Symbiotics have been advocated as being beneficial to patients undergoing surgery. Postoperative complications are major concerns that directly affect the clinical evolution and hospital costs of surgical patients. The patient’s nutritional status and the ‘gut origin of sepsis’ theory have been suggested to explain these complications as the result of bacterial translocation (BT), whereby the altered intestinal permeability facilitates the passage of indigenous microbiota to mesenteric lymph nodes and the systemic circulation( Reference Correia, Liboredo and Consoli 1 ). Undernutrition, a condition widely present among hospitalised patients, further impairs the immunologic/inflammatory response, thus increasing the risk of infections( Reference van Bokhorst-De van der Schuer, von Blomberg-van der Flier and Riezebos 2 ).
Symbiotics, ‘a mixture of probiotics and prebiotics that beneficially affects the host by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract, by selectively stimulating the growth and/or by activating the metabolism of one or a limited number of health-promoting bacteria, and thus improving host welfare’( Reference Gibson and Roberfroid 3 ), have been reported to affect intestinal function and permeability( Reference Bazzocchi, Giovannini, Giussani, Brigidi and Turroni 4 ). The mechanisms of action can be related to the modulation of the intestinal barrier function, the production of cytokines and immunoglobulins with anti-inflammatory properties, and the capacity to compete against pathogenic bacteria and inhibit their adherence, thus reducing gut permeability and decreasing BT.
Beneficial effects of symbiotics in surgical patients have been shown for over a decade, both pre- and postoperatively, in patients undergoing laparotomy, liver transplantation and resection, colectomy, esophagectomy, pancreatoduodenectomy or other pancreas ressection( Reference Rayes, Hansen and Seehofer 5 – Reference Reddy, Macfie, Gatt, Larsen, Jensen and Leser 11 ). Rayes et al. reported that the use of symbiotics was associated with decreased infection and complication rates, less use of antibiotics and shorter length of hospital stay( Reference Rayes, Hansen and Seehofer 5 – Reference Rayes, Seehofer and Theruvath 7 ). However, other authors have not shown the same positive effects( Reference Krebs 12 , Reference Anderson, McNaught, Jain and MacFie 13 ) and, to our knowledge, no study has assessed the use of symbiotics in head and neck cancer patients.
It was our goal to evaluate the postoperative use of symbiotics in non-abdominal surgical patients (head and neck cancer patients) for intestinal function, intestinal permeability and postoperative outcomes. Thereby, diamine oxidase (DAO) enzyme was chosen for intestinal permeability assessment, as it has a relationship with intestinal maturity and integrity and can be considered a regulatory enzyme for intestinal mucosal proliferation( Reference Bieganski, Kusche, Lorenz, Hesterberg, Stahlknecht and Feussner 14 , Reference Takagi, Nakao, Ogura, Nabeshima and Kunii 15 )
Methods
This double-blind randomised controlled trial (RCT) was conducted at the Alfa Institute of Gastroenterology, Hospital das Clínicas da Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil. Patients were assessed for eligibility if they met the inclusion criteria: older than 18 years, undergoing elective head and neck cancer operations, and having access to the gastrointestinal tract after surgery. Exclusion criteria included the following: presence of inflammatory bowel disease, liver disease or other cancers; undergoing neoadjuvant chemotherapy; use of antibiotics or probiotics 1 month before the trial; and no access to the gastrointestinal tract after surgery. All patients gave written informed consent to enter the study, which was approved by the university ethics committee (CAAE 24375713.0.0000.5149) and registered at Clinical Trials (NCT02654652).
