid band differing or not in color from cloak of last whorl. Filament channel well defined, free on most spire whorls” (Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, p. 526). The shell is less glossy than in Oliva, with a taller, more acuminate spire and slightly flaring outer apertural lip. López et al. (Reference López, Montoya and López1988, p. 296) suggested that the “count of lirae [on the inner lip of the aperture]” and the “height and shape of the spire” provide useful specific characters in Agaronia.
Agaronia was originally described by Gray (Reference Gray and Beechey1839) as a subgenus of Olivancillaria, which was accepted by some later authors. It was elevated to a separate genus by Olsson (Reference Olsson1931), and this has been more widely accepted. Agaronia is most often placed in a monotypic subfamily, Agaroniinae (Olsson, Reference Olsson1956; Ponder and Warén, Reference Ponder and Warén1988; Sterba, Reference Sterba2003; Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017), although Bouchet and Rocroi (Reference Bouchet and Rocroi2005) and Cilia (Reference Cilia2012) placed it in Olivinae. The majority of the ~20 described extant species occur on low-latitude coasts of west Africa, western Central America, and the eastern Indian Ocean (see López et al., Reference López, Montoya and López1988; Cilia, Reference Cilia2012). The oldest recognized species is Agaronia bombylis (Conrad, Reference Conrad1833) from the Lower Eocene (Ypresian) (see below).
We recognize four species of Agaronia in the Paleogene of the Coastal Plain and Florida: A. alabamensis (Conrad, Reference Conrad1833), A. bombylis (Conrad, Reference Conrad1833), A. media (Meyer, Reference Meyer1885), and A. inglisia (Palmer in Richards and Palmer, Reference Richards and Palmer1953). We follow Garvie (Reference Garvie2021) in placing the species A. mediavia (Harris, Reference Harris1896) in the genus Eoancilla Stephenson, Reference Stephenson1941.
Our phylogenetic analysis (see below) indicates that Agaronia is paraphyletic and includes the ancestry of Oliva mississippiensis. Since a thorough phylogenetic analysis of all fossil and extant Agaronia is beyond the scope of this paper, we use the name Agaronia sensu lato to include all Coastal Plain Paleogene species.
Agaronia alabamensis (Conrad, Reference Conrad1833)
Figure 6.7–6.14
- non Reference Broderip and Sowerby1829
Oliva gracilis; Broderip and Sowerby, p. 379.
- Reference Conrad1833
Oliva alabamensis Conrad, p. 32.
- Reference Lea1833
Oliva Greenoughi Lea, p. 183, pl. 6, fig. 197.
- Reference Lea1833
Oliva dubia Lea, p. 183, pl. 6, fig. 198.
- Reference Lea1833
Oliva Phillipsii Lea, p. 184, pl. 6, fig. 199.
- Reference Lea1833
Oliva gracilis Lea, p. 182 [in part], pl. 6, fig. 196.
- Reference Conrad and Morton1834b
Oliva Phillipsii; Conrad, p. 5.
- Reference Conrad and Morton1834b
Oliva alabamensis; Conrad, p. 5.
- Reference Conrad1835
Oliva alabamensis; Conrad, p. 41, pl. 16, fig. 3.
- non Reference Deshayes1835
Ancillaria dubia; Deshayes, p. 734.
- non Reference Deshayes1835
Oliva nitidula Deshayes, p. 741.
- Reference Duclos1835
Oliva alabamiensis [sic]; Duclos, pl. 18, figs. 13, 14.
- Reference Duclos1844
Oliva alabamiensis [sic]; Duclos, p. 11, pl. 20, figs. 13, 14.
- Reference Conrad1846
Oliva alabamensis; Conrad, p. 220.
- Reference Lea1849
Oliva alabamensis; Lea, p. 103.
- Reference Lea1849
Oliva Greenoughi; Lea, p. 103.
- Reference Lea1849
Oliva dubia; Lea, p. 103.
- Reference Lea1849
Oliva Phillipsii; Lea, p. 103.
- Reference Lea1849
Oliva gracilis; Lea, Reference Lea1849, p. 103.
- Reference d'Orbigny1850
Oliva Phillipsii; d'Orbigny, p. 351.
- Reference d'Orbigny1850
Oliva alabamensis; d'Orbigny, p. 351.
- Reference Tuomey1858
Oliva alabamensis; Tuomey, p. 266.
- Reference Conrad1865a
Lamprodoma alabamiensis [sic]; Conrad, p. 22.
- Reference Conrad1865a
Lamprodoma gracilis; Conrad, p. 22.
- Reference Conrad1865a
Lamprodoma Phillipsii; Conrad, p. 22.
- Reference Conrad1866
Lamprodoma alabamiensis [sic]; Conrad, p. 17.
- Reference Conrad1866
Lamprodoma gracilis; Conrad, p. 17.
- Reference Conrad1866
Lamprodoma Phillipsii; Conrad, p. 17.
- Reference de Gregorio1890
Oliva Phillipsii; de Gregorio, p. 53, pl. 3, fig. 66 [copied Lea, Reference Lea1833].
- Reference de Gregorio1890
Oliva gracilis; de Gregorio, p. 52, pl. 3, fig. 50, 51 [copied Lea, Reference Lea1833].
- Reference de Gregorio1890
Oliva nitidula; de Gregorio, p. 51, pl. 3, figs. 36–42.
- Reference de Gregorio1890
Oliva mitreola Lamarck; de Gregorio, p. 51, pl. 3, fig. 47, 48 [not Lamarck, Reference Lamarck1803, p. 391].
- Reference de Gregorio1890
Oliva antelucana; de Gregorio, p. 54, pl. 3, figs. 58–61.
- Reference de Gregorio1890
Oliva pinaculica; de Gregorio, p. 54, pl. 3, figs. 63–65.
- Reference Heilprin1891
Oliva gracilis; Heilprin, p. 397.
- Reference Cossmann1893
Olivella alabamiensis [sic]; Cossmann, p. 40.
- Reference Cossmann1893
Olivella Phillipsi; Cossmann, p. 40.
- Reference Harris1895b
Oliva alabamensis; Harris, p. 3.
- Reference Cossmann1899
Olivancillaria (Agaronia) alabamiensis [sic]; Cossmann, p. 51.
- non Reference Cossmann1899
Oliva parisiensis; Cossmann, p. 178.
- Reference Cooke1926a
Oliva alabamensis; Cooke, pl. 95, fig. 5.
- Reference Davies1935
Olivancillaria (Agaronia) alabamiensis [sic]; Davies, p. 306.
- Reference Palmer1937
Agaronia alabamensis; Palmer, p. 431, pl. 68, figs. 14–16, 18–22, pl. 89, fig. 5.
- non Reference Palmer1937
Oliva parnensis; Palmer, p. 431.
- Reference Shimer and Shrock1944
Olivella (Agaronia) alabamensis; Shimer and Shrock, p. 511, pl. 210, fig. 13 [copied Conrad, 1935a].
- Reference Harris and Palmer1947
Agaronia alabamensis; Harris and Palmer, p. 408.
- Reference Brann and Kent1960
Agaronia alabamensis; Brann and Kent, p. 29.
- Reference Glibert1960
Olivancillaria (Agaronia) alabamiensis [sic]; Glibert, p. 19.
- Reference Palmer and Brann1966
Agaronia alabamensis; Palmer and Brann, p. 484.
Type material
Lectotype + 8 specimens ANSP 14649; holotype Oliva greenoughi ANSP 5916; holotype Oliva dubia ANSP 5920; holotype Oliva phillipsii ANSP 5926; holotype Oliva gracilis ANSP 5914; hypotypes Agaronia alabamensis (Palmer, Reference Palmer1937) PRI 3288, 3289, 3290, 3291, 3292, 3293.
Occurrence
Alabama: middle Eocene (Lutetian–Bartonian), Lisbon Formation, Gosport Sand (Locs. AL-CL-1, AL-MO-2a, b); South Carolina: middle Eocene (Bartonian), McBean Formation (Loc. SC-OR-1); Texas, Louisiana, Mississippi: middle Eocene (Lutetian–Bartonian), Cook Mountain Formation (see Palmer, Reference Palmer1937, p. 434).
Revised description
Shell large. Protoconch of one and a half or two smooth whorls, and the sutures are indistinct, not channeled as on teleoconch whorls. Spire up to ~0.25 total height in adults, shorter in juveniles. Sutures channeled. Shell smooth. Callus extends only slightly laterally out of aperture over body whorl and posteriorly toward spire, creating narrow callus band, often of lighter color, above sutures. Aperture ~0.6 total height, narrowing posteriorly into a sharp channel and widening anteriorly to a broad channel. Olivoid band distinct and continuing on dorsal side, with sharp posterior margin. Another band often present posterior to olivoid band, consisting of smooth stripe or slight concavity on body whorl, bounded posteriorly by slight rounded ridge. Anterior band distinct, separated from olivoid band by faint line. Plication plate distinct, slightly inflated, bearing multiple plications, separated from anterior band by deep groove.
Other material examined
MCZIP 29246 (5 specimens); PRI 14142 (172 specimens); PRI 104503 (2 specimens); PRI 104693 (2 specimens).
Remarks
This species is one of the most common large gastropods in the upper middle Eocene Gosport Sand of Alabama (CoBabe and Allmon, Reference CoBabe and Allmon1994; Pietsch et al., Reference Pietsch, Harrison and Allmon2016). Kelley and Swan (Reference Kelley and Swann1988) noted that Agaronia alabamensis shows a single pigmented spiral band parallel to the suture. Gosport specimens are larger than those from other stratigraphic units (Haveles and Ivany, Reference Haveles and Ivany2010).
Agaronia bombylis (Conrad, Reference Conrad1833)
Figure 6.3, 6.4
- non Reference Broderip and Sowerby1829
Oliva gracilis; Broderip and Sowerby, p. 379 [fide Palmer and Brann, Reference Palmer and Brann1966, p. 486].
- Reference Conrad1833
Oliva bombylis Conrad, p. 32.
- Reference Lea1833
Oliva constricta Lea, p. 182, pl. 6, fig. 195.
- Reference Lea1833
Oliva gracilis; Lea, p. 182 (part).
- Reference Conrad1835
Oliva bombylis; Conrad, p. 42, pl. 16, fig. 4.
- Reference Duclos1835
Oliva bombylis; Duclos, pl. 18, figs. 7, 8.
- Reference Conrad1846
Oliva bombylis; Conrad, p. 220.
- Reference Lea1849
Oliva bombylis; Lea, p. 103.
- Reference Lea1849
Oliva constricta; Lea, p.103.
- Reference d'Orbigny1850
Oliva bombylis; d'Orbigny, p. 351.
- Reference Conrad1865a
Lamprodoma bombylis; Conrad, p. 22.
- Reference Conrad1866
Lamprodoma bombylis; Conrad, p. 17.
- Reference Heilprin1879
Oliva bombylis; Heilprin, p. 223.
- non Reference Aldrich1886
Oliva bombylis; Aldrich, p. 53 [fide Palmer and Brann, Reference Palmer and Brann1966, p. 485].
- non Reference Aldrich1886
Oliva gracilis; Aldrich, p. 56 [fide Palmer and Brann, Reference Palmer and Brann1966, p. 486].
- Reference de Gregorio1890
Oliva bombylis; de Gregorio, p. 52, pl. 3, fig. 49, [copied Conrad, Reference Conrad1835], fig. 52 [copied Oliva constricta Lea, Reference Lea1833].
- Reference Cossmann1893
Olivella bombylis; Cossmann, p. 40.
- Reference Harris1895b
Oliva bombylis; Harris, p. 8.
- Reference Cossmann1899
Olivella bombylis; Cossmann, p. 54.
- Reference Palmer1937
Agaronia bombylis; Palmer, p. 434, pl. 68, figs. 12, 13.
- Reference Glibert1960
Olivancillaria (Agaronia) bombylis; Glibert, p. 19.
- Reference Palmer and Brann1966
Agaronia bombylis; Palmer and Brann, p. 485.
Type material
Lectotype ANSP 14627; holotype Oliva constricta ANSP 5911; hypotypes (Palmer, Reference Palmer1937) PRI 3286, 3287.
Occurrence
Texas: middle Eocene (Ypresian–Bartonian), Weches Formation, Stone City Formation, Cook Mountain Formation (Locs. TX-BA-1); Alabama: middle Eocene (Lutetian–Bartonian), Upper Lisbon Formation, Gosport Sand (Locs. AL-MO-2a, AL-MO-5); South Carolina: middle Eocene (Bartonian), McBean Formation (Loc. SC-OR-1).
Revised description
Shell small and elongate. Protoconch of one and one-half or two whorls. Spire 0.2–0.25 total height. Sutures channeled. Callus extends only slightly laterally out of aperture over body whorl and posteriorly toward spire, creating wide callus band, usually of lighter color, above sutures. Shell smooth. Aperture 0.5–0.6 total height. Aperture narrow, pinching to sharp channel posteriorly and wider anteriorly. Olivoid band distinct, bounded posteriorly by a sharp line or groove. Anterior band distinct, bounded posteriorly by rounded ridge. Plication plate distinct with multiple plications. Columellar terminus pointed.
Other material examined
PRI 56684 (1 specimen), PRI 56028 (35 specimens).
Remarks
As noted by Palmer (Reference Palmer1937, p. 434–435), juvenile A. alabamensis and A. bombylis may be confused with each other, but are distinguishable by overall shell shape, with A. bombylis being consistently more slender in its bodywhorl. In A. bombylis, the callus band above the suture is also relatively wider and more conspicuous. Agaronia bombylis does not attain the size or abundance of A. alabamensis. Kelley and Swan (Reference Kelley and Swann1988) noted that Agaronia bombylis shows a single pigmented spiral band parallel to the suture. Palmer (Reference Palmer1937, p. 435; Palmer and Brann, Reference Palmer and Brann1966, p. 486) stated that it occurs in the Weches and Stone City formations of Texas, but we have not been able to locate these specimens in the PRI collection. These reported occurrences are important because they considerably extend the stratigraphic range of the species downward (see Fig. 2).
Agaronia inglisia Palmer in Richards and Palmer, Reference Richards and Palmer1953
Figure 6.18–6.21
- Reference Richards and Palmer1953
Agaronia inglisia Palmer in Richards and Palmer, p. 31, pl. 6, figs. 5, 8, 13.
- Reference Palmer and Brann1966
Agaronia inglisia; Palmer and Brann, p. 486.
Type material
Holotype FGS I-7604 (UF 108756); paratypes FGS I-7605 (UF 108760), FGS I-7606 (UF 108764).
Occurrence
Florida: upper Eocene (Bartonian–Priabonian), Inglis Formation (Loc. FL-LE-1).
Revised description
Shell medium-sized. Protoconch bulbous, of ~1.5 whorls. Spire <0.2 total height. Sutures deeply grooved. Callus extends posteriorly from aperture about half-way to suture. Body whorl smooth, unsculptured. Aperture narrow, ~0.6 total height. Olivoid and anterior bands marked by strong grooves. Plication plate relatively wide.
Other material examined
UF 5396 (1 specimen), UF 5448 (2 specimens), UF 5455 (2 specimens), UF 6794 (2 specimens), UF 12753 (1 specimen), UF 19132 (2 specimens), UF 66680 (1 specimen), UF 106738 (1 specimen), UF 107439 (1 specimen).
Remarks
This is the only species of Agaronia known from the Eocene of Florida.
Agaronia media (Meyer, Reference Meyer1885)
Figure 6.15–6.17
- Reference Meyer1885
Oliva media Meyer, p. 465.
- Reference Cooke1926b
Olivella jacksonensis Cooke, p. 134, fig. 5.
- Reference Harris and Palmer1947
Agaronia jacksonensis; Harris and Palmer, pl. 63, fig. 10.
- Reference Harris and Palmer1947
Agaronia media; Harris and Palmer, p. 407, pl. 63, figs. 7, 9, 11–13.
- Reference Palmer and Brann1966
Agaronia media; Palmer and Brann, p. 486.
- Reference Dockery1977
Agaronia media; Dockery, p. 79, pl. 11, figs. 1A, B, 2A, B.
Type material
Syntypes and lectotype GSA-I17375 (includes “holotype” listed in Palmer and Brann, Reference Palmer and Brann1966, p. 486, as GSATC 78); hypotypes (Harris and Palmer, Reference Harris and Palmer1947) PRI 20009, (Dockery, Reference Dockery1977) MGS 2073, 2074.
Occurrence
Mississippi: upper Eocene (Bartonian–Priabonian), Moodys Branch Formation (Locs. MS-CL-2, MS-HI-3, MS-HI-4); Arkansas, Louisiana, Texas: (see Palmer and Brann, Reference Palmer and Brann1966, p. 486).
Revised description
Shell small. Protoconch spherical. Spire ~0.25 total height. Suture strongly channeled. Callus minimal. Shell smooth, shiny, unsculptured. Aperture narrow, ~0.5 total height. Olivoid and anterior bands well marked. Plication plate narrow.
Remarks
Meyer (Reference Meyer1885) did not figure the species when he described it, nor did he designate a type specimen. According to Palmer (in Harris and Palmer, Reference Harris and Palmer1947, p. 408), the collection in the Alabama Museum of Natural History included eight specimens labeled as “types,” probably by Alabama State Paleontologist Winnie McGlamery. From among these, Palmer selected one as a lectotype. Unfortunately, this specimen was not kept separate and was recombined with 52 others in a single vial, all being given the number GSATC 78; they have since been given the new number GSA-I17375 (T.L. Harrell, personal communication, October 21, 2021). From these, one specimen was identified by T.L. Harrell as the most likely to have been Palmer's lectotype, and it is figured here (Fig. 6.15). Harris and Palmer (Reference Harris and Palmer1947, p. 407) reported this species to be “very common” in the Moodys Branch Formation at Jackson, MS.
Genus Bulovia Palmer, Reference Palmer1937
Type species
Bulovia weisbordi Palmer, Reference Palmer1937, by original designation.
Remarks
The shell is very distinctive, which led Palmer to put it in a new monotypic genus. It resembles species of Agaronia in its strong olivoid and anterior bands, aperture shape, and strongly channeled suture, and we have been tempted to place it in Agaronia. In our phylogenetic analyses (Fig. 5), however, Bulovia weisbordi consistently falls outside of Agaronia because of the unique shape of the anterior end of the shell, especially the deep groove separating the plication plate and anterior band. Despite it being represented by a single specimen, we therefore retain it in Palmer's monotypic genus Bulovia.
Bulovia weisbordi Palmer, Reference Palmer1937
Figure 6.5, 6.6
- Reference Palmer1937
Bulovia weisbordi Palmer, p. 293, pl. 40, figs. 10, 11.
- Reference Wenz and Schindewolf1943
Bulovia weisbordi; Wenz, p. 1226, fig. 3489 [copied Palmer, Reference Palmer1937, pl. 40, fig. 10].
- Reference Brann and Kent1960
Bulovia weisbordi; Brann and Kent, p. 140.
- Reference Palmer and Brann1966
Bulovia weisbordi; Palmer and Brann, p. 546.
- Reference Cernohorsky, Wagner and Abbott1982
Bullia (Bulovia) weisbordi; Cernohorsky, p. 17.
- Reference Allmon1990
Bulovia weisbordi; Allmon, p. 60, pl. 9, fig. 5.
Type material
Holotype PRI 3048.
Revised description
Shell small and slender. Protoconch unknown. Spire ~0.2 total height. Sutures are callused, with a prominent sutural band and the last suture deeply channeled. Callus extends posteriorly from aperture almost to suture, and laterally over more than half of body whorl. Growth lines have prominent relief on spire and body whorl beneath a prominent smooth subsutural band. Aperture wide, just over half total height, with a wide anterior canal. Olivoid and anterior bands very prominent. Plication plate narrow and smooth, separated from anterior band by a very deep groove, almost a pseudoumbilicus.
Occurrence
Texas: middle Eocene (Ypresian), Weches Formation (Loc. TX-BA-1).
Remarks
Bulovia weisbordi is known only from its holotype specimen, from the now-inaccessible Smithville outcrop of the Weches Formation in Texas.
Family Ancillariidae Swainson, Reference Swainson1840
(= Ancillinae Adams and Adams, Reference Adams and Adams1853)
Diagnosis
(Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017, p. 530) “Shell glossy or mat, lacking periostracum, fusiform to narrowly fusiform, with high last whorl, and medium broad-to-narrow aperture tapering adapically. Siphonal canal absent, anterior end of shell distinctly notched. Anterior shell end with well-defined anterior band, raised above the shell cloak and often strongly shagreened. Olivoid groove present (at least in some species) in all genera. Plication plate limited to columella, usually with spiral plicae. Primary spire callus well defined, covering most of, or even completely, the shell. Secondary spire callus from poorly defined to very strong. Suture always overlaid by the callus.”
Genus Ancillopsis Conrad, Reference Conrad1865
Type species
Ancillopsis altilis Conrad, Reference Conrad1865a, by subsequent designation (Cossmann, Reference Cossmann1899, p. 45).
Diagnosis
Shell medium to very large. Spire in juveniles one-fourth or less of total height; spire in adults may be only a tiny point above the expanded callus, which may make shell subspherical. Aperture one-half to two-thirds total height. Sutures simple in juveniles, heavily callused on adults. Shell in juveniles lanceolate in overall shape; in adults shell is oval to almost circular and may be dorso-laterally flattened. Olivoid band and anterior bands pronounced. Plication plate narrow and simple and usually callused. Anterior end of columella a simple point.
Remarks
When he first introduced the name Ancillopsis, Conrad (Reference Conrad1865a, p. 22) did not provide a description (he also erroneously gave the date of its introduction as 1864), but listed four species (altile, scamba, subglobosa, and tenera) (he had earlier [Conrad, Reference Conrad1832, Reference Conrad1834a] placed these in Ancillaria, but this name was already preoccupied by Ancillaria Lamarck, Reference Lamarck1799). These species were allied with Nassariidae by Cossmann (Reference Cossmann1893), who placed them in the genus Buccinanops. Palmer (Reference Palmer1937) agreed with this familial placement but moved them all into the nassariid genus Bullia. Gardner (Reference Gardner1945, p. 199) rejected Palmer's judgement, suggesting that the “much smaller protoconch and the banding of the body by the change in direction of the growth lines are probably significant characters in separating Ancillopsis from Bullia.” Allmon (Reference Allmon1990) similarly argued that Ancillopsis and associated forms were not closely related to Bullia, but did not assign them to another group. Pacaud and Cazes (Reference Pacaud and Cazes2014) reiterated the case for an assignment of altilis and similar forms to Bullia. Dockery (Reference Dockery1980) figured a small specimen with axial ribs on early whorls from the Cook Mountain Formation of Mississippi, referring it to “Bullia sp.”, which may belong to the species A. altilis.
Species assigned here to Ancillopsis have in common with other species of Ancillariidae the presence of olivoid and anterior bands, which are not present in Recent species of Bullia (Fig. 7). Furthermore, the form of the anterior end of the columella is different between Ancillopsis altilis and extant Bullia species (Fig. 7): in A. altilis, the end comes to an acute point, while in Bullia, it is terminated by a fold. For these reasons, altilis and related forms can be placed in the genus Ancillopsis in the family Ancillariidae.

Figure 7. Comparison of the anterior ends of the shell in three living species of Bullia and specimens of Ancillopsis, which have been placed by other authors in Bullia. The Bullia specimens (1–3) all show a terminal columellar fold (arrows), whereas the specimens of Ancillopsis (4, 5) do not. (1) Bullia vittata (Linnaeus, Reference Linnaeus1767), Sri Lanka, PRI 104508. (2) Bullia laevissima (Gmelin, Reference Gmelin and Gmelin1791), South Africa, PRI 104509. (3) Bullia annulata (Lamarck, Reference Lamarck1816), South Africa, PRI 104507. (4) Ancillopsis altilis, Gosport Sand, Alabama (Loc. AL-MO-2a), PRI 83941. (5) Ancillopsis patula, Eocene, Ducy, France (Loc. FR-1), PRI 83935. All scale bars = 1 cm.
Pacaud and Cazes (Reference Pacaud and Cazes2014) reported preserved color patterns on specimens of the two species here included in this genus (A. altilis and A. patula).
Ancillopsis altilis (Conrad, Reference Conrad1832)
Figures 8.1–8.21, 9.1, 9.10, 9.13, 9.14, 9.16, 9.17, 10
- Reference Conrad1832
Ancillaria altile Conrad, p. 24, pl. 10, fig. 2.
- Reference Conrad1832
Ancillaria subglobosa Conrad, p. 25, pl. 10, fig. 3.
- Reference Lea1833
Anolax gigantea Lea, p. 180, pl. 6, fig. 193.
- Reference Lea1849
Ancillaria subglobosa; Lea, p. 96.
- Reference d'Orbigny1850
Ancyllaria subglobosa; d'Orbigny, p. 352.
- Reference Conrad1862
Tritia altilis; Conrad, p. 562.
- Reference Conrad1865a
Ancillopsis subglobosa; Conrad, p. 22.
- Reference Conrad1866
Ancillopsis subglobosa; Conrad, p. 17.
- Reference Gill1867
Ptychosalpinx altilis; Gill, p. 154.
- Reference Heilprin1880
Ancillaria (Ancillopsis) subglobosa; Heilprin, p. 364.
- Reference Aldrich1886
cf. Ancillaria subglobosa; Aldrich, p. 50, 51, 58.
- Reference Aldrich1886
Expleritoma prima; Aldrich, p. 29, pl. 5, fig. 1.
- non Reference Aldrich1886
Ancillaria expansa; Aldrich, p. 28, pl. 5, fig. 11.
- Reference de Gregorio1890
Ancilla altilis; de Gregorio, p. 55, pl. 3, figs. 21, 22, 57, 62, 67.
- Reference de Gregorio1890
Ancilla subglobosa; de Gregorio, p. 56, pl. 4, figs. 3,4,19,20.
- Reference de Gregorio1890
Expleritoma prima; de Gregorio, p. 108, pl. 8, figs. 26, 27.
- non Reference de Gregorio1890
Ancilla expansa; de Gregorio, p. 55, pl. 4, fig. 1 [copied Aldrich, Reference Aldrich1886].
- Reference Cossmann1893
Buccinanops altile; Cossmann, p. 33.
- Reference Cossmann1893
Buccinanops subglobosum; Cossmann, p. 33.
- Reference Harris1895b
Ancillaria subglobosa; Harris, p. 43.
- Reference Cossmann1899
Buccinanops altile; Cossmann, p. 45.
- Reference Cossmann1901b
Buccinanops (Brachysphingus) subglobosa; Cossmann, p. 221, pl. 9, fig. 14 [captions for figs 14 and 23 reversed].
- Reference Veatch and Stephenson1911
cf.? Buccinanops altile; Veatch and Stephenson, p. 295.
- Reference Aldrich1921
Ancillopsis Tuomoyi [sic]; Aldrich, p. 12, pl. 1, figs. 23, 24.
- Reference Price and Palmer1928
Bullia altile harrisi Palmer in Price and Palmer, p. 29, pl. 7, figs. 7, 11, 12, 15.
- Reference Price and Palmer1928
Bullia altile; Palmer in Price and Palmer, p. 28, pl. 6, figs. 13, 14, 16.
- Reference Price and Palmer1928
Bullia altile (B. subglobosum form); Palmer in Price and Palmer, p. 29, pl. 7, figs. 13, 14, 16.
- Reference Palmer1937
Bullia altilis; Palmer, p. 287, pl. 39, figs. 7–9.
- Reference Palmer1937
Bullia altilis subglobosa; Palmer, p. 289, pl. 39, figs. 1, 4, 5, 6, 11, 12, pl. 40, figs. 1–3, 5.
- Reference Palmer1937
Bullia altilis harrisi; Palmer, p. 290, pl. 39, figs. 2, 3, 10, 13.
- Reference Palmer1937
Lisbonia expansa Palmer [in part], p. 295, pl. 40, figs. 8, 12, 13.
- Reference Wenz and Schindewolf1943
Lisbonia expansa; Wenz, p. 1227, fig. 3491 [copied Palmer, Reference Palmer1937].
- Reference Gardner1945
Ancillopsis subglobosa; Gardner, p. 199, pl. 22, figs. 20, 21.
- Reference Gardner1945
Ancillopsis harrisi; Gardner, p. 200, pl. 22, figs. 22, 23.
- Reference Harris and Palmer1947
cf. Bullia altilis; Harris and Palmer, p. 347, pl. 45, figs. 22, 23.
- Reference Harris and Palmer1947
cf. Bullia altilis subglobosa; Harris and Palmer, p. 348, pl. 45, fig. 24.
- Reference Wilbert1953
Bulla [sic] altilis subglobosa; Wilbert, p. 99.
- Reference Brann and Kent1960
Bullia altilis harrisi; Brann and Kent, p. 139.
- Reference Brann and Kent1960
cf. Bullia altilis subglobosa; Brann and Kent, p. 139.
- Reference Brann and Kent1960
Lisbonia expansa [in part]; Brann and Kent, p. 500.
- Reference Palmer and Brann1966
Bullia altilis harrisi; Palmer and Brann, p. 543.
- Reference Palmer and Brann1966
Bullia altilis subglobosa; Palmer and Brann, p. 543.
- Reference Palmer and Brann1966
Bullia tuomeyi; Palmer and Brann, p. 545.
- Reference Palmer and Brann1966
Lisbonia expansa [in part]; Palmer and Brann, p. 740.
- Reference Dockery1977
Bullia altilis; Dockery, p. 73, pl. 14, figs. 8, 9.
- Reference Toulmin1977
Bullia altilis; Toulmin, p. 276, pl. 45, fig. 9.
- Reference Toulmin1977
Bullia altilis subglobosa; Toulmin, p. 205.
- Reference Dockery1980
Bullia calluspira Dockery, p. 109, pl. 3, figs. 4–7.
- Reference Allmon1990
“Bullia” altilis; Allmon, p. 56, pl. 9, fig. 10.
- Reference Allmon1990
“Bullia” tuomeyi; Allmon, p. 59, pl. 9, fig. 13.
- Reference Garvie1996
Bullia altilis harrisi; Garvie, p. 74, pl. 15, figs. 1, 2.
- Reference Pacaud and Cazes2014
Bullia altilis subglobosa; Pacaud and Cazes, p. 18, pl. 1, figs. 4, 5, pl. 2, figs. 10, 11.
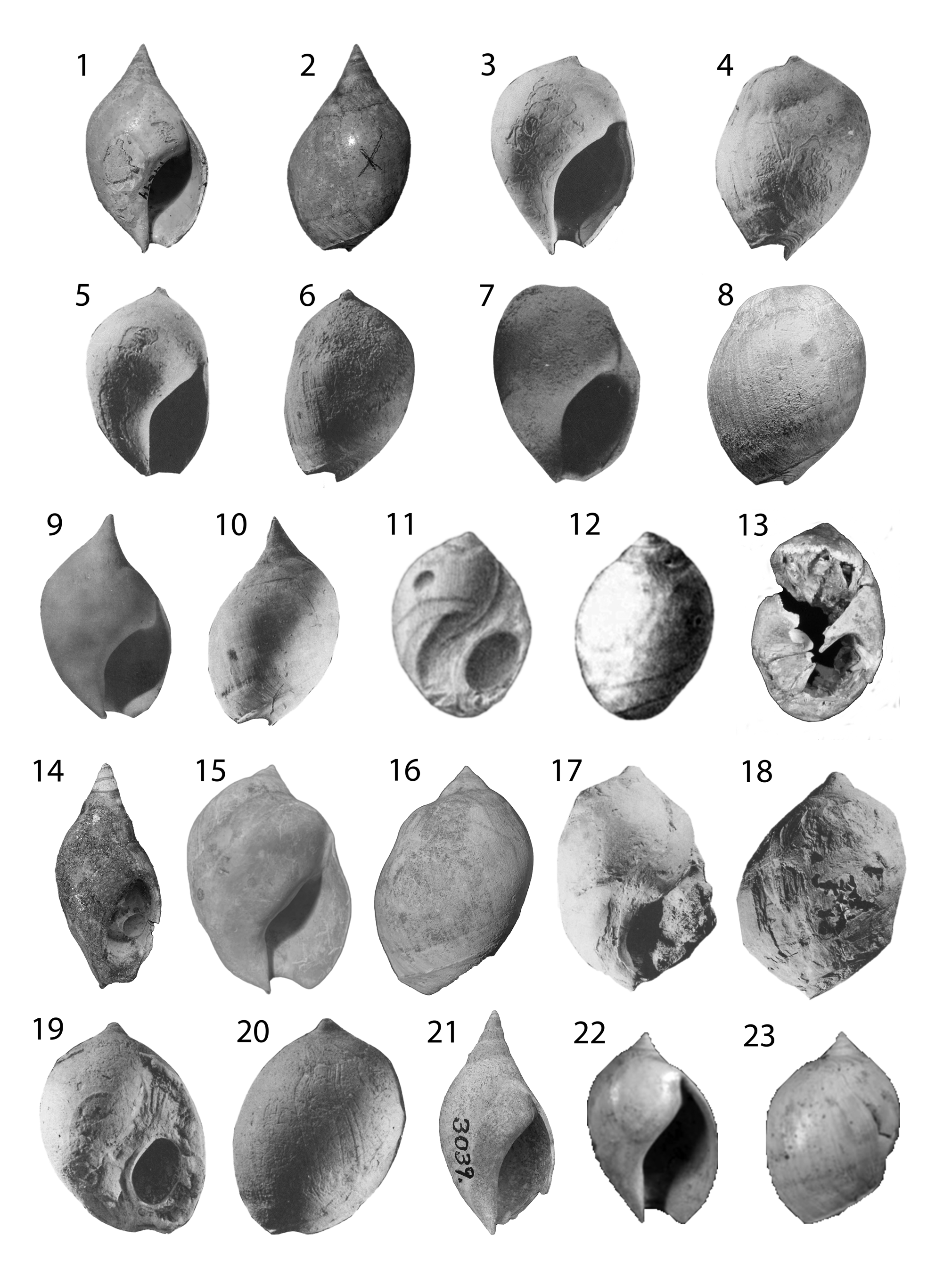
Figure 8. Ancillopsis. (1–20) Ancillopsis altilis: (1, 2) Ancillaria altile lectotype ANSP 14644; height 37.7 mm. (3, 4) Bullia altilis subglobosa hypotype PRI 3044; height 20.0 mm. (5, 6) Bullia altilis subglobosa hypotype PRI 3043; height 31.0 mm. (7, 8) Bullia calluspira holotype PRI 30022; height 27.0 mm. (9, 10) Bullia altilis hypotype PRI 3040; height 44.7 mm. (11–13) Expleritoma prima holotype USNM 638776: (11, 12) drawings from Aldrich (Reference Aldrich1886); (13) photo of broken specimen; height 36.0 mm. (14) Ancillopsis altilis (juvenile) PRI 4659; height 15.2 mm. (15, 16) Ancillopsis altilis ALMNH 15246; height 69.2 mm. (17, 18) Lisbonia expansa hypotype PRI 3047; height 78.4 mm. (19, 20) Bullia altilis subglobosa hypotype PRI 3037; height 26.6 mm. (21) Bullia altilis (juvenile) hypotype PRI 3039; height 27.0 mm. (22, 23) Ancillopsis patula (Bullia patula lectotype UCBL EM30549; height 28.0 mm; from Pacaud and Cazes, Reference Pacaud and Cazes2014).