Patients were initially evaluated for baseline characteristics (age, sex, time since diagnosis, T staging of the tumour( Reference Edge and Compton 16 ) and tumour localisation) and for nutritional parameters (weight, subjective global nutritional assessment( Reference Detsky, McLaughlin and Baker 17 ) and hand grip strength( Reference Budziareck, Pureza Duarte and Barbosa-Silva 18 )). Patients were allocated by block randomisation into (A) treatment or (B) placebo groups. Patients and the research team were blinded to the interventions. After the operation, patients started receiving 6 g of treatment samples (109 colony-forming units/ml each of Lactobacillus paracasei LPC-31, L. rhamnosus HN00l, L. acidophilus NCFM, and Bifidobacterium lactis HN019 plus 6 g of fructo-oligosaccharides) or placebo samples (maltodextrin) packed in identical sachets (Invictus® Farmanutrition FQM Group), on the first postoperative day. The sachets were similarly diluted in 20 ml of water and administered twice a day, for at least 5 d and up to 7 d, through a nasoenteric catheter placed intraoperatively. Patients in both groups also received enteral nutritional therapy with a standard polymeric formula (4 kJ/ml (1 kcal/ml), 15 % protein, 55 % carbohydrate, 30 % lipids and 220 mOsm/l) on the 1st postoperative day, starting with 40 % of the estimated energetic goal (calculated by using 30 kcal/kg per d, as standardised by the hospital nutrition team) and continuing until 100 % of the energetic requirements were met on the third postoperative day.
Outcomes
The primary outcomes were intestinal function and permeability. Intestinal functional comprised the day of first evacuation, the number of total stool episodes within intervention days, stool consistency and gastrointestinal tract adverse symptoms and signs, such as flatulence, abdominal bloating, and cramps. Stool consistency and frequency data were collected daily questioning the patient, and the Bristol stool form scale( Reference Lewis and Heaton 19 ) was used to score the data. Gastrointestinal adverse effects were considered as the total number of symptoms reported by patients throughout the intervention days.
Secondary outcomes were postoperative complications such as wound infection, salivary fistula, pneumonia indicated by pulmonary infiltrates on chest X-ray associated with fever and positive culture results from sputum, bacteraemia with fever and positive blood culture results, sepsis identified as low blood pressure associated with fever and positive blood culture results, other infections with a temperature higher than 38·5°C treated with antibiotics, and haemodynamic instability with use of vasopressors( Reference Dindo, Demartines and Clavien 20 ). Length of hospital stay, calculated from admission day to discharge day; mortality within the first 30 d after surgery; and length of antibiotic therapy were also collected.
Laboratory data
Blood samples were drawn preoperatively and at the end of the intervention period to assess TNF-α cytokine levels, C-reactive protein (CRP) levels, global leucocyte count, and intestinal permeability by DAO.
All blood samples were centrifuged at 1000 g for 10 min and the serum was stored at −80°C until analysis. Serum TNF-α cytokine concentrations were measured by a high-sensitivity sandwich ELISA kit (DY210 Lot 1325880), in accordance with the manufacturer’s specifications (R&D Systems®). The serum DAO enzyme concentration was also determined by a sandwich ELISA kit (SEA656Hu), in accordance with the manufacturer’s specifications (Cloud-Clone Corporation®). Serum CRP and leucocyte levels were analysed by the hospital laboratory and recorded in the medical records.
Statistical analysis and sample size calculation
The sample size was calculated on the basis of an expected 30 % increase in DAO concentration after intervention, according to the data of Kamiya et al. ( Reference Kamiya, Nagino and Kanazawa 21 ) from a randomised trial. Adopting the criteria of Armitage & Berry( Reference Armitage and Berry 22 )(5 % significance and power of 80 %), we calculated the required sample size to be eighteen participants in each group.
Group comparisons were performed by using the Student’s t test and the Mann–Whitney U test (expressed as means and standard deviations and medians and ranges) for parametric and non-parametric data, respectively, and the χ 2 test for proportions. Between-treatment effects were evaluated with the Student’s paired t test and the Wilcoxon test for parametric and non-parametric variables, respectively. ANCOVA was used to compare median values between groups postoperatively, after adjusting for preoperative median values. A P value of <0·05 was considered statistically significant. Statistical analysis was performed with SPSS software, version 19.0.