Figure 9. Ancillopsis altilis (continued) and Ancillaria expansa. (1–10, 13, 14, 16, 17) Ancillopsis altilis: (1) Bullia altilis harrisi holotype PRI 360; height 15.3 mm. (2) Bullia altilis harrisi paratype PRI 356; height 16.8 mm. (3) Bullia altilis harrisi paratype PRI 357; height 20 mm. (4, 5) Ancillopsis altilis from Hatchetigbee Bluff, Alabama (Loc. AL-WA-1) PRI 104694; height 27.2 mm. (6–8) Ancillopsis tuomeyi holotype GSA-I17344; height 28 mm. (9, 10) Ancillopsis tuomeyi cotype GSA-I17579; height 23.2 mm. (11, 12) Ancillaria expansa holotype USNM 638775; height 51.4 mm. (13) Scanning electron micrograph of shell apex, Bullia altilis (juvenile) hypotype PRI 3039; height 27.0 mm. (14, 17) Scanning electron micrographs of shell apex, Ancillopsis altilis (juvenile) PRI 4659. (15) Scanning electron micrograph of shell apex, Ancillopsis patula PRI 83935. (16) Scanning electron micrograph of shell apex, Ancillopsis altilis PRI 83944.
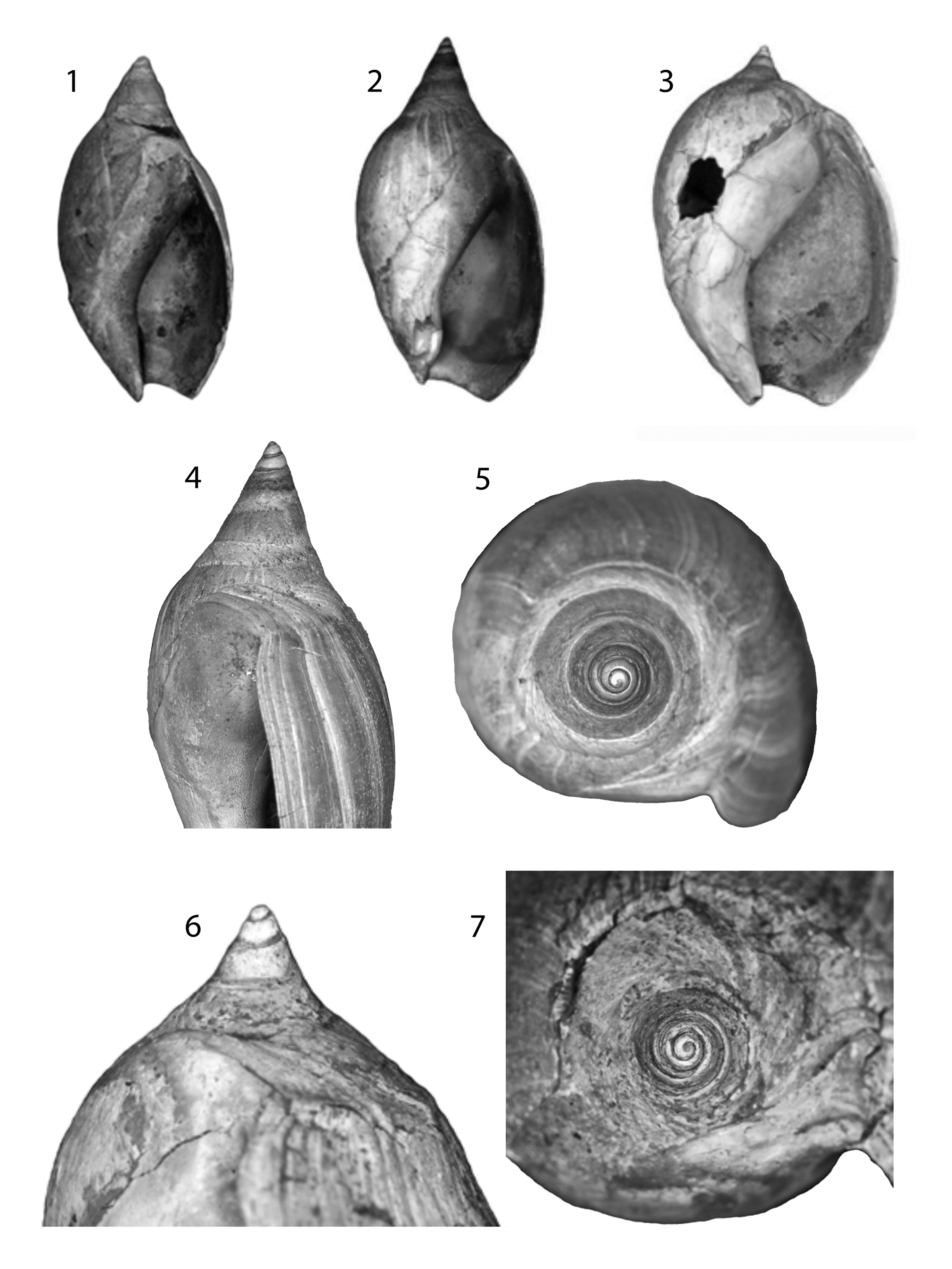
Figure 10. Ancillopsis altilis (continued), Moodys Branch Formation, Mississippi (Loc. MS-YA-1). (1) MGS 2103 Height 25.0 mm. (2, 4, 5) MGS 2104 Height 29.0 mm. (3, 6, 7) MGS 2386 Height 36.0 mm. Photos provided by David Dockery.
Type material
Lectotype (plus 8 specimens) Ancillaria altile (selected by Palmer, Reference Palmer1937, p. 289 [fide Moore, Reference Moore1962, p. 36]) ANSP 14644; holotype Anolax gigantea Lea, Reference Lea1833, ANSP 5909 (lost; J. Sessa, personal communication, 11/12/21); holotype B. altilis harrisi PRI 360; paratypes PRI 356, 357; hypotype (Garvie, Reference Garvie1996) PRI 33127; holotype B. calluspira PRI 30022; hypotypes Lisbonia expansa (Palmer, Reference Palmer1937) PRI 3046, 3047; hypotypes B. altilis (Palmer, Reference Palmer1937) PRI 3038, 3040, 3042, juvenile specimen 3039; juvenile specimen (Harris and Palmer, Reference Harris and Palmer1947) PRI 4659; hypotypes B. altilis subglobosa (Harris and Palmer, Reference Harris and Palmer1947) PRI 4660; (Palmer, Reference Palmer1937) PRI 3037, 3038, 3043; holotype Ancillopsis tuomeyi GSA-I17344, cotype GSA-I17579; holotype Expleritoma prima Aldrich, Reference Aldrich1886, USNM 638776.
Occurrence
Alabama: upper Paleocene (Thanetian), Nanafalia Formation, Bells Landing Marl, (AL-MO-3), lower Eocene (Ypresian), Bashi Marl, Hatchetigbee Formation (Locs. AL-CH-1, AL-CL-2, AL-CL-6, AL-WA-1), middle Eocene (Lutetian–Bartonian), Lisbon Formation, Gosport Sand (Locs. AL-CL-1, AL-CH-4, AL-MO-2, AL-MO-5, AL-PA-1); Mississippi: lower Eocene (Ypresian), Bashi Marl (Locs. MS-LA-1, MS-LA-2), upper Eocene (Bartonian–Priabonian), Moodys Branch Formation (Loc. MS-YA-1); Texas: middle Eocene (Lutetian–Bartonian), Cook Mountain, Reklaw, Weches formations (Locs. TX-BA-4, TX-MI-1); Arkansas: upper Eocene (Priabonian), White Bluff Formation (Loc. AR-ST-1). Mexico: middle Eocene (Bartonian), Laredo Formation (Loc. MX-NL-1), upper Eocene (Priabonian), Jackson Formation (MX-TA-1).
Revised description
Adult shell small to very large. Protoconch of 2–3 smooth whorls. Shell lanceolate with acute spire as juvenile, becoming rounded with lower spire with age. Spire in juveniles up to 0.25 total height, sometimes with faint axial ribs. In mature individuals, almost the entire ventral surface of shell covered by callus, with the early spire whorls sometimes barely or not at all protruding, producing a subspheroidal shape. Aperture lanceolate, 0.5–0.7 total shell height and ~0.5 maximum width. Posterior canal usually conspicuous. Shell smooth except for growth lines. Anterior and olivoid bands covered by callus near aperture, well developed on dorsal side of body whorl, with pronounced ridge between them. Growth lines prominent, straight, and sharply angled in olivoid band, deeply curved concavely toward the anterior notch in the anterior band. Plication plate covered by callus and not visible. Anterior tip of columella simple and pointed. Some large individuals show slight shouldering on posterior of body whorl.
Other material examined
PRI 64338 (1 specimen); PRI 83922 (10 specimens); PRI 83923 (1 specimen); PRI 83924 (1 specimen); PRI 83925 (4 specimens); PRI 83926 (2 specimens); PRI 83928 (1 specimen); PRI 83929 (1 specimen); PRI 83930 (1 specimen); PRI 83931 (15 specimens); PRI 83932 (3 specimens); PRI 83933 (1 specimen); PRI 83934 (16 specimens); PRI 83938 (4 specimens); PRI 83939 (1 specimen); PRI 83940 (1 specimen); PRI 83941 (1 specimen); PRI 83942 (1 specimen); PRI 83943 (2 specimens); PRI 83944 (1 specimen); PRI 83945 (1 specimen); PRI 83946 (12 specimens); PRI 104694 (1 specimen); ALMNH 15245 (27 specimens); ALMNH 15246 (1 specimen); MCZIP 24244 (53 specimens); MCZIP 29243 (21 specimens); MCZIP 29245 (1 specimen).
Morphometrics
We measured 10 variables on a total of 211 specimens from localities in Alabama, Mississippi, and France (Fig. 11; Supplement 2). Measurements were taken with digital calipers. Data were analyzed by factor analysis, using the 4M program in the BMDP statistical package (Dixon, Reference Dixon1993). The first three factors reported explained 91.6% of the total variation in the dataset. The results (Fig. 12) indicate that the specimens cannot be clearly separated morphologically, and therefore reasonably can be included in a single species-level taxon. The specimens measured included the type specimen of Bullia calluspira Dockery, Reference Dockery1980 (from the Bashi Formation), and the European species Buccinum patulum Deshayes, Reference Deshayes1835 (see below), both of which are morphometrically clustered among the other specimens.
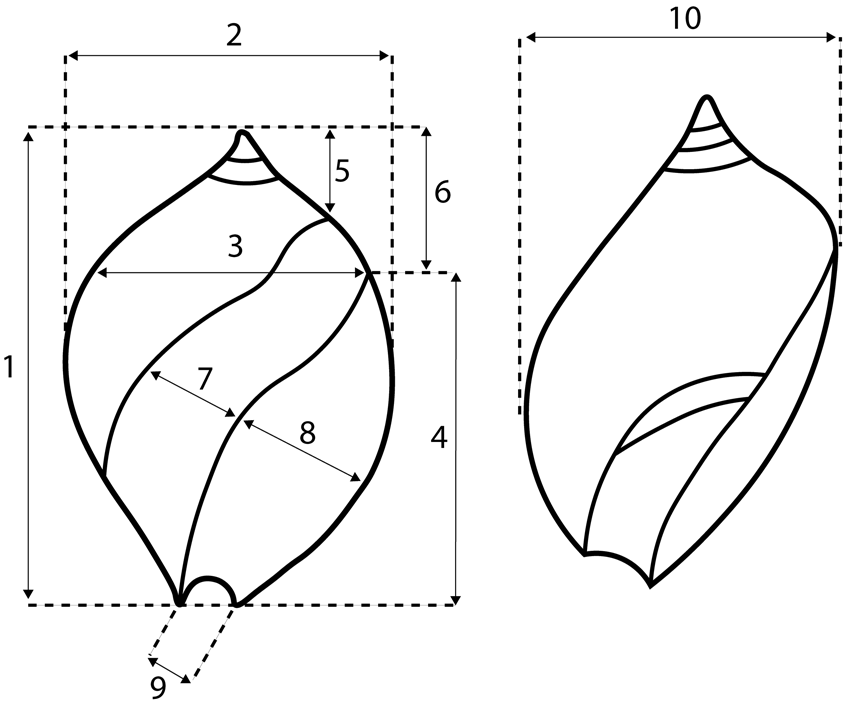
Figure 11. Measurements taken on specimens of Ancillopsis altilis for morphometric analysis. 1. Maximum height. 2. Maximum width in apertural view. 3. Width at posterior end of aperture. 4. Aperture length. 5. Height from posteriormost point of parietal callus. 6. Maximum height minus aperture length. 7. Maximum width of callus on ventral side. 8. Maximum width of aperture. 9. Width of anterior canal. 10. Maximum width from left side.
Specimens from early in the history of the lineage (from the Tuscahoma, Bashi, and Hatchetigbee formations) do, however, differ in size and shape from those in the later Gosport Sand and Moodys Branch formations. Older specimens are smaller, and Gosport/Moodys specimens are larger (similar to the pattern reported in Agaronia alabamensis and other taxa; see Haveles and Ivany, Reference Haveles and Ivany2010) (Figs. 13, 14). Price and Palmer (Reference Price and Palmer1928) described harrisi as a subspecies of altilis from the Queen City Formation at Smithville, Bastrop County, TX (Loc. TX-BA-4) (see Molineaux et al., Reference Molineux, Zachos, Karadker, Hunt and Catlos2013, about this locality), and Garvie (Reference Garvie1996) reported it from the Reklaw Formation in Texas. Specimens of this form are especially small.

Figure 13. Anagenetic change in Ancillopsis altilis through time (formations as indicated in Fig. 2). (1) Shell height vs. shell width; (2) shell height vs. callus width; (3) shell height vs. aperture length. Measurements are in mm. See text for further discussion.

Figure 14. Height of Ancillopsis altilis through time (mean and +/− one standard deviation). Formations as indicated in Figure 2.
Shell shape and degree of callus lateral expansion over the body whorl also vary with time (Fig. 12). Specimens from the Bashi and Gosport are more inflated and have callus covering about half to three-fourths of the ventral side, while those from the Hatchetigbee are flatter and have callus on the entire ventral side and lapping over onto the dorsal side. Specimens from the Bashi, Hatchetigbee, and Queen City/Reklaw formations have low spires even as juveniles. The earliest known specimens, from the Greggs Landing bed of the Tuscahoma Formation (described as Ancillopsis tuomeyi Aldrich, Reference Aldrich1921), are also distinctive in being dorso-ventrally flattened (Fig. 9.6–9.10).
Remarks
This is one of the most distinctive gastropods in the Eocene of the Gulf Coastal Plain. It has received a large number of names, which has unfortunately obscured rather than clarified its manifest morphological variability and disparity through its extended stratigraphic range. Significantly, the numerous named forms do not overlap with each other in time, suggesting a single variable lineage showing considerable anagenetic change through time rather than multiple separate taxa (Figs. 13, 14).