Results
In all, forty-six patients were assessed for entry into the study from October 2014 until April 2016. Six were ineligible: one with oesophageal cancer, one with cirrhosis, and four because they had inoperable cancers. A total of forty were randomised (nineteen to the symbiotic group and twenty-one to the control group), as depicted in Fig. 1.
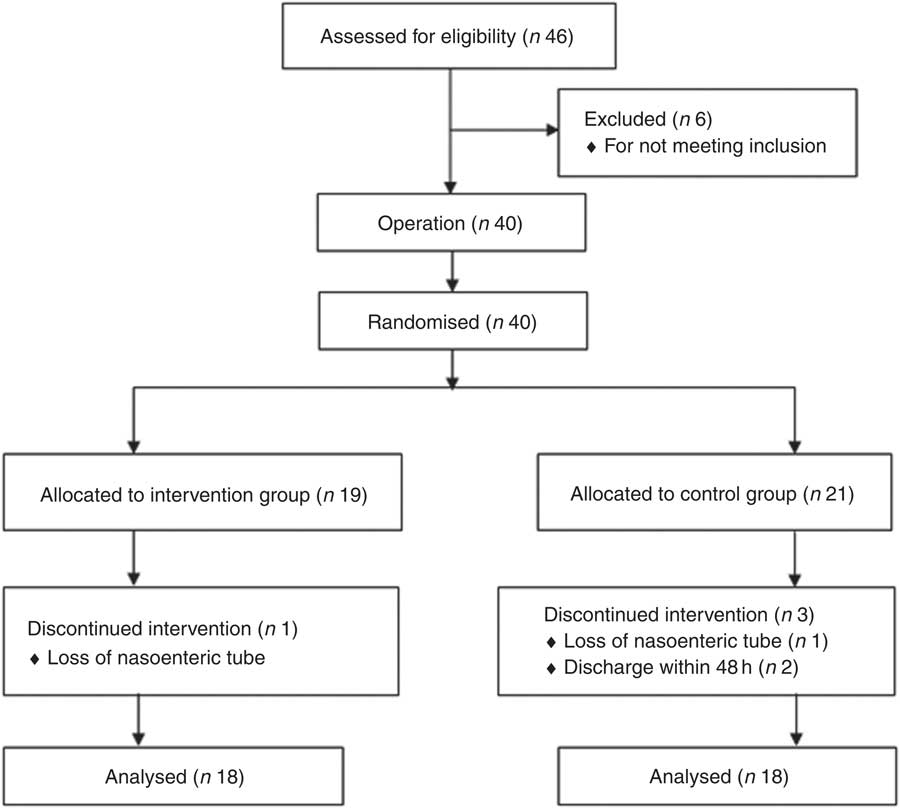
Fig. 1 Trial flow diagram.
Groups were homogeneous in terms of baseline characteristics and nutritional parameters (Table 1). Nutritional therapy was similarly provided in both groups. Patients received a mean of 4958 (sd 3531) kJ/d (1185·3 (sd 844·0) kcal/d) and 43 (sd 15·6) g/d of protein, which represents <70 % of mean nutritional requirements.
Table 1 Baseline characteristics and nutritional parameters (Mean values and standard deviations; numbers and ranges)
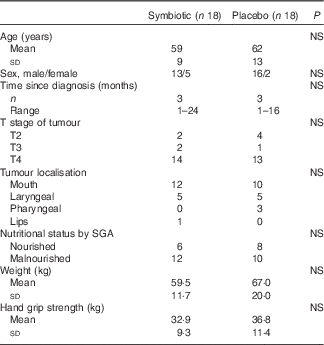
SGA, subjective global assessment.
Both groups presented with similar intestinal function after the intervention (Table 2). Fig. 2 contains data on stool consistency according to the Bristol stool form scale. Intestinal permeability by DAO activity is depicted in Table 3.
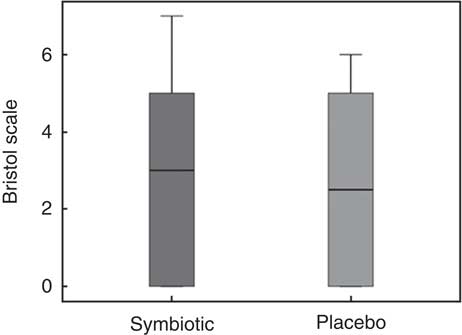
Fig. 2 Stool consistency by the Bristol Stool Scale, a visual scale that categorises stool into seven different consistencies, representing dried stool (1) to liquid stool (7); 0 represents no evacuation at all within the intervention period. Non-significant statistical results were found.
Table 2 Intestinal function (Medians and ranges)

Table 3 Intestinal permeabilityFootnote * (Mean values and standard deviations)
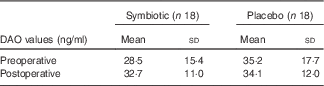
DAO, diamine oxidase.
* Statistical results were non-significant between pre- and postoperative periods and between symbiotic and placebo groups.
The number of patients with postoperative complications was similar in both groups (P>0·05), as shown in Table 4. There was a higher incidence of fistula in the symbiotic group (n 8) than in the control group (n 2; P<0·05). However, of the eight patients who had fistula in the symbiotic group, three were diagnosed within the first 48 h after surgery.
Table 4 Postoperative complications
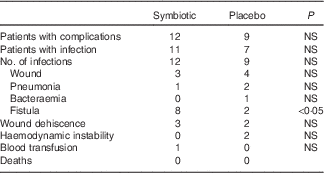
A total of eleven patients in the symbiotic and eight in the control group used antibiotics for therapeutic reasons (P>0·05). Patients in the symbiotic group had a median of 10·5 (range 3–90) d of hospitalisation compared with 9·0 (range 5–21) d in the control group (P>0·05). Mortality was null within 30 d of discharge.
Inflammatory markers were also similar in both groups. TNF-α cytokine concentrations had mean values in the symbiotic group ranging from 16·4 (sd 4·3) pg/ml in the preoperative period to 14·9 (sd 4·7) pg/ml in the postoperative period and in the control group ranging from 15·2 (sd 4·1) pg/ml preoperatively to 15·4 (sd 4·7) pg/ml postoperatively, without statistical significance between groups or between periods. For CRP levels (symbiotic: 3038·1 (SD 4847·6) to 10485·7 (SD 6676·2) nmol/l; control: 2933·3 (SD 3695·2) to 13571·4 (SD 9590·4) nmol/l and global leucocyte counts (symbiotic: 8·4 (sd 2·9) to 11·2 (sd 3·1) × 109/l; control: 8·7 (sd 3·0) to 11·5 (sd 5·0) × 109/l), there was an increase between pre- and postoperative periods (P<0·05), but no statistical difference between groups. Results are shown as means and standard deviations.
Discussion
Symbiotics are expected to have a positive impact on intestinal permeability, improving intestinal function and lowering postoperative complications. It has been postulated that they can inhibit the adherence and growth of pathogenic microorganisms through the production of immunomodulatory metabolites, antimicrobial substances and the formation of biofilms over the gut wall. Through a synergic effect, probiotics strains ferment prebiotics and produce SCFA that have immunomodulatory effects and suppress TNF-α production. Symbiotics can also induce the expression of the tight junction protein occludin, increasing the stability of tight junction proteins and intestinal barrier function( Reference Suchodolski and Jergens 23 ). Intestinal permeability can be negatively affected by metabolically stressful situations such as operations, some diseases, and nutritional status, and symbiotics can be used as potential modulators in this situation.