One of the most conspicuous characteristics of these forms is the greatly expanded parietal callus on adult individuals, frequently extending over the apex giving the shells an almost spherical overall shape (see Pietsch et al., Reference Pietsch, Anderson, Maistros, Padalino and Allmon2021) (e.g., Figs. 8, 9). Juveniles, in contrast, have attenuated spires and only narrow extent of callus on the body whorl and spire (Fig. 8.14, 8.21). A series of specimens from the Moodys Branch Formation shows this ontogenetic transition particularly well (Fig. 10).
Several specimens from the Gosport Sand also show enormously thickened shell inside the last whorl ending at the aperture. This includes the type specimen of Explerotoma prima (USNM 638776; Fig. 8.11–8.13), which is now unfortunately badly damaged, and a specimen that Palmer assigned to Bullia altilis subglobosa (PRI 3037; Fig. 8.19, 8.20). Palmer (Reference Palmer1937, p. 289) described these specimens as “injured or diseased” individuals of B. altilis subglobosa.
Palmer (Reference Palmer1937) named the genus Lisbonia for Ancillaria expansa Aldrich, Reference Aldrich1886. She stated that young specimens had axial ribs on their early whorls and were relatively uncallused, but that adult specimens, “rivalling in size B. altilis” were heavily callused. Indeed, a large specimen assigned to L. expansa by Palmer (Reference Palmer1937; Fig. 8.16, 8.17) is almost identical to large specimens of altilis. Palmer noted that such ribbing did not occur on early whorls of altilis, and that “[t]he life histories of the two species are different and show that the two belong to two different genera” (Palmer, Reference Palmer1937, p. 295). She stated that the holotype of expansa (Fig. 9.11, 9.12) “has longitudinal nodes and fine, spiral lines on the apical whorls” (Palmer, Reference Palmer1937, p. 295). This is true, but these nodes are not the same as the longer longitudinal ribs present in other specimens, which are herein assigned to Anbullina elliptica (Whitfield, Reference Whitfield1865) (see below). The holotype of Ancillaria expansa Aldrich (Fig. 9.11, 9.12), furthermore, has a very different overall shell shape compared to specimens assigned here to Ancillopsis altilis. The former has a very prominent and sharp rear edge of the anterior band, and no olivoid band. The widest part of the body whorl is just beneath the spire, rather than adjacent to the aperture. It is clearly not Ancillaria (see discussion below), and is more similar to Pseudoliva, except that it does not have the “pseudolivid groove” (see Vermeij, Reference Vermeij1998), and may belong in the family Pseudolividae.
Ancillopsis patula (Deshayes, Reference Deshayes1835)
Figures 8.22, 8.23, 9.15
- non Reference Linnaeaus1758
Buccinum patulum Linnaeus, Reference Linnaeaus1758 (see Pacaud and Cazes, Reference Pacaud and Cazes2014, p. 17).
- Reference Deshayes1835
Buccinum patulum; Deshayes, p. 646, pl. 88, figs. 5, 6.
- Reference Deshayes and Milne Edwards1844
Buccinum patulum; Deshayes and Milne Edwards, p. 211, n. 10.
- Reference d'Orbigny1850
Buccinanops palulum [sic]; d'Orbigny, p. 420, n. 1556.
- Reference Sowerby and Dixon1850
Pseudoliva ovalis Sowerby, p. 106, pl. 7, fig. 13.
- Reference Morris1854
Pseudoliva ovalis; Morris, p. 274.
- Reference Edwards1854
Pseudoliva ovalis; Edwards, p. 451.
- Reference Pictet1855
Buccinum patulum; Pictet, p. 44, pl. 67, fig. 4.
- Reference Deshayes1865
Buccinum patulum; Deshayes, p. 495, n. 2.
- Reference Briart and Cornet1871
Pseudoliva ovalis; Briart and Cornet, p. 40.
- Reference Mayer-Eymar1889
Ancillaria cossmanni Mayer-Eymar, p. 324, n. 88, pl. 14, fig. 1.
- Reference Cossmann1889
Buccinanops (Bullia) palulum [sic]; Cossmann, p. 134.
- Reference de Gregorio1890
Ancilla cossmanni; de Gregorio, p. 56.
- Reference Newton1891
Pseudoliva ovalis; Newton, p. 167.
- Reference Cossmann1893
Buccinanops (Bullia) palulum [sic]; Cossmann, p. 33.
- Reference Dollfus1900
Buccinum (Buccinanops) palulum [sic]; Dollfus, p. 135.
- Reference Cossmann1901a
Buccinanops (Brachysphingus) patulum; Cossmann, p. 48.
- Reference Cossmann1901b
Buccinanops (Brachysphingus) palulum [sic]; Cossmann, p. 222.
- Reference Cossmann1901b
Buccinanops patulum; Cossmann, p. 222.
- Reference Cossmann and Pissarro1911
Buccinanops (Brachysphingus) palulum [sic]; Cossmann and Pissarro, pl. 36, fig. 175-1.
- Reference Palmer1937
Bullia patula; Palmer, p. 289.
- Reference Gardner1945
Ancillopsis patula; Gardner, p. 199.
- Reference Glibert1963
Bullia patula; Glibert, p. 98.
- Reference Allmon1990
“Ancillopsis” patula; Allmon, p. 86, pl. 9, fig. 12.
- Reference Le Renard and Pacaud1995
Bullia patula; Le Renard and Pacaud, p. 114.
- Reference Pacaud and Le Renard1995
Bullia patula; Pacaud and Le Renard, p. 167.
- Reference Tracey, Todd, Le Renard, King and Goodchild1996
Ancillopsis patula; Tracey et al., p. 120.
- Reference Squires1997
Ancillopsis patula; Squires, p. 850.
- Reference Pacaud and Cazes2014
Bullia patula; Pacaud and Cazes, p. 17, text-fig. 1, pl. 1, figs. 1–3; pl. 2, figs. 1–9.
Type material
Lectotype UCBL EM30549.
Occurrence
France: upper Eocene (Auversian); UK: upper Eocene, Bracklesham Beds, Selsey Formation (Loc. UK-WS-1).
Revised description
The shell is medium in size, oval, plump, with rounded curve at the back, dorso-ventrally depressed, with thick test. The spiral is short, pointed, composed of 3–4 very narrow whorls, separated by simple sutures and disturbed by the increments (disrupted by growth lines?). The whole of the teleoconch is devoid of sculpture; we observe only strong and numerous streaks of weakly opisthocyrtic growth lines, strongly sinuous in the peri-sutural adapical region, intersected by very fine barely visible spiral streaks. The body whorl, very large, constituting by itself almost the entire total height, shows a particularly convex profile; it ends without a neck, by a broad, clearly delimited fasciole. The body whorl presents in the abapical region above the fasciole, a wide band, slightly depressed, inducing a wide furrow on the edge of the labrum corresponding to the deviation of the streaks of growth. This band is separated from the fasciole by a space equal in width to the abapical band. The opening is large, ovoid, dilated, broad in front, narrow in the back, and terminated by a short and narrow anal canal. The columella, clearly excavated over the entire height, ends in an acute point; the columella also is cut by a wide and deep siphonal notch. The parietal and columellar calluses are thick, very widely spread laterally. The labrum is thin, smooth on the inside, slightly prosocline (translation of Pacaud and Cazes, Reference Pacaud and Cazes2014, p. 17–18).
Other material examined
PRI 83935 (1 specimen) (Loc. FR-1).
Remarks
As noted by Palmer (Reference Palmer1937, p. 289), Allmon (Reference Allmon1990, p. 86), and Squires (Reference Squires1997), Ancillopsis patula is almost identical to Ancillopsis altilis from the U.S. Gulf Coast in its subspherical but dorsoventrally flattened shape, minute spire, inflated, unsculptured body whorl, large aperture, expanded callus, and lack of terminal columellar fold; and in our morphometric analysis, it falls among Coastal Plain specimens (Fig. 12). It differs in being smaller than specimens of A. altilis of similar geological age and having a shinier shell (which might be partly an artifact of preservation). The most significant difference between the two species may be their pattern of remnant color on the body whorl; A. patula shows an olivoid band that appears purplish under UV light, whereas A. altilis does not (Pacaud and Cazes, Reference Pacaud and Cazes2014, p. 21).
As noted by Pacaud and Cazes (Reference Pacaud and Cazes2014, p. 16), the species also exists in the Bartonian in England where it had been erroneously assigned to the genus Pseudoliva and described as Pseudoliva ovalis (Briart and Cornet, Reference Briart and Cornet1871; Newton, Reference Newton1891). As the only representative of this clade outside of the Gulf Coast, this species has interesting paleobiogeographic implications.
Pacaud and Cazes (Reference Pacaud and Cazes2014) argued that patula should be retained in Bullia in Nassariidae. Neither patula nor altilis, however, have terminal columellar folds, which are characteristic of all modern members of Nassariidae (Allmon, Reference Allmon1990; see Fig. 7).
Genus Anbullina Palmer, Reference Palmer1937
Type species
Ancillaria ancillops Heilprin, Reference Heilprin1891, by original designation (Palmer, Reference Palmer1937, p. 292).
Diagnosis
Shell oval to lanceolate; spire low but acute. First three or four teleoconch whorls longitudinally ribbed, ribs becoming obsolete on later whorls of spire and body whorl. Spire and body whorls frequently slightly shouldered. Body whorl bears narrow band below suture, which bears sigmoidal growth lines of growth. Plication plate and anterior band faint to pronounced. Parietal callus extends less than halfway across ventral surface of body whorl, and only slightly posterior of aperture. Olivoid band present but faint. Anterior notch moderate to deep.
Remarks
Palmer (Reference Palmer1937) named Anbullina for the distinctive species Ancillaria ancillops Heilprin, Reference Heilprin1891. This species was allied with the Bullia group in Nassariidae by Cossmann (Reference Cossmann1901b), who placed it in the genus Buccinanops, and Palmer proposed Anbullina as a subgenus within Bullia Gray, Reference Gray, Griffith and Pidgeon1834. Its similarities to these genera of Nassariidae, however, consist of little more than overall shape (Allmon, Reference Allmon1990, p. 59). On the other hand, it shares with other ancillariids an (albeit very faint) olivoid band and (well-developed) anterior band. It therefore seems more likely assignable to the ancillariids, but does not agree with any other genus in that family. Recognition of a second species, Anbullina elliptica (Whitfield, Reference Whitfield1865), further justifies continued recognition of a separate genus-level taxon.
Anbullina ancillops (Heilprin, Reference Heilprin1891)
Figure 15.1, 15.2
- Reference Heilprin1891
Ancillaria ancillops Heilprin, p. 398, pl. 11, fig. 4.
- Reference Cossmann1901b
Buccinanops (Bullia) ancillopsis [sic]; Cossmann, p. 223, pl. 9, fig. 24.
- non Reference Cossmann1901b
Anaulax ancillopsis; Cossmann, p. 223.
- Reference Palmer1937
Bullia (Anbullina) ancillops; Palmer, p. 292, pl. 40, figs. 4, 6.
- Reference Wenz and Schindewolf1943
Bullia (Anbullina) ancillops; Wenz, p. 1226, fig. 3488 [copied Palmer, Reference Palmer1937, pl. 40, fig. 6].
- Reference Dockery1980
Bullia cf. B. (Anbullina) ancillops [misspelled in plate caption as “Bucilla cf. (Anbullina) Ancillops”]; Dockery, p. 110, pl. 17, fig. 4.
- Reference Allmon1990
“Bullia” (Anbullina) ancillops; Allmon, p. 59, pl. 9, fig. 4.

Figure 15. Anbullina. (1, 2) Anbullina ancillops: Bullia (Anbullina) ancillops hypotype PRI 3045; height 28.8 mm. (3–10, 12, 16–18) Anbullina elliptica: (3) Anbullina elliptica (Buccinanops ellipticum hypotype [Barry and LeBlanc, Reference Barry and LeBlanc1942] LSU 6023; height 27.5 mm). (4, 5) Pseudoliva elliptica, holotype FMNH-UC 24670; height 17 mm. (6) Bullia sp. (from Dockery, Reference Dockery1980, pl. 37, fig. 7), MGS 523; height 11 mm. (7, 8, 18) “Buccinanops” ellipticum reklawensis holotype PRI 30410; height 23.5 mm); (18) scanning electron micrograph of shell apex. (9, 10) Anbullina elliptica (Lisbonia expansa hypotype [Palmer, Reference Palmer1937] PRI 3046; height 25 mm). (11) Bucilla [sic] cf. B. (Anbullina) ancillops (from Dockery, Reference Dockery1980, pl. 17, fig. 4), MGS 110; height 20.7 mm. (12, 16, 17) Anbullina elliptica, specimen from Bells Landing, AL (Loc. AL-MO-3), PRI 83937; height 18.4 mm; (16, 17) scanning electron micrographs of shell apex. (13) Anbullina elliptica? (Pseudoliva ostrarupis pauper holotype NPL 35590); height 18 mm. (14, 15) Anbullina elliptica? (Lisbonia pauper NPL 37825); height 13.2 mm.
Type material
Holotype lost (fide Palmer, Reference Palmer1937, p. 293); hypotype (Palmer, Reference Palmer1937) PRI 3045.
Occurrence
Texas: middle Eocene, Weches Formation (Loc TX-BA-1).
Revised description
Shell lanceolate; spire low but acute. Protoconch of 1.5 whorls, smooth, rounded; first protoconch whorl flatly convex; first three or four teleoconch whorls longitudinally ribbed, the ribs becoming obsolete on the later whorls of the spire and the body whorl, which are smooth. Body whorl with narrow band below suture, which bears sigmoidal growth lines. Plication plate with sharp rear edge forming slight false umbilicus and square anterior edge; anterior notch deep.
Other material examined
PRI 57311 (1 specimen).
Remarks
Anbullina ancillops is known only from one locality, the now-inaccessible Smithville outcrop of the Weches Formation in Texas (Loc. TX-BA-1). Dockery (Reference Dockery1980, p. 110, pl. 17, fig. 4) figured a poorly preserved specimen from the Doby's Bluff Tongue (see Fig. 3) in Mississippi and assigned it to “Bullia cf. B. (Anbullina) ancillops.” This specimen (see Figure 15.11), however, has a relatively longer and wider aperture than the type of ancillops, the spire appears to be partially covered with parietal callus, and it lacks the distinctive anterior end of the columella. It somewhat resembles modern and fossil species of Baryspira from New Zealand (see Beu et al., Reference Beu and Maxwell1990), and resembles no other form in the Coastal Plain. No other similar specimens have been found in the Doby's Bluff (Dockery, personal communication, November 2, 2021). It may represent yet another otherwise unrecorded ancillariid lineage in the region.
Anbullina elliptica (Whitfield, Reference Whitfield1865)
Figure 15.3–15.10, 15.12, 15.16–15.18
- Reference Whitfield1865
Pseudoliva elliptica Whitfield, p. 260.
- Reference Aldrich1886
Pseudoliva elliptica; Aldrich, p. 56.
- Reference Aldrich1887
Pseudoliva elliptica; Aldrich, p. 80 [not “1897” as in Harris, Reference Harris1899a, and Barry and LeBlanc, Reference Barry and LeBlanc1942].
- non Reference Harris1895a
Pseudoliva ostrarupis pauper Harris, p. 76, pl. 8, fig. 4.
- Reference Harris1896
Pseudoliva ostrarupis pauper; Harris, p. 99, pl. 9, fig. 20.
- Reference Harris1899a
Buccinanops ellipticum; Harris, p. 30, pl. 3, figs. 14, 15.
- Reference Harris1899b
Buccinanops ellipticum; Harris, p. 305, pl. 54, figs. 4, 5.
- Reference Trowbridge1923
Pseudoliva ostrarupis pauper; Trowbridge, p. 96.
- Reference Plummer, Sellards, Adkins and Plummer1933
Pseudoliva ostrarupis pauper; Plummer, p. 581.
- Reference Gardner1935
Pseudoliva ostrarupis pauper; Gardner, p. 317.
- Reference Palmer1937
Lisbonia expansa (Aldrich) [in part]; Palmer, p. 295, pl. 40, figs. 8, 12, 13.
- Reference Barry and LeBlanc1942
Buccinanops ellipticum; Barry and LeBlanc, p. 117, pl. 15, figs. 1, 2.
- Reference Gardner1945
Pseudoliva elliptica; Gardner, p. 195, pl. 27, figs. 3, 4.
- Reference Gardner1945
Pseudoliva ostrarupis pauper; Gardner, p. 195.
- Reference Brann and Kent1960
Buccinanops ellipticum; Brann and Kent, p. 134.
- Reference Palmer and Brann1966
Buccinanops ellipticum; Palmer and Brann, p. 533.
- Reference Allmon1990
“Buccinanops” ellipticum; Allmon, p. 59, pl. 9, fig. 8.