For head and neck cancers, surgical treatment is the primary option in most cases( Reference Galbiatti, Padovani-Junior, Maniglia, Rodrigues, Pavarino and Goloni-Bertollo 24 ). Patients with this type of cancer are at higher risk of postoperative complications, as they are usually malnourished, a condition known to directly impact on the immunologic system and the development of infection( Reference van Bokhorst-de van der Schueren, van Leeuwen, Sauerwein, Kuik, Snow and Quak 25 ). A high prevalence of malnutrition, about 60 %( Reference Jager-Wittenaar, Dijkstra, Vissink, van der Laan, van Oort and Roodenburg 26 ), is usually shown in the head and neck cancer population, data similar to our findings. Most likely, this depleted nutritional status is related to food restriction due to mechanical obstruction and odynophagia( Reference Gourin, Couch and Johnson 27 ). In addition, patients in this study had been diagnosed with cancer with an average of 3 months, ranging up to 24 months, which may have contributed to a worst nutritional status due to the longer food deprivation. However, there was no difference in the presence of malnutrition between the groups, which represents similar intestinal functionality, as, undernutrition can also alter intestinal permeability( Reference Takimoto, Yoshiuchi, Shimodaira and Akabayashi 28 ), affecting a patient’s intestinal health. The surgical procedure per se is another risk factor related to intestinal health.
Therefore, we analysed serum DAO concentrations, but found no differences between the symbiotic and control groups. Other authors who compared symbiotic use with a placebo in surgical patients also found no differences in permeability between groups( Reference Sugawara, Nagino and Nishio 29 , Reference Kanazawa, Nagino and Kamiya 30 ). Moreover, intestinal function, complications of infection, and inflammatory and immunomodulation markers were not different between our study groups.
There was a higher incidence of fistula in the symbiotic group, but most occurred in the first 48 h and were thus usually related to technical issues. Therefore, the impact of symbiotic treatment on these fistulas should be interpreted with caution, as complications related to the surgical technique cannot be modified by nutritional treatment or the modulation of microbiota( Reference Levine and Alverdy 31 , Reference Shogan, Smith, Christley, Gilbert, Zaborina and Alverdy 32 ).
Rammohan et al. ( Reference Rammohan, Sathyanesan and Rajendran 8 ), indicated that, contrary to our results, perioperative symbiotic administration was beneficial when compared with placebo in patients undergoing the Frey procedure for chronic pancreatitis. Patients received symbiotics orally three times daily, initiated 5 d preoperatively and up to 10 d after surgery. The infection rate was three times higher in the placebo group, with decreased antibiotic use and length of hospital stay in the symbiotic group( Reference Rammohan, Sathyanesan and Rajendran 8 ). A recent meta-analysis on the effects of perioperative probiotics and symbiotics on postoperative infections after gastrointestinal surgery also indicated beneficial effects of the administration of both probiotics and symbiotics( Reference Yang, Wu, Liu and Fan 33 ). It showed a reduction in postoperative infections, shorter lengths of hospital stay, and decreased antibiotic therapy for patients. Even after stratification of the studies assessing probiotics v. symbiotics, there were still beneficial results in both treatment modalities.
We hypothesise that several potential factors might explain why we obtained different results from those of the above-mentioned studies. First, the administration of symbiotics in the postoperative period in our study might not have had an impact on microbiota homoeostasis, the mucosal barrier, or endothelial function because of the timing of administration. In most RCT with beneficial symbiotic effects, the treatment was initiated before the operation, thus showing a preventive role. Second, because head and neck procedures do not directly involve bowel manipulation, symbiotics may have had a restricted impact compared with symbiotics used in RTCs conducted in patients who underwent major abdominal surgery. Moreover, in our study, the symbiotic combination was fructo-oligosaccharides plus Lactobacillus acidophilus, L. rhamnosus, L. paracasei and Bifidobacterium lactis, in contrast to the most frequently used prebiotic in other studies that showed positive effects, galacto-oligosaccharides, and the most frequently used probiotic bacteria: L. plantarum, L. acidophilus, L. casei and B. breve. These considerable differences raise an important point: the results may differ because of the probiotic species recommendation. Some studies report that some probiotic supplements may not modify the microbiota profile, especially the lactobacillus strains( Reference Wang, Chen and Jin 34 – Reference Gorshein, Wei and Ambrosy 37 ).