- Reference Garvie1996
“Buccinanops” ellipticum reklawensis Garvie, p. 74, pl. 15, figs. 14, 15.
- ?Reference Garvie2013
Lisbonia pauper; Garvie, p. 4, pl. 7, figs. 14, 15.
Type material
Holotype FMNH-UC 24670; hypotype (Barry and LeBlanc, Reference Barry and LeBlanc1942) LSU 6023; holotype “Buccinanops” ellipticum reklawensis, PRI 30410; holotype Pseudoliva ostrarupis pauper TMM BEG 35590; hypotypes (Garvie, Reference Garvie2013) TMM NPL 37825, 37826.
Occurrence
Texas: upper Paleocene (Selandian), Solomon Creek Member, Seguin Formation (Loc.TX-BA-2), upper Paleocene (Thanetian), Pendleton Formation (Loc. TX-SA-1), lower Eocene (Ypresian), Reklaw Formation (Loc. TX-MI-1); Louisiana: upper Paleocene (Selandian), Marthaville Formation (Locs. LA-NA-1, LA-SA-1, LA-SA-2); Alabama: upper Paleocene (Thanetian), Bells Landing Marl (Loc. AL-MO-3); middle Eocene (Lutetian–Bartonian), Lisbon Formation (Loc. AL-MO-5); Mississippi: upper Eocene (Bartonian–Priabonian), Moodys Branch Formation (Loc. MS-NE-1).
Revised description
Shell medium sized, lanceolate to elliptical in shape, with an evenly curved profile attenuated at both apical and anterior ends. Protoconch incompletely known, but probably of 2–3 smooth whorls. Spire relatively low, comprising not more than one-fourth the total height, while the aperture comprises more than one-half the total height. Spire usually bears numerous faint straight axial ribs on early teleoconch whorls. Sculpture on body whorl lacking, other than growth lines. Olivoid band faint to pronounced. In the holotype, this band takes the form of an adapertural angular deflection of the growth lines, forming shallow chevrons. Specimens from the Moodys Branch Formation of Mississippi show a single shallow groove 1–2 mm wide. Body whorl may show minor shouldering beneath spire or be smoothly tapered. Posterior margin of parietal callus usually even with posterior end of aperture, rarely extending to spire. Anterior notch deep.
Other material examined
PRI 83936 (3 specimens); PRI 83937 (1 specimen).
Remarks
Whitfield (Reference Whitfield1865) stated that his type specimen (Fig. 15.4, 15.5) came from Vicksburg, Mississippi, but Aldrich (Reference Aldrich1887, p. 80; see Palmer and Brann, Reference Palmer and Brann1966, p. 533) argued that it likely came from the Bells Landing Marl Member of the Tuscahoma Formation in Alabama (AL-MO-3) (see Fig. 2), where other very similar specimens have been found (see Fig. 15.12). This variable species includes specimens that have been placed in a variety of taxa, including those identified by Palmer (Reference Palmer1937) as juveniles of her Lisbonia expansa (see above, under Ancillopsis altilis).
Adults of Anbullina elliptica are similar to juveniles of Ancillopsis altilis (compare Figs. 8.14, 8.21, 10.1, 10.2 with 15.1–10, 15.12). Our phylogenetic analysis shows that the two species are closely related (Fig. 5).
Price and Palmer (Reference Price and Palmer1928, p. 23) listed but did not figure “Bullia sp. aff. ellipticum Whitefield” (sic) from Smithville, TX (Loc. TX-BA-1). Garvie (Reference Garvie2013, p. 44–45) placed Pseudoliva ostrarupis pauper in the genus Lisbonia, arguing (based on material he said was in his collection but did not figure) that the genus is valid (see discussion of Lisbonia above under Ancillopsis altilis). The hypotype of Lisbonia pauper (NPL 37825) figured by Garvie (Reference Garvie2013) shows axial ribs on the early teleoconch whorls, and may belong here, but the holotype of Pseudoliva ostrarupis pauper Harris, Reference Harris1895a (NPL 35590; Fig. 15.13) lacks axial ribs, and may belong to Pseudoliva.
Genus Eoancilla Stephenson, Reference Stephenson1941
Type species
Eoancilla acutula Stephenson, Reference Stephenson1941, by original designation.
Diagnosis
From Garvie's (Reference Garvie2013, p. 59) diagnosis: “Shell with high, smooth, evenly tapering spire; protoconch smooth, blunt, of 2 ¾ whorls; tip minute, partially immersed; callus band covering approximately lower 70% of spire whorls; columella strongly twisted; fasciolar band with 5–8 oblique narrow lirae, usually posterior ancillid band, and groove; anterior notch deep, internally thickened with callus; small low ridge of callus continuing posteriorly up inside of outer lip for ca. 1/3 of its height; small labral denticle present at end of line or kink in growth lines running from posterior end of aperture.”
Remarks
Stephenson described Eoancilla based on a Late Cretaceous species from Texas. As summarized by Garvie (Reference Garvie2013, p. 59–60), Sohl (Reference Sohl1964) synonymized Eoancilla with Ancillus Montfort, Reference Montfort1810, the type species of which is A. buccinoides Lamarck, Reference Lamarck1803, from the Lutetian of the Paris Basin, “on the basis of the shared glazed whorls, the blunted apex, and apertural features.” Garvie (Reference Garvie2013, Reference Garvie2021) argued that Eoancilla was distinct from Ancilla. He also described two additional Paleocene species from Texas and Alabama, assigned the Paleocene species Olivella mediavia Harris, Reference Harris1896, to Eoancilla, and said that he had “several specimens of Eoancilla, or a close relative thereof, from the middle Claibornian Weches Formation” (Garvie, Reference Garvie2013, p. 61), which he did not figure. He suggested that Eoancilla can “be taken as an ancestral Upper Cretaceous ancillid taxon that by Middle Eocene times had already spread to the Nangulaan Eocene of Java, because A. songoensis Martin, Reference Martin1914… is remarkably close to A. mediavia” (Garvie, Reference Garvie2013, p. 61).
Eoancilla acutula Stephenson, Reference Stephenson1941
Figure 16.1, 16.2
- Reference Stephenson1941
Eoancilla acutula Stephenson, p. 361, pl. 69, figs. 8, 9.
- Reference Sohl1964
Ancilla (Ancillus) acutula; Sohl, p. 248, pl. 36, figs. 1–7, 10.

Figure 16. Eoancilla. (1, 2) Eoancilla acutula holotype USNM 77126 (from Stephenson, Reference Stephenson1941); height 9.3 mm. (3, 4) Eoancilla lapicidina holotype NPL 93694 (from Garvie, Reference Garvie2021); height 11.1 mm. (5) Eoancilla mediavia (Olivella mediavia, drawing from Harris, Reference Harris1896, of specimen in USNM). (6, 7) Eoancilla mediavia PRI 57647; height 17.4 mm. (8, 9) Eoancilla hordea holotype NPL 37709 (from Garvie, Reference Garvie2013); height 11.5 mm.
Type material
Holotype USNM 77126; paratype USNM 77127; hypotypes (Sohl, Reference Sohl1964) USNM 130465–130467.
Occurrence
Texas: Upper Cretaceous (Maastrictian), Kemp Clay (Loc. TX-TR-1); Mississippi: Upper Cretaceous (Maastrictian), Owl Creek Formation (Locs. MS-TI-1, MS-TI-2); Tennessee: Upper Cretaceous (Maastrictian), Clayton Formation (Owl Creek Formation reworked into base) (Loc. TN-HA-1).
Original description
(Stephenson, Reference Stephenson1941, p. 361) “Shell small, polished, with maximum inflation at about the midheight, from which region the surface slopes gently toward each extremity. Protoconch small smooth, trochoid, coiled about twice. Whorls four. Spire acute and a little less than half the total height of the shell; spiral angle about 45 degrees at the tip decreasing to about 40 degrees on the whorls below. Sides of whorls of spire nearly flat; the lower 7/10 of the surface of the penultimate whorl is covered with a smooth, nontumid, closely appressed band of callus, which is separated from the upper edge of the body whorl by a fine, sharp, slightly incised, but not canaliculate, suture; the upper edge of the band is gently undulating, but the band extends with about the same proportional width all the way back to the protoconch. The main surface of the shell is smooth, except for growth lines and an exceedingly faint indication of fine spiral lines, and one fine spiral groove at about the position of the periphery. The growth lines cross the body whorl in a gently sinuous trend, bending sharply backward before they join the suture above, and more gently backward near their junction with a sharply incised groove on the base below. The aperture is lenticular with a narrow, sharply upturned, posterior canal, and widens anteriorly to a short, wide, deeply notched, siphonal canal. Outer lip broadly arcuate and notched at the suture above; inner lip broadly excavated and forming on the parietal wall a band of callus which spreads forward a little and extends upward, becoming thicker in front of the posterior canal; this callus spreads upward across about 7/10 of the surface of the penultimate whorl and is continued backward forming the band of callus on that whorl already described. The columella is flattened anteriorly and is ornamented with a band of 7 or 8 closely spaced, small, narrow oblique ridges which continue forward on the sharply twisted anterior fasciole to the terminus of the shell. The anterior fasciole is bordered on the outer side by a deep, wide, round-bottomed spiral sulcus which is traceable backward until it is covered by the callus of the lip; the anterior edge of the callus of the inner lip follows down the bottom of the sulcus to the terminus of the shell; the sulcus is bordered in front on the base of the shell by a wide, smooth band which is limited both above and below by narrow sharply incised grooves.”
Remarks
This is the only Cretaceous species treated here and may be among the oldest known species of Ancillariidae.
Eoancilla hordea Garvie, Reference Garvie2013
Figure 16.8, 16.9
- Reference Garvie2013
Eoancilla hordea Garvie, p. 61, pl. 11, figs. 6, 7.
Type material
Holotype TMM NPL 37709; paratype TMM NPL 37710.
Occurrence
Texas: upper Paleocene (Selandian), Seguin Formation (Loc. TX-BA-2).
Original description
(Garvie, Reference Garvie2013, p. 60) “Shell small, subcylindrical, smoothly rounded, barely contracted at suture; protoconch of ca. 2 whorls; tip somewhat oblique, partially immersed, with no demarcation transition to teleoconch whorls; suture defined by impressed line; spire whorls mostly covered with enamel-callus band; aperture slightly larger than ½ shell Height; columella spirally twisted; fasciolar band with 6 oblique narrow lirae; ancillid band wide; groove prominent; anterior notch deep, internally thickened with callus; thin line of callus continuing posteriorly up inside of outer lip; labral denticle small.”
Remarks
This species is known from 23 specimens from the type locality (Garvie, Reference Garvie2013, p. 61).
Eoancilla lapicidina Garvie, Reference Garvie2021
Figure 16.3, 16.4
- Reference Garvie2021
Eoancilla lapicidina Garvie, p. 138, pl. 14, figs. 11, 12.
Type material
Holotype TMM NPL 93694; paratype TMM NPL 93695.
Occurrence
Texas: lower Paleocene (Danian), Kincaid Formation (Loc. TX-FA-1).
Original description
(Garvie, Reference Garvie2021, p. 139) “Shell small to medium sized, whorls feebly concave on upper half, feebly convex below; whorls covered with a light coating of callus; columella not or only weakly twisted, with 7 spiral ridges margined by a deep sulcus, sulcus forming the anterior part of the lower anterior band, upper anterior band well defined and posteriorly margined by a minute, impressed line, line only visible near the aperture, rapidly becoming obsolete adaperturally; olivid groove and band not visible; secondary callus thick where margining the upper part of the aperture, rapidly thinning and becoming the convex part of the spire, although not easily differentiated; protoconch of 2 whorls, somewhat flattened, and set at a slight angle to the shell axis.”
Remarks
This species is known from 18 specimens from the type locality (Garvie, Reference Garvie2021, p. 139).
Eoancilla mediavia (Harris, Reference Harris1896)
Figure 16.5–16.7
- Reference Harris1896
Olivella mediavia Harris, p. 80, pl. 7, fig. 19.
- non Reference Harris1897
Olivella mediavia; Harris, p. 29, pl. 3, fig. 12 (fide Palmer and Brann, Reference Palmer and Brann1966, p. 486).
- Reference Cossmann1899
Ancilla (Sparella) mediavia; Cossmann, p. 62.
- Reference Gardner1935
Olivella mediavia; Gardner, p. 230.
- Reference Palmer and Brann1966
Agaronia mediavia; Palmer and Brann, p. 486.
- Reference Garvie2021
Eoancilla mediavia; Garvie, p. 139.
Type material
Holotype lost (fide Palmer and Brann, Reference Palmer and Brann1966, p. 487).
Occurrence
Alabama: upper Paleocene (Selandian–Thanetian), Matthews Landing Marl, Bells Landing Marl (Locs. AL-MO-3, AL-SU-3, AL-WI-1, AL-WI-2).
Original description
(Harris, Reference Harris1896, p. 80) “…whorls about 7; the first extremely small, the second much larger, and the third still greater, producing a blunt appearance; remaining spiral whorls nearly or quite covered by the sutural callosity; body whorl smooth, but the direction of the lines of growth can be traced with a glass; growth lines slightly geniculated about three-fourths of the way from the suture to the anterior folds at a faint depression which produces a faint tooth on the margin of the outer lip; columella well twisted below where it is 7–8 striate; above on the columella there is often a large obtuse fold which marks a former position of the upper margin of the slit for the anterior canal.”
Other material examined
PRI 57647 (Bell's Landing, AL; Loc. AL-MO-3).
Remarks
The type specimen was from Matthews Landing, AL (Loc. AL-WI-2). Gardner (Reference Gardner1935, p. 230) said that this species “is widespread and fairly common” and Palmer and Brann (Reference Palmer and Brann1966) listed it as coming from several other Alabama localities. Garvie (Reference Garvie2013, p. 60–61) argued that its multispiral protoconch, callus that covers only a portion of the teleoconch whorls, and the lower inner lip callus support placing it in Eoancilla. Our phylogenetic analysis (see below) indicates that this species may be ancestral to Olivula staminea (Conrad, Reference Conrad1832).
Genus Monoptygma Lea, Reference Lea1833
Type species
Monoptygma alabamiensis Lea, Reference Lea1833, by subsequent designation (Cossmann, Reference Cossmann1899).
Remarks
The name Monoptygma has a complicated history. It was first proposed by Isaac Lea (Reference Lea1833) for two fossil species from the Eocene of Alabama (M. alabamiensis and M. elegans), which do not especially resemble each other (Fig. 17.7, 17.8). G.B. Sowerby II (Reference Sowerby1839, p. 66) listed “Monoptygma Lea”, but as including only “M. elegans,” with a copy of Lea's illustration. Four lines later, he listed “Monotigma Gray” with no species name and referenced his figure 371, which shows a very different shell. According to van Aartsen and Hori (Reference van Aartsen and Hori2006, p. 3), however, “there is no indication of involvement of Gray in Sowerby's Manual,” and so “one has to consider Sowerby, Reference Sowerby1839, as the author of Monotigma.” Gray (Reference Gray1847, p. 140, 159), citing “J. Lea,” distinguished “Monop. alabamiensis, J. Lea” and “?Monoptygma sp. Lea” from “Monotigma or Monotygma, G. Sowerby,” the latter containing “Mon. elegans, Lea,” and assigned the former to Pyramidellidae. Adams (Reference Adams1853, Reference Adams and Sowerby1854), however, used Monoptygma for several modern species in Pyramidellidae. This was repeated by Smith (Reference Smith1872) and Mörch (Reference Mörch1875). van Aartsen (Reference van Aartsen1986) untangled these names, clarifying that Monoptygma Lea is a valid genus, and that Monotygma and Monotigma are both valid and distinct genera of pyramidellids.

Figure 17. Monoptygma lymneoides. (1, 2) Monoptygma leai PRI 3026; height 22 mm. (3, 4) Monoptygma lymneoides PRI 3036; height 35 mm. (5, 6) Monoptygma crassiplica ANSP 13274; height 17 mm. (7) Monoptygma alabamiensis, drawing from Lea (Reference Lea1833). (8) Monoptygma elegans, drawing from Lea (Reference Lea1833) (not Monoptygma). (9) Monoptygma crassiplica, drawing by G.D. Harris (from Palmer, Reference Palmer1937, pl. 38, fig. 4) of USNM specimen. (10) Monoptygma curta holotype ANSP 15618; height 11.6 mm. (11, 12) Monoptygma crassiplica hypotype PRI 3027; height 22.4 mm. (13, 14) Monoptygma alabamiensis paratype ANSP 5930; height 8.2 mm. (15, 16) Monoptygma alabamiensis holotype ANSP 5929; height 12 mm. (17) Monoptygma leai syntype FMNH 24671; height 19 mm. (18) Monoptygma crassiplica, drawing from Gabb (Reference Gabb1860).
Although some authors (e.g., Gabb, Reference Gabb1872) placed Monoptygma in Olividae, Palmer (Reference Palmer1937, p. 296) allied it with Bullia in Nassariidae, writing that “[t]he columella is smooth as in Bullia.” This was accepted by Glibert (Reference Glibert1963). Cernohorsky (Reference Cernohorsky1984, p. 27) seemed to be agnostic about the placement in Nassariidae, writing that “Monoptygma lacks any characters which would suggest a relationship with the Dorsaninae” in Nassariidae.
Monoptygma (which means “single fold”) is characterized by a single (very rarely double) fold or plication on the inner apertural lip. All species also show an olivoid band wrapping around the lower part of the body whorl. This combination of characters is unique and makes this taxon somewhat puzzling. Careful examination of all available specimens, however, indicates that the fold is continuous with the plication plate, and is therefore not homologous to the “columellar folds” of other taxa, such as species of Volutidae. We therefore conclude that it is assignable to Olividae.
The genus Monoptygma has been oversplit, and several species are represented by few or poorly known specimens. We synonymize all described forms into one somewhat variable species.
Monoptygma lymneoides (Conrad, Reference Conrad1833)
Figure 17.1–17.7, 17.9–17.18
- Reference Conrad1833
Ancillaria lymneoides Conrad, p. 44.
- Reference Lea1833
Monoptygma alabamiensis Lea, p. 186, pl. 6, fig. 201.
- Reference Conrad and Morton1834b
Ancillaria lymneoides; Conrad, p. 5.
- Reference Conrad1835
Ancillaria lymneoides; Conrad, p. 42, pl. 16, fig. 6.
- Reference Lea1849
Ancillaria lymneoides; Lea, p. 96.
- Reference d'Orbigny1850
Ancyllaria [sic] lymneoides; d'Orbigny, p. 352.
- Reference Conrad1854
Ancilla lymneoides; Conrad, p. 30.
- Reference Gabb1860
Monoptygma crassiplica Conrad in Gabb, p. 384, pl. 67, fig. 37.
- Reference Conrad1865a
Monoptygma crassiplica; Conrad, p. 22.
- Reference Conrad1865a
Monoptygma alabamiensis; Conrad, p. 22.
- Reference Conrad1865a
Monoptygma curta Conrad, p. 22.
- Reference Conrad1865a
Monoptygma lymneoides; Conrad, p. 23.
- Reference Conrad1865b
Monoptygma curta; Conrad, p. 143, pl. 11, fig. 8.
- Reference Whitfield1865
Monoptigma [sic] leai Whitfield, p. 261, pl. 27, fig. 7.
- Reference Conrad1866
Monoptygma curta; Conrad, p. 17.
- Reference Conrad1866
Monoptygma lymneoides; Conrad, p. 17.
- Reference Conrad1866
Monoptygma curta; Conrad, p. 17.
- Reference Conrad1866
Monoptygma alabamiensis; Conrad, p. 17.
- Reference Conrad1866
Monoptygma crassiplica; Conrad, p. 17.
- Reference Tryon1883
Monoptygma lymneoides; Tryon, p. 61, pl. 3, fig. 23.
- Reference Aldrich1887
Monoptygma leai; Aldrich, p. 80.
- Reference de Gregorio1890
Monoptygma alabamiensis; de Gregorio, p. 58, pl. 4, fig. 10.
- Reference de Gregorio1890
Ancilla (Monoptygma) curta; de Gregorio, p. 58, pl. 4, fig. 11 [copied Conrad, Reference Conrad1865b, pl. 11, fig. 8].
- Reference de Gregorio1890
Ancilla (Monoptygma) Alabamiensis; de Gregorio, p. 58, pl. 4, fig. 10 [copied Lea, Reference Lea1833, pl. 6, fig. 201].
- Reference de Gregorio1890
Ancilla (Monoptygma) lymneoides; de Gregorio, p. 58, pl. 4, fig. 14 [copied Conrad, Reference Conrad1835, pl. 16, fig. 6].
- Reference de Gregorio1890
Monoptygma curta; de Gregorio, p. 58, pl. 4, fig. 11.
- Reference Heilprin1891
Monoptygma crassiplica; Heilprin, p. 398.
- Reference Cossmann1893
Monoptygma limneoides [sic]; Cossmann, p. 41.
- Reference Harris1895b
Monoptygma curta; Harris, p. 14.
- Reference Harris1895b
Ancillaria lymneoides; Harris, p. 26.
- Reference Cossmann1899
Monoptygma curta; Cossmann, p. 72.
- Reference Cossmann1899
Monoptygma limneoides [sic]; Cossmann, p. 71, pl. 3, figs. 24, 25.
- Reference Palmer1937
Monoptygma crassiplica; Palmer, p. 298, pl. 38, figs. 3–5.
- Reference Palmer1937
Monoptygma lymneoidies [sic]; Palmer, p. 296, pl. 38, figs. 19, 20, pl. 85, figs. 3, 7.
- Reference Palmer1937
Monoptygma curta; Palmer, p. 298, pl. 85, fig. 8.
- Reference Palmer1937
Monoptygma leai; Palmer, p. 297, pl. 38, figs. 1, 2, 6, 8.
- Reference Wenz and Schindewolf1943
Monoptygma lymneoides; Wenz, p. 1227, fig. 3492 [copied Palmer, Reference Palmer1937, pl. 38, fig. 19].
- Reference Gardner1945
Monoptygma leai; Gardner, p. 195, pl. 27, figs. 2, 5.
- Reference Brann and Kent1960
Monoptygma leai; Brann and Kent, p. 567.
- Reference Palmer and Brann1966
Monoptygma leai; Palmer and Brann, p. 779.
- Reference Palmer and Brann1966
Monoptygma curtum; Palmer and Brann, p. 779.
- Reference Palmer and Brann1966
Monoptygma crassiplicum; Palmer and Brann, p. 778.
- Reference Allmon1990
Monoptygma crassiplicum; Allmon, p. 61.
- Reference Allmon1990
Monoptygma curtum; Allmon, p. 61.
- Reference Allmon1990
Monoptygma leai; Allmon, p. 60, pl. 9, fig. 9.
- Reference Allmon1990
Monoptygma lymneoides; Allmon, p. 60.
Type material
Conrad (Reference Conrad1832–1835) apparently did not designate a holotype for M. lymneoides (see Moore, Reference Moore1962, p. 72); lectotype (ANSP 15619) selected by Palmer (Reference Palmer1937, p. 297) with eight other specimens under the same number (all apparently lost; J. Sessa, personal communication, 11/12/21); holotype M. alabamiensis Lea, ANSP 5929; paratype ANSP 5930; hypotype (Palmer, Reference Palmer1937), PRI 3036; holotype M. curta ANSP 15618; syntypes Monoptygma leai FMNH-UC 24671 (5 specimens); hypotype (Palmer, Reference Palmer1937) PRI 3026; Conrad's holotype Monoptygma crassiplica probably lost (fide Palmer, Reference Palmer1937, p. 298; Moore, Reference Moore1962, p. 51); hypotype (Palmer, Reference Palmer1937) PRI 3027.
Occurrence
Alabama: middle Eocene (Lutetian–Bartonian), Upper Lisbon Formation, Gosport Sand (Locs. AL-MO-2a, AL-MO-5); Texas: middle Eocene (Ypresian–Luettian), Weches Formation, Stone City Beds (Loc. TX-BA-1, TX-RO-1); Louisiana: middle Eocene (Lutetian–Bartonian), Cook Mountain Formation (LA-BI-1, LA-OU-1).
Revised description
Shell lanceolate in shape, with aperture equal to about two-thirds of shell height. Protoconch unknown. Body whorl profile usually grades smoothly into spire profile, but occasionally slightly shouldered. Single distinct columellar fold on the middle of the parietal lip. Spire slightly acuminate, with faint axial ribbing on adapical margins of the whorls. Spire sutures moderately callused. Parietal callus moderately developed, extending about one-half to one-third of the way across the body whorl and about halfway between posterior end of aperture and suture. Faint anterior band, consisting of slight deflection of the growth lines, on body whorl, sometimes with faint ridge on posterior edge. Anterior end of body whorl ends in a simple tapered point.
Other material examined
PRI 56353 (7 specimens); MCZIP 29252 (1 specimen); MCZIP 29247 (1 specimen); ANSP 13274 (1 specimen); PRI 104504 (2 specimens); PRI 83948 (1 specimen); PRI 104514 (2 specimens).
Remarks
Monoptygma lymneoides has been oversplit; the various named forms mostly do not overlap in time and grade into one another, forming a single variable lineage. Monoptygma lymneoides from the upper middle Eocene Gosport Sand is the largest form. Monoptygma curta, also from the Gosport, is known only from the holotype. Palmer (Reference Palmer1937, p. 298) said that it “differs from the young of M. lymneoides in being broader and shorter” but also closely resembles some specimens of M. leai from the underlying Cook Mountain/Lisbon formations. Palmer (Reference Palmer1937, p. 297) described M. leai as “beautiful and distinct,” but it intergrades with specimens of M. lymneoides (compare Fig. 17.1–17.4). Monoptygma crassiplica occurs in the middle Eocene Weches and Stone City beds in Texas and the Cook Mountain Formation of Louisiana. It also intergrades with M. lymneoides. Palmer (Reference Palmer1937, p. 298) mentioned that a specimen of crassiplica “in the U.S. Nat. Museum from Holstein's well, 5 miles southeast of Gibbsland, Bienville Parish, La. was drawn by G.D. Harris for his Texas Eocene MS” and published that figure as her pl. 38, fig. 4. We have not been able to locate Harris’ specimen, and the drawing is reproduced here as Figure 17.19. The specimen ANSP 5929 (Figure 17.15, 17.16) was listed as the holotype of Monoptygma alabamiensis by Palmer (Reference Palmer1937, p. 297). A query (“?”) was added to this designation in Palmer and Brann (Reference Palmer and Brann1966, p. 780). The specimen generally resembles Lea's figure (Reference Lea1833, pl. 6, fig. 201), but the apex may have been damaged.
As explained by Wheeler (Reference Wheeler1935, p. 103–105), Conrad (Reference Conrad1833) was published on August 29, while Lea (Reference Lea1833) was published on December 2, therefore Conrad's name lymneoides has priority.
Genus Olivula Conrad, Reference Conrad1832
Type species
Ancillaria staminea Conrad, Reference Conrad1832, by subsequent designation Cossmann (Reference Cossmann1899, p. 70).
Remarks
Lamarck (Reference Lamarck1811) proposed the name Ancillaria, but it is generally synonymized with Ancilla Lamarck, Reference Lamarck1799 (e.g., Kilburn, Reference Kilburn1981, p. 358). In 1832, Conrad proposed the species Ancillaria staminea from the Claibornian Eocene of Alabama and said that it closely resembled Ancillaria canalifera (Lamarck, Reference Lamarck1803), from the Eocene of France. But he then suggested (Conrad, Reference Conrad1832, p. 25) that “[t]hese two species do not correspond entirely with the genus Ancillaria, as the aperture is much longer, the shells are striated, and the suture is somewhat channeled;” he therefore stated that these two species “might constitute a separate genus by the name of Olivula.” Cossmann (Reference Cossmann1899, p. 70) designated A. staminea Conrad as the type species of Olivula. Wenz (Reference Wenz and Schindewolf1943, p. 1277) and Glibert (Reference Glibert1960, p. 19) also placed it there, as did Tracey et al. (Reference Tracey, Todd, Le Renard, King and Goodchild1996) and Garvie (Reference Garvie1996, p. 87), who made Olivula a subgenus of Ancilla. Meanwhile, as noted by Palmer (Reference Palmer1937, p. 429), Bellardi (Reference Bellardi1882) had used canalifera as the type species of his genus Ancillarina. Palmer argued that canalifera and staminea differ enough to be separated at “sectional” (i.e., subgeneric) rank, and so retained staminea in Olivula, which she treated as a subgenus of Ancilla.
In his comprehensive review of the genus Ancilla, Kilburn (Reference Kilburn1981, p. 356) treated Ancillarina as a separate genus (possibly “a sister group” of Olivula) containing “Ancilla-like species with a similarly divided fasciolar band but a total lack of callus on the spire whorls and sutures,” and represented by fossils from the Eocene of Java and possibly the Cretaceous of Burma.
Even though canalifera lacks the callused sutures characteristic of staminea, Garvie (Reference Garvie2013, p. 60) assigned both canalifera and staminea to Ancillarina, suggesting that it be given subgeneric rank in Olivula. Garvie (Reference Garvie2013, p. 60) also noted a change in the form of the suture callus or “collar band” over time in the three named subspecies of staminea, with punctulifera from the middle Eocene Claibornian and maternae from the lower Eocene showing “a steady decrease in the strength and sagittal angle of the [growth] lines” on the callus.
The marked callusing of the suture in staminea distinguishes it from canalifera, to which it is otherwise quite similar in overall shape, so we do not combine the two species in Ancillarina. It nevertheless seems useful to retain Olivula as a separate genus-level taxon, with a single, somewhat variable, species extending throughout much of the Gulf Coast Eocene.
The cancellate sculpture on the body whorl of O. staminea separates it from all other Coastal Plain olivoids. If, as implied by our phylogenetic analysis (Fig. 5), it is derived from Eoancilla (see Fig. 20), this would make Eoancilla paraphyletic. Further exploration of late Paleocene faunas in the Coastal Plain might further elucidate this relationship.
Olivula staminea (Conrad, Reference Conrad1832)
Figure 19.4–19.10
- Reference Conrad1832
Ancillaria staminea Conrad, p. 25, pl. 10, fig. 5.
- Reference Conrad and Morton1834b
Ancillaria staminea; Conrad, p. 5.
- Reference Duclos1835
Oliva staminea; Duclos, pl. 18, figs. 9, 10.
- Reference Duclos1844
Oliva staminea; Duclos, p. 11, pl. 20, figs. 9, 10.
- Reference Conrad1846
Ancillaria staminea; Conrad, p. 220.
- Reference Lea1849
Ancillaria staminea; Lea, p. 96.
- Reference d'Orbigny1850
Ancyllaria staminea; d'Orbigny, p. 352.
- Reference Tuomey1858
Ancillaria staminea; Tuomey, p. 264.
- Reference Conrad1858
Anaulax staminea; Conrad, p. 166.
- Reference Gabb1860
Agaronia punctulifera Gabb, p. 381, pl. 67, fig. 22.
- Reference Conrad1865a
Olivula punctulifera; Conrad, p. 22.
- Reference Conrad1865a
Olivula staminea; Conrad, p. 22.
- Reference Conrad1866
Olivula staminea; Conrad, p. 17.
- Reference Conrad1866
Olivula punctulifera; Conrad, p. 17.
- Reference Tryon1883
Olivula staminea; Tryon, p. 61, pl. 3, figs. 24, 25.
- Reference Aldrich1886
Ancillaria staminea; Aldrich, p. 51.
- Reference de Gregorio1890
Agaronia punctulifera; de Gregorio, p. 54.
- Reference de Gregorio1890
Ancilla (Olivula) staminea; de Gregorio, p. 57, pl. 4, figs. 5–8, 17, 18 [copied Conrad, Reference Conrad1832, in part].
- Reference Heilprin1891
Olivula punctulifera; Heilprin, p. 398.
- Reference Cossmann1893
Olivula staminea; Cossmann, p. 41.
- Reference Harris1895b
Ancillaria staminea; Harris, p. 42.
- Reference Harris1899a
Ancilla (Olivula) staminea; Harris, p. 30, pl. 3, fig. 13.
- Reference Cossmann1899
Ancilla (Olivula) staminea; Cossmann, p. 70, pl. 3, figs. 10, 11.
- Reference Palmer1937
Ancilla staminea; Palmer, p. 428, pl. 68, figs. 7, 9, 11.
- Reference Palmer1937
Ancilla staminea maternae Palmer, p. 430, pl. 68, figs. 3, 8.
- Reference Palmer1937
Ancilla staminea punctulifera; Palmer, p. 429, pl. 68, figs. 10, 17.
- Reference Wenz and Schindewolf1943
Ancilla (Olivula) staminea; Wenz, p. 1277, fig. 3635 [copied Cossmann, Reference Cossmann1899].
- Reference Shimer and Shrock1944
Olivula staminea; Shimer and Shrock, p. 511, pl. 210, fig. 16 [copied Conrad, 1832].
- Reference Brann and Kent1960
Ancilla staminea; Brann and Kent, p. 44.
- Reference Brann and Kent1960
Ancilla staminea maternae; Brann and Kent, p. 44.
- Reference Brann and Kent1960
Ancilla staminea punctulifera; Brann and Kent, p. 44.
- Reference Glibert1960
Ancilla (Olivula) staminea punctulifera; Glibert, p. 19.
- Reference Glibert1960
Ancilla (Olivula) staminea; Glibert, p. 19.