We analysed the bacterial profile by real-time PCR to identify potential pathogen colonisation (data not shown) that could explain some of the results. Although we found that Enterobacteriaceae species were increased after surgery in both the symbiotic and the control group, there was no statistical difference between them. Enterococcus species increased postoperatively in the symbiotic group, but this was also not statistically different from the numbers in the preoperative period or in the control group. These findings show there was an ongoing microbiota imbalance in these surgical patients despite interventions. Studies by Anderson et al. ( Reference Anderson, McNaught, Jain and MacFie 13 ) and McNaught et al. ( Reference McNaught, Woodcock, MacFie and Mitchell 38 ), with different study populations but similar results to ours, indicate the need for more trials. Anderson et al. compared two groups, symbiotic (L. acidophilus, L. bulgaricus, B. lactis, S. thermophilus+oligofructose) and placebo, aiming to evaluate the impact of symbiotics on BT, systemic inflammation and postoperative complications in patients undergoing elective laparotomies and starting the intake of symbiotics 12 d before the operation until the fourth postoperative day. There were no differences between groups in BT, systemic inflammation, length of hospital stay, incidence of postoperative infections or mortality. McNaught et al. followed patients who underwent major elective abdominal surgery and who received a probiotic drink (L. plantarum) 9 d preoperatively and 5 d postoperatively and assessed the impact on BT and septic morbidity. The authors found no differences in the number of positive mesenteric lymph nodes between groups, nor in the CRP values or the incidence of septic morbidity.
Limitations of the current study may be related to the method used to assess intestinal permeability. DAO activity seems to be a good test to evaluate intestinal permeability in humans when compared with urinary sugar excretion tests, such as the lactulose-mannitol ratio test, or with mesenteric lymph node cultures (analyses that require a minimum of 6 h of urinary collection and a surgical procedure, respectively). However, the DAO activity test may not be the most sensitive for identifying alterations in intestinal permeability in surgical patients, as antibiotic use and aging may interfere with its activity( Reference Maintz and Novak 39 , Reference Xie and Gan 40 ). In addition, our sample size was calculated to evaluate intestinal permeability, related to differences in DAO concentrations. Thus, this study was not statistically powered to compare groups in terms of complications and other assessed variables. Furthermore, it is important to address the postoperative administration of the symbiotics in which a shorter period of treatment may have been crucial to our results, in consideration of the mechanisms of action proposed to avoid postoperative complications( Reference Correia, Liboredo and Consoli 1 , Reference Suchodolski and Jergens 23 , Reference Jeppsson, Mangell and Thorlacius 41 ). It seems that a preventive preoperative approach could help minimise the negative impact caused by the surgical stress on the intestinal barrier. Prebiotics, probiotics, and symbiotics seem to present better results when administered preoperatively.
Conclusions
Postoperative symbiotics did not impact on intestinal function and postoperative outcomes of head and neck surgical patients.
Acknowledgements
The authors would like to thank the head and neck surgery team at the Hospital das Clínicas da Universidade Federal de Minas Gerais for cooperation during the research and for helping the development of this project, specially professor José Maria Porcaro Salles, surgeons Alexandre de Andrade Sousa and Guilherme de Souza Silva, and the residente Lívio Bruno Santos Cunha. The authors also thank Invictus® Farmanutrition FQM Group for providing the study samples and Fundação de Amparo e Apoio à Pesquisa de Minas Gerais (FAPEMIG) for the financial support.
The authors contributed to all research phases, including the formulation of the research question, designing the study, carrying it out, data analysis and writing.