- Reference Brann and Kent1960
Ancilla (Olivula) staminea; Brann and Kent, p. 44.
- Reference Palmer and Brann1966
Ancilla (Olivula) staminea; Palmer and Brann, p. 492.
- Reference Palmer and Brann1966
Ancilla staminea maternae; Palmer and Brann, p. 492.
- Reference Palmer and Brann1966
Ancilla staminea punctulifera; Palmer and Brann, p. 493.
- Reference Dockery1980
Ancilla staminea punctulifera; Dockery, p. 114, pl. 176, fig. 3.
- Reference Garvie1996
Ancilla (Olivula) staminea reklawensis Garvie, p. 87, pl. 19, figs. 15, 16.
Type material
Lectotype (Palmer, Reference Palmer1937, p. 429) ANSP 14670; hypotypes (Palmer, Reference Palmer1937) PRI 3284, 3285; holotype Ancilla staminea maternae PRI 3282; holotype Agaronia punctulifera ANSP 30729; hypotype (Palmer, Reference Palmer1937) PRI 3283; holotype Ancilla staminea reklawensis PRI 30425; paratype PRI 30426.
Occurrence
South Carolina: middle Eocene (Bartonian), McBean Formation (Loc. SC-OR-1); Alabama: lower Eocene (Ypresian), Bashi Formation (Loc. AL-CL-2), middle Eocene (Lutetian–Bartonian), Lisbon Formation, Gosport Sand (Locs. AL-MO-2a, AL-MO-5); Mississippi: middle Eocene (Lutetian–Bartonian), Dobys Bluff Tongue, Cook Mountain Formation (Locs. MS-CL-1, MS-NE-1, MS-NE-2, MS-NE-3); Louisiana: middle Eocene (Lutetian–Bartonian), Cook Mountain Formation (Locs. LA-BI-2, LA-OU-2, LA-OU-3, LA-SA-3); Texas: middle Eocene (Ypresian–Lutetian), Stone City Beds, Reklaw Formation, Wheelock Member, Cook Mountain Formation (Locs. TX- RO-1, TX-BA-1).
Revised description
(Revised by Palmer, Reference Palmer1937, p. 428–429) “Nucleus consists of two and a half smooth whorls; whorls of the spire crowded, those of the apex enveloped in the lower whorls; heavy, sutural callus collar extends over the upper margin of the lower whorl and lower margin of the preceding whorl with the suture a groove along the midline of the collar; the callus has deep sagittate longitudinal lines; in most cases the sutural collar covers most of the surface of the whorls of the spire’ shell covered with coarse, longitudinal lines crossed by coarse, spiral lines which give the surface a fine, cancellated appearance.”
Other material examined
PRI 104505 (17 specimens); PRI 56421 (77 specimens).
Remarks
Olivula staminea is a distinctive, abundant, long-lived, and widespread species. From youngest to oldest, in addition to Ancilla staminea s.s. from the Bartonian Gosport Sand, it includes three named temporal subspecies: A. s. punctulifera from the Lutetian Stone City Beds and the Wheelock Member of the Cook Mountain Formation in Texas; A. s. reklawensis from the upper Ypresian Reklaw Formation of Texas; and A. s. maternae from the lower Ypresian Bashi Formation of Alabama (Fig. 20).
Our phylogenetic analysis (see below) suggests that this species may have been derived from a species of Eoancilla, perhaps E. mediavia.
Genus Palmoliva new genus
Type species
Ancillaria tenera Conrad, Reference Conrad1834a, by original designation herein.
Diagnosis
Spire one-third or less of total shell height. Aperture one-half to one-third total shell height. Protoconch incompletely known, but probably of 2–3 smooth whorls. Sutures callused. Spire and body whorl strongly to moderately shouldered, with shoulders bearing faint to moderate axial sculpture. Shell otherwise smooth. Olivoid band moderate to faint, weakening but persisting on dorsal side. Anterior band pronounced, with posterior margin marked by a sharp ridge. Plication plate narrow and simple. Anterior end of columella a simple point.
Etymology
Named in honor of Katherine Palmer, author of many of the taxa discussed in this paper.
Remarks
Palmer (Reference Palmer1937) placed two similar species, Ancillaria tenera Conrad, Reference Conrad1834a, and Ancillaria scamba Conrad, Reference Conrad1832, in Bullia, but they do not belong there because, although they both have simple, pointed anterior columellar ends, they both show well-developed olivoid and anterior bands, which are not present in Bullia. These two species share pronounced shouldering on spire and body whorls and faint to moderate axial sculpture on those shoulders, features that are not present together in any other taxa discussed here. We therefore place them both in a new genus, Palmoliva.
Palmoliva scamba (Conrad, Reference Conrad1832) new combination
Figure 18.10–18.13
- Reference Conrad1832
Ancillaria scamba Conrad, p. 25, pl. 10, fig. 4.
- Reference Lea1833
?Anolax plicata Lea, p. 181, pl. 6, fig. 194.
- Reference Lea1849
?Anolax plicata; Lea, p. 96.
- Reference Conrad1854
Ancilla scamba; Conrad, p. 30.
- Reference Conrad1865a
Ancillopsis scamba; Conrad, p. 22.
- Reference Conrad1865a
Olivula ? plicata; Conrad, p. 22.
- Reference Conrad1866
Ancillopsis scamba; Conrad, p. 17.
- Reference Conrad1866
Olivula ? plicata; Conrad, p. 17.
- Reference Tryon1883
Ancillaria (Ancillopsis) scamba; Tryon, p. 61, pl. 3, fig. 26.
- Reference de Gregorio1890
Ancilla scamba; de Gregorio, p. 55, pl. 4, figs. 12, 13, 15, 16 [copied Conrad, Reference Conrad1832, in part].
- Reference de Gregorio1890
Ancilla (Olivula) plicata; de Gregorio, p. 57, pl. 4, fig. 9 [copied Lea, Reference Lea1833, pl. 6, fig. 194].
- Reference Cossmann1893
Ancillina scamba; Cossmann, p. 40.
- Reference Cossmann1893
Ancillina ? plicata; Cossmann, p. 40.
- Reference Harris1895b
?Anolax plicata; Harris, p. 35.
- Reference Cossmann1901b
Ancilla (Olivula) plicata; Cossmann, p. 223.
- Reference Cossmann1901b
Buccinanops (Bullia) scambum; Cossmann, p. 223, pl. 9, fig. 23 [plate captions for figs. 23 and 14 are reversed].
- Reference Palmer1937
Bullia scamba; Palmer, p. 290, pl. 44, figs. 2, 7.
- Reference Brann and Kent1960
Bullia scamba; Brann and Kent, p. 140.
- Reference Glibert1963
Bullia scamba; Glibert, p. 98.
- Reference Palmer and Brann1966
Bullia scamba; Palmer and Brann, p. 544.
- Reference Allmon1990
“Bullia” scamba; Allmon, p. 58, pl. 9, fig. 2.
Type material
Lectotype (plus 10 specimens) (selected by Palmer, Reference Palmer1937, p. 291 [fide Moore Reference Moore1962, p. 95]) ANSP 14647; hypotype (Palmer, Reference Palmer1937) PRI 3082.
Occurrence
Alabama: middle Eocene; Gosport Sand (Locs. AL-CL-1; AL-MO-2a).
Revised description
Protoconch unknown. Earliest known whorls smooth. Spire up to one-third of total height. Callus extending adapically of posterior end of aperture, giving sutures a callused form. Spire and body whorl moderately shouldered, with shoulders bearing faint to moderate axial sculpture. Posterior edge marked by ridge. Olivoid band faint and weakens but persists on dorsal side. Anterior band marked by strong growth lines, which are concave anteriorly. Posterior margin of anterior band is a sharp ridge, more pronounced in juvenile specimens. Plication plate narrow and simple. Anterior end of columella a simple point. Aperture height usually about half of total height; aperture usually about half or less of total maximum width.
Other material examined
PRI 57505 (8 specimens); PRI 57499 (2 specimens); PRI 63642 (4 specimens); PRI 104511 (3 specimens); PRI 104512 (1 specimen); PRI 104513 (3 specimens).
Remarks
Palmer (Reference Palmer1937) noted that scamba is similar to Monoptygma lymneoides in having a similar overall shell shape and similar anterior notch and callused sutures but differs in lacking the single well-developed plication on the columella. Palmer (Reference Palmer1937, p. 291) suggested that Anolax plicata Lea, Reference Lea1833 (the lectotype of which, ANSP 5910, is lost; J. Sessa, personal communication, 11/12/21) may actually have been a juvenile of tenera.
Palmoliva tenera (Conrad, Reference Conrad1834) new combination
Figure 18.1–18.9
- Reference Conrad1834a
Ancillaria tenera Conrad, p. 147.
- Reference Conrad1835
Ancillaria tenera; Conrad, p. 42, pl. 16, fig. 5.
- Reference Conrad1865a
Ancillopsis tenera; Conrad, p. 22.
- Reference Conrad1866
Ancillopsis tenera; Conrad, Reference Conrad1866, p. 17.
- Reference de Gregorio1890
Ancilla tenera; de Gregorio, p. 56, pl. 4, fig. 2 [copied Conrad, 1835, pl. 16, fig. 5]
- Reference Palmer1937
Bullia tenera; Palmer, p. 291, pl. 42, figs. 7–13.
- Reference Brann and Kent1960
Bullia tenera; Brann and Kent, p. 140.
- Reference Palmer and Brann1966
Bullia tenera; Palmer and Brann, p. 54.
- Reference Allmon1990
“Bullia” tenera; Allmon, p. 58, pl. 9, figs. 1, 3.

Figure 18. Palmoliva n. gen. (1–9) Palmoliva tenera n. comb.: (1, 2) Ancillaria tenera holotype ANSP 14646; height 29.7 mm. (3, 4) Bullia tenera hypotype PRI 3065 (from Palmer, Reference Palmer1937); height 23.3 mm. (5–7) Bullia tenera hypotype PRI 3064; height 26 mm; scale bar on (7) = 500 μm. (8, 9) Bullia tenera hypotype PRI 3066 (from Palmer, Reference Palmer1937); height 41 mm. (10–13) Palmoliva scamba n. comb.: (10, 11) Bullia scamba hypotype PRI 3082; height 35.9 mm. (12, 13) Ancillaria scamba lectotype ANSP 14647; height 36.7 mm.
Type material
Holotype ANSP 14646; hypotypes (Palmer, Reference Palmer1937) PRI 3064 (not “3074” as in Palmer, Reference Palmer1937, p. 634), 3065, 3066.
Occurrence
Alabama: middle Eocene (Bartonian), Gosport Sand (Loc. AL-MO-2a); Texas: middle Eocene (Lutetian), Stone City Beds (Loc. TX-RO-1); Louisiana: middle Eocene (PRI collection, exact localities unknown).
Revised description
Protoconch incompletely known but probably of 2–3 smooth whorls. Spire less than one-fifth of total height. Callus extending adapically of posterior end of aperture, giving sutures a callused form. Spire and body whorl strongly shouldered, with shoulders bearing faint to moderate axial sculpture. Olivoid band more marked in juveniles. Posterior edge marked by ridge, more pronounced on posterior end. Olivoid band weakens on dorsal side but persists as a broad depression with moderately deflected growth lines. Anterior band marked by strong growth lines that are concave anteriorly. Posterior margin of anterior band is a sharp ridge, more pronounced in juvenile specimens. Plication plate narrow and simple. Anterior end of columella a simple point. Aperture width at least half of total maximum width.
Remarks
The ANSP holotype is from the Gosport Sand of Alabama. PRI specimens are from older deposits to the west, including the Stone City Beds of Texas (PRI 3066) and “Louisiana. Exact data lost” (Palmer, Reference Palmer1937, p. 10, 292) (PRI 3064, 3065).
Palmoliva tenera n. comb. differs from P. scamba n. comb. mainly in having a lower spire, wider aperture, and more pronounced shouldering. Palmer (Reference Palmer1937) noted that there was considerable variation in form among individuals in this species, but most of this variation appears to be ontogenetic. Younger individuals have lower spire, wider aperture, more pronounced olivoid and anterior bands, and more pronounced shoulders with stronger axial sculpture.
Genus Micrancilla Maxwell, Reference Maxwell1992
Type species
By original designation, Amalda (Micrancilla) granum Maxwell, Reference Maxwell1992 (Priabonian, New Zealand).
Other included species
Micrancilla alibamasiana Pacaud, Merle, and Pons, Reference Pacaud and Cazes2013 (Bartonian, Alabama); M. antipodarum Pacaud, 2014 (Lutetian, France); M. dilatata (Cossmann, Reference Cossmann1886) (Lutetian, France); M. guanensis Pacaud, Merle, and Pons, 2013 (Ypresian, France); M. oesiensis Pacaud, Merle, and Pons, Reference Pacaud and Cazes2013 (Lutetian, France). Maxwell (Reference Maxwell1992, p. 143) stated that there are also undescribed species from New Zealand that belong to this taxon.
Original diagnosis
(Maxwell, Reference Maxwell1992, p. 143) “Shell very small for subfamily, narrowly ovate, spire elevated, apex rather broad, well-rounded. Parietal callus thin, ascending almost vertically from top of columella then bending back sharply, running parallel to and at some distance from base of callus band on posterior portion of whorls. Suture barely hidden by callus. Aperture small, narrowly ovate, columella short, nearly vertical with a few narrow plaits.”
Micrancilla alibamasiana Pacaud, Merle, and Pons, Reference Pacaud and Cazes2013
Figure 19.1–19.3

Figure 19. Micrancilla and Olivula. (1–3) Micrancilla alibamasiana holotype MNHN.F.J13251 (from Pacaud, Reference Pacaud2014); height 5 mm. (4–10) Olivula staminea: (4, 5) Ancillaria staminea lectotype ANSP 14670; height 31.8 mm. (6, 7) Ancilla staminea maternae holotype PRI 3282; height 25.3 mm. (8, 9) Ancilla staminea reklawensis holotype PRI 30425; height 15.4 mm. (10) Agaronia punctulifera holotype ANSP 30729; height 6.8 mm.
Type material
Holotype MNHN.F.J13251.
Occurrence
Alabama: middle Eocene (Bartonian); Gosport Sand (Locs. AL-CL-1; AL-MO-2a).
Original description
(Translation from Pacaud et al., Reference Pacaud and Cazes2013.) Small, narrow, elongated shell, cylindrical, thick test, consisting of about 5 whorls which are separated by indistinct suture lines in adulthood. The protoconch, with a pointed “button”, consists of 2 whorls. The apical angle is 35°. The spire, high, is covered by a thick callus which hides the suture on the last turn, and which extends to the adapical part of the final whorl by forming a bead. The parietal callus is thin, rising almost vertically from the top of columella and then bending sharply backwards, runs parallel, at a certain distance from the boundary of the spiral callus, and produces a narrow and unglazed spiral band on the adapical part of the spire. The callus extends over the neck, up to the fasciolar groove. The last whorl is elongated, cylindrical. The unglazed band is wide, well demarcated, strongly marked by the increments (growth lines), separated from the whorl by a clear limit of the spiral callus. The ancilline groove is well developed, narrow, linear, and deep. The ancilline band is very narrow, depressed, marked by the increases (growth lines). The neck is covered by a broad undivided fasciole. The posterior area is provided with a weak median ridge, blunt. Columellar torsion, short, slightly sinuous, not truncated and separated from the fasciole by a wide and deep fasciole groove, is decorated with 5 folds, wide and flat. The aperture, occupying a little less than half of the total height, is olivoid, contracted at its parietal angle. The siphonal notch is sinuous and widely marked. The labrum, beveled, orthoclinically oriented, is thickened in its terminal part. It presents an opisthocyrt outline in its adapical part, above the parietal angle of the opening. Exposure to UV light does not show residual colored pattern.
Remarks
This is the only species of this genus in the Americas. It is apparently rare, as we have not encountered any specimens of it in the Gosport Sand, despite intensive sampling (e.g., CoBabe and Allmon, Reference CoBabe and Allmon1994; Pietsch et al., Reference Pietsch, Harrison and Allmon2016).
Discussion and conclusions
An evolutionary tree based on the cladogram in Figure 5.2 and the stratigraphic ranges shown in Figure 2 are presented in Figure 20; they suggest that the basal diversification of ancillariids in the Coastal Plain occurred in the early to middle Paleocene. There are several significant ghost ranges, suggesting that the pre-middle Eocene record is less complete than that from the middle Eocene. This species-level diversification is slightly earlier than the Eocene global diversification of olivoid and ancillariid genera (Fig. 21).

Figure 20. Evolutionary tree of the taxa discussed here, based on the cladogram in Figure 5.2 and the stratigraphic ranges in Figure 2.

Figure 21. Global diversity of genera and subgenera since the late Cretaceous of (1) Olivoidea, and (2) only Ancillariidae. Data from Table 4.
Table 4. Described genera/subgenera (n = 84) in the Superfamily Olivoidea (sensu Kantor et al., Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017). References: (1) Kilburn (Reference Kilburn1981); (2) Sepkoski (Reference Sepkoski2002); (3) Kollman and Peel (Reference Kollman and Peel1983); (4) Ninomiya (Reference Ninomiya1990); (5) Ninomiya (Reference Ninomiya1988); (6) Kilburn (Reference Kilburn1977); (7) this paper; (8) Kantor et al. (Reference Kantor, Fedosov, Puillandre, Bonillo and Bouchet2017); (9) Petuch and Sargeant (Reference Sterba1986); (10) Vermeij (Reference Vermeij1998); (11) Olsson (Reference Olsson1956); (12) Absalão and Pimenta (Reference Absalão and Pimenta2003); (13) Watters and Fleming (Reference Watters and Fleming1972); (14) Pacaud et al. (Reference Pacaud, Merle and Meyer2000); (15) Tracey et al. (Reference Tracey, Todd, Le Renard, King and Goodchild1996); (16) Pacaud et al. (Reference Pacaud, Merle and Pons2013); (17) Kantor and Bouchet (Reference Kantor and Bouchet2007); (18) Klappenbach (Reference Klappenbach1962); (19) Petuch (Reference Petuch1988); (20) Drez (Reference Drez1981); (21) Voskuil (Reference Voskuil2018).

Olivoids appear to have originated in the Cretaceous in the eastern Tethys, the region that now includes Madagascar, South Asia, and Japan, and soon spread to the Gulf Coast of North America and western Europe. These early olivoids were stem group ancillariids. The genus Micrancilla appears to have been part of this basal ancillariid radiation, perhaps originating in western Europe and spreading to New Zealand and America (Pacaud et al., Reference Pacaud, Merle and Pons2013; Pacaud, Reference Pacaud2014). This biogeographic history may explain the absence of Micrancilla in the Coastal Plain prior to the late middle Eocene.
The relative stratigraphic position of the four species of Eoancilla is consistent with them comprising a single ancestor-descendant lineage, perhaps including the ancestors of both Olivula staminea and all of the other species considered here.
The genus Agaronia, as presented here, is paraphyletic, and includes the ancestry of the oldest known species of the genus Oliva. Agaronia is widely distributed beyond the Coastal Plain up to the Recent; its comprehensive phylogenetic analysis is beyond the scope of this paper, so we have not subdivided it at the genus level.
Most of the species treated here have durations of <10 my (7 of 19 are known from a single formation), but three species are relatively long-lived (Anbullina elliptica, ca. 20–23 my; Ancillopsis altilis, ca. 20 my; Olivula staminea, ca. 18 my), and all three appear to show noticeable anagenetic change through these durations.
The Paleogene gastropods of the Coastal Plain are relatively well studied, but our analysis indicates that a significant number of taxonomic assignments should be changed. Nine of the 19 Coastal Plain species listed in Table 1 are here assigned to different genera and nine to different families than they were in the most recent authoritative summary more than 50 years ago (Palmer and Brann, Reference Palmer and Brann1966).
Acknowledgments
We are grateful to P. Ippel, C. Lenard, E. Schuster, and C. Wooten for assistance with morphometric analysis; to P. Callomon, K. Estes-Smargiassi, G. Rosenberg, and J. Sessa (Academy of Natural Sciences), R. Portell (Florida Museum of Natural History), W. Blow, M. Florence, and J. Nakano (National Museum of Natural History), J. Cundiff (Museum of Comparative Zoology), C. Copeland, A. Rindsberg, and T.L. Harrell (Geological Survey of Alabama), A. Klompmaker and M.B. Prondzinski (Alabama Museum of Natural History), S. Lidgard and P. Mayer (Field Museum), L. Appleton (University of Texas at Austin), and L. Skibinski (PRI) for facilitating access to collections under their care; to C. Hensen for discussion and assistance in the lab and field; to L. Dolin for donating a specimen; to B. Anderson, L. Ivany, P. Mikkelsen, C. Pietsch, and T. Yancey for discussion; to D. Dockery for discussion and providing specimen images; to K. Crowley and V. Wang for assistance with figures; to C. Matteson and K. Crowley for editorial assistance; and to two anonymous reviewers for comments on an earlier draft. Work on this project was supported in part by NSF-EAR grant 0719642, and by the donors to the Director's Discretionary Fund of the Paleontological Research Institution.
Declaration of competing interests
The authors declare none.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ffbg79cz6.
Appendix
Locality register
GSA = Geological Survey of Alabama localities; MGS = Mississippi Geological Survey localities (see Dockery, Reference Dockery1980); PRI = Paleontological Research Institution station numbers (see Palmer, Reference Palmer1937); TBEG = Texas Bureau of Economic Geology localities; USGS = U.S. Geological Survey station numbers.
Alabama
Twenty-two localities.
AL-CH-1.—Choctaw County. Left (north) bank of Tuckabum Creek under Highway 114 bridge, between Pennington and Lavaca; Nanafalia Formation (PRI collection).
AL-CH-2.—Choctaw County. Jackson (Geological Survey of Alabama collection).
AL-CH-3.—Choctaw County. “West end of Butler Road bed and bank by Judge Lindsey's farm” (Geological Survey of Alabama collection).
AL-CH-4.—Choctaw County. “1 mile E of Butler on Mt. Sterling Road” (Geological Survey of Alabama collection).
AL-CL-1.—Clark County. Little Stave Creek. ~3 miles north of Jackson, ~0.75 mile west of Highway 43. Gosport Sand (MGS 29).
AL-CL-2.—Clarke County. Woods Bluff, left bank of Tombigbee River; Bashi Marl (PRI 749; USGS 262, 2667, 3099, 3100, 5470, 6205, 6206, 6207, 7482).
AL-CL-3.—Clarke County. Bashi Creek; Bashi Marl (PRI collection).
AL-CL-4.—Clarke County. Knight's Branch; Bashi Formation (Geological Survey of Alabama collection).
AL-CL-5.—Clarke County. “1 mile N of Campbell, Highway 79 roadcut” (Geological Survey of Alabama collection).
AL-CL-6.—Clarke County. Satilpa Creek (Aldrich, Reference Aldrich1886; Palmer, Reference Palmer1937, p. 289).
AL-CL-7.—Clarke County. Cave Branch, several caves along a fork in a creek within the western half of S10-T11N-R2E; Bashi Marl (GSA 67).
AL-CO-6.—Coffee County. Elba Dam on Pea River; Bashi Marl (USGS 10013,10780).
AL-MA-1.—Marengo County. Nanafalia Landing, Tombigbee River; Nanafalia Formation (USGS 271, 5641).
AL-MO-2.—Monroe County. Claiborne Landing and Bluff, left bank Alabama River, downstream from Highway 84 bridge; 2a = Gosport Sand in bluff (MGS 28; PRI 104, 140; USGS 263, 2391, 2867); 2b = Upper Lisbon Formation exposed at base of bluff on river bank (PRI 103, 139; USGS 2395, 2396, 12171).
AL-MO-3.—Monroe County. Bell's Landing, left bank of Alabama River; Bells Landing Marl Member, Tuscahoma Formation (PRI 752; USGS 260, 2669, 3098, 5593, 5594, 5595).
AL-MO-4.—Monroe County. Gregg's Landing, right bank Alabama River just downstream of island; Greggs Landing Marl Member, Tuscahoma Formation (PRI 751; USGS 268, 2670, 3117, 3118, 3604, 5642).
AL-MO-5.—Monroe County. Lisbon Landing, Alabama River; Upper Lisbon Formation (USGS 3105, 5511, 6086).
AL-PE-1.—Perry County. “E.R. Showalter, Uniontown” (Geological Survey of Alabama collection).
AL-SU-3.—Sumter County. Black Bluff, Tombigbee River (PRI collection).
AL-WA-1.—Washington County. Hatchetigbee Bluff, right bank Tombigbee River; Hatchetigbee Formation (type section) (Toulmin, Reference Toulmin1977, loc. Awa-1).
AL-WI-1.—Wilcox County. One mile W of Oak Hill, Naheola Formation (PRI collection).
AL-WI-2.—Matthews Landing. Nine miles W of Camden, right bank of Alabama River at bend; Matthews Landing Marl (USGS 3116, 2671, 5596).
Arkansas
One locality.
AR-ST-1.—St. Francis County. Crow Creek. At bridge on Highway 70 ~2 miles east of Forest City (PRI 894, 1046).
Florida
One locality.
FL-LE-1.—Levy County. Quarry 2.9 miles S of town of Gulf Hammock, SW of state road 55 (UF collection).
Louisiana
Twelve localities.
LA-BI-1.—Bienville Parish. “Holstein's well, 5 miles southeast of Gibbsland” (Palmer, Reference Palmer1937, p. 298).
LA-BI-2.—Bienville Parish. Hammetts Branch, ~2 miles NW of Mt. Lebanon (PRI 730).
LA-GR-1.—Grant Parish. Montgomery Landing, Moodys Branch Formation (PRI 11).
LA-NA-1.—Natchitoches Parish. “Cultivated hill on L.E. Place's farm in the NE¼ NW¼ of sec. 22, T9N, R10W” (Barry and LeBlanc, Reference Barry and LeBlanc1942, p. 34).
LA-NA-2.—“Hillside at end of an old road in the NW¼ SE¼ NW¼ of sec. 36, T9N, R9W” (Barry and LeBlanc, Reference Barry and LeBlanc1942, p. 39).
LA-NA-3.—“Road cut along local road ion NE¼ SW¼ NE¼ of sec. 19, T9N, R8W” (Barry and LeBlanc, Reference Barry and LeBlanc1942, p. 39)
LA-OU-1.—Ouachita Parish. Monroe (PRI 735).
LA-OU-2.—Ouachita Parish. East bank, Ouashita River, Lapiniere Landing (PRI 756).
LA-OU-3.—Ouachita Parish. Brewer's, 1200 ft., Monroe (PRI 735).
LA-SA-1.—Sabine Parish. “About ¼ of a mile upstream from the bridge over the Sabine River on Louisiana Highway 6” (Barry and LeBlanc, Reference Barry and LeBlanc1942, p. 37).
LA-SA-2.—Sabine Parish. South bank of Slaughter Creek. In approximately the NW¼ SE¼ of sec 34, T6N, R13W (Barry and LeBlanc, Reference Barry and LeBlanc1942, p. 37).
LA-SA-3.—Sabine Parish. Sabine River bank (PRI 724, 725?).
Mississippi
Fifteen localities.
MS-CL-1.—Clarke County. Doby's Bluff. East side of Chickasawhay River (MGS 26).
MS-CL-2.—Clarke County. Garland Creek. Moodys Branch Formation (MGS 9).
MS-LA-1.—Lauderdale County. Low bluff behind Red Hot Truck Stop parking lot, on Interstate 10, east of Meridian; Bashi Marl (MGS 19).
MS-LA-2.—Lauderdale County. Large concretions placed along 31st Street exit, south of I-20, Meridian; Bashi Marl (MGS 20).
MS-HI-1.—Hinds County. Town Creek, Jackson (MGS 1).
MS-HI-2.—Hinds County. Riverside Park, Jackson (MGS 2).
MS-HI-3.—Hinds County. Moodys Branch, Jackson (MGS 3).
MS-HI-4.—Hinds County. Sewer excavation across Town Creek, Jackson (MGS 7).
MS-NE-1.—Newton County. “Hill on south side of county road paralleling Interstate 20 along north side, 0.3 mile west of Mississippi Highway 15, just north of Newton” (TU 923; MGS 68).
MS-NE-2.—Newton County. Hickory (PRI 728).
MS-NE-3.—Newton County. Two miles N of Newton, on Rt. 15 (PRI 803).
MS-TI-1.—Tippah County. Roadcuts on north-facing slope of a tributary of Fourth Creek, 0.9 mile north of Providence School. Owl Creek Formation (USGS 25422).
MS-TI-2.—Tippah County. Bluffs on right bank of Owl Creek, 2.5 miles northeast of Ripley. Owl Creek Formation (USGS 541, 546, 594, 707, 6464, 6876, 25423).
MS-WA-23.—Warren County. “Kings Crossing. Four miles N of Kings Crossing, Vicksburg, MS. Road cut ~3 miles N of Mint Spring Bayou entrance to National Cemetery” (PRI 887).
MS-YA-1.—Yazoo County. Techeva Creek (MGS 11).
South Carolina
One locality
SC-OR-1.—Near Orangeburg (PRI 707, 708).
Tennessee
One locality.
TN-HA-1.—Hardeman County. Roadcut on Tennessee State Route 57, on west-facing slope of Muddy Creek valley, near Trimm's old mill site, 3.3 miles east of the road junction that is 1.5 miles south of Middleton. Clayton Formation, basal beds containing reworked Late Cretaceous fossils (Sohl, Reference Sohl1964, p. 325) (USGS 25420).
Texas
Sixteen localities.
TX-BA-1.—Bastrop County. Bluff on right bank of Colorado River, ~200 m downstream from Highway 71 bridge at Smithville, Viesca Member, Weches Formation (PRI 733, 767; TBEG loc 11-T-2; USGS 6088, 10386).
TX-BA-2.—Bastrop County. Dry creek at mouth of Colorado River (Loc. 11-T-101 of Garvie, Reference Garvie2013).
TX-BA-3.—Bastrop County. Solomon's Farm (Locs. 11-T-3, 11-T-13 of Garvie, Reference Garvie2013).
TX-BA-4.—Bastrop County. East bank of mouth of Gazley Creek, south side of Colorado River, Smithville, Queen City Formation (PRI 776; Price and Palmer, Reference Price and Palmer1928; Molineux et al., Reference Molineux, Zachos, Karadker, Hunt and Catlos2013).
TX-BA-5.—Bastrop County. Colorado River, 4 ± miles below Webberville, bed No. 3, Kincaid Formation (USGS 11696?, 11914, 12112) (Gardner, Reference Gardner1935, p. 231).
TX-BE-1.—Bexar County. Smith Tract, Somerset field, 659–680 feet and 769–782 feet (USGS 8656) (Gardner, Reference Gardner1935, p. 231).
TX-BR-1.—Brazos County. Little Brazos River, 2.5 miles above Stone City (PRI 727).
TX-BU-1.—Burleson County. Moseleys Ferry, Brazos River (PRI 723).
TX-FA-1.—Falls County. Quarry of Frost Crushed Stone Company, 1 km (0.62 mile) south of Highway 7 and ~17 km (10.6 miles) east of Marlin. Kincaid Formation, Tehuacana Limestone Member (Loc. FQ of Garvie, Reference Garvie2021).
TX-KA-1.—Kaufman County. Water Hill, 5 miles northeast of Kemp. Kincaid Formation (USGS 11665?) (Gardner, Reference Gardner1935, p. 231).
TX-MI-1.—Milam County. Joe Taylor Branch (Locality 20 of Garvie, Reference Garvie1996).
TX-MI-2.—Milam County. U.S.G.S. Station 11921, Brazos River, 1 mile below the Falls County line, Kincaid Formation (USGS 11921) (Gardner, Reference Gardner1935, p. 231).
TX-RO-1.—Robertson County. “Big Branch of Cedar Creek, east of Mr. Pollard's (deceased) farm, 3 miles N.W. of Stone City”; Stone City Beds (Palmer, Reference Palmer1937, p. 11) (PRI 766). (Palmer's listing of this location as in Burleson County was in error. She corrected it to Robertson County in Palmer and Brann [Reference Palmer and Brann1966, p. 779], citing Stenzel et al. [Reference Stenzel, Krause and Twining1957, p. 11]).
TX-SA-1.—Sabine County. Pendleton Bluff, Pendleton Formation (Locality 40 of Barry and LeBlanc, Reference Barry and LeBlanc1942).
TX-TR-1.—Travis County. Webberville, Kemp Clay (USGS 7601).
TX-WI-1.—Williamson County. Lower bed, Dry Brushy Creek, 6 miles south of Thrall on Taylor-Beaukiss road Wills Point Formation (USGS 10420) (Gardner, Reference Gardner1935, p. 231).
France
One locality.
FR-1.—Ducy, near Montepilloy (PRI collection).
Mexico
Two localities.
MX-NL-1.—Nuevo Leon. “On southeast slope of low hill 1 km east of triangulation point Palma, Carlos Cantu, General Bravo” (USGS 13554).
MX-TA-1.—Tamaulipas. 15.9 km SE of Ciudad Camargo (USGS 13504).
United Kingdom
One locality.
UK-WS-1.—West Sussex. Selsey Peninsula. Bracklesham Beds, Selsey Formation (Tracey et al., Reference Tracey, Todd, Le Renard, King and Goodchild1996; Squires, Reference Squires1997).





























