INTRODUCTION
Since the 1990s, nosocomial outbreaks due to extended spectrum β-lactamase-producing enterobacteria (ESBL-E) have been increasingly reported worldwide. Knowing that intestinal carriage is the main reservoir of these organisms, it has been suggested that gut colonization is associated with a high risk for developing self- and cross-infections with ESBL-E producers [Reference Smith1, Reference Castillo-Garcia2]. Although carriers of ESBL-E producers are expected to be present in general practice, there are few studies conducted in the community or in hospital settings during non-outbreak situations [Reference Kader and Kamath3]. In developing countries, prevalence studies on the faecal carriage of ESBL-E are scarce, whereas the burden of linked infections is increasing [Reference Mirellis4, Reference Andriatahina5]. Bacterial infections are much more frequent in cirrhotic patients than in the general population [Reference Gustot6], with episodes of infection reaching up to 40% of hospitalized patients [Reference Borzio7]. In a previous study [Reference Fam8] on ESBL-E isolates causing infection in liver disease patients the proportion of E. coli ESBL producers did not differ significantly between hospitalized and outpatients (20% vs. 17%) which showed that ESBLs were equally pervasive in both hospital and community settings and responsible for the possible high prevalence of ESBL faecal carriers in the community [Reference Fam8]. This study aimed to assess and compare the epidemiology and molecular types of ESBL-E in digestive carriage of patients hospitalized for more than 24 h in the Hepatology department of two hospitals specializing in liver disease in Egypt and France, i.e. Theodor Bilharz Research Institute (TBRI), Cairo, Egypt and Beaujon Hospital (Bj), Clichy, France.
METHODS
Rectal swabs were collected in parallel over a period of 30 and 40 days from all patients admitted to the Hepatology departments of two hospitals: TBRI (Cairo, Egypt) and Beaujon hospital (Clichy, France), respectively. Samples were obtained in the first 48 h of hospitalization.
Microbiological detection of ESBL-E
TBRI
At TBRI rectal swabs were cultured on Drigalski agar containing 0·5 mg/l cefotaxime. Growing colonies were tested by the double disc synergy test on Muller–Hinton agar. Briefly, amoxicillin-clavulanic acid (AMC) disc was placed in the centre and discs of ceftazidime (CAZ), cefotaxime (CTX) and cefipime (FEP) were placed 1·5 cm and 2·5 cm, respectively, distal from the AMC disc. All samples showing positive synergy were identified by API 20E (bioMérieux, France).
Beaujon Hospital
At Bj rectal swabs were cultured on chrom IDIM ESBL agar (bioMérieux). In case of growth of pink colonies, an indole-positive test confirmed the diagnosis of E. coli. For non-pink colonies or pink colonies with negative indole test, bacterial identification was performed using API 20E (bioMérieux). ESBL detection was tested by double disc diffusion test performed on Muller–Hinton agar using two discs of FEP placed at 1·5 cm and 2·5 cm, respectively, distal from the AMC disc. The appearance of synergy between AMC and FEP confirmed ESBL.
Antibiotic susceptibility testing was applied to all identified ESBL isolates according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [9]. ESBL-producing strains were conserved on conservation agar medium (Bio-Rad, France) for molecular study.
Characterization of ESBLs
CTX–M enzyme groups were characterized as described previously [Reference Leflon-Guibout10], TEM and SHV derivatives were identified using the check-point system/KPC® (Check-points, The Netherlands, [email protected]).
Molecular analysis of E. coli strains
Phylogenetic groups of E. coli were determined as described previously [Reference Clermont, Bonacorsi and Bingen11]. Molecular typing of strains was performed by using ERIC-2 PCR [Reference Nicolas-Chanoine12].
Representatives of previously published E. coli clones were used as controls in the ERIC-2 PCR analysis: for group A (ST709, ST10, ST744, ST167, ST606); for group B2 isolates (ST95, ST131, ST141) and for group D isolates (ST117, ST393, ST69-CGA). In order to confirm that E. coli group B2 belonged to clone ST131 a PCR specific for clone ST131 (pab B gene) was performed on group B2 E. coli isolates as described previously [Reference Clermont13].
Epidemiological data
Demographic and clinical data were collected on the designed sheet from all patients in the study including: age, sex, date of admission and of sample collection, previous hospitalization, underlying disease, ascites and comorbidity.
Statistical analysis
Statistical analysis was performed by analytical epidemiological software Epi Info v. 6 (CDC, USA). Statistical data were analysed using χ 2 test and Fisher's exact test.
RESULTS
Prevalence of digestive carriage of ESBL-E in TBRI and Bj patients
In TBRI, the period of study, initially designed for 2 months, was shortened as the prevalence of ESBL-E carriers was significantly higher than in Bj. During the study period, 58 patients were screened and 45 (77·6%) were ESBL carriers, four of whom carried two different strains. Therefore for the study performed in TBRI, 49 strains were isolated from 45 patients. In Bj, 199 patients were screened and 13 patients (6·5%) were ESBL-E carriers. Of these patients, one was a carrier of two different ESBL-E strains. These 14 strains isolated from 13 patients were retained for the study. The difference in prevalence of digestive carriage observed in the two study populations was statistically significant (P < 10−7) (Table 1).
Table 1. Prevalence of patients with faecal ESBL-E at admission

ESBL-E, Extended spectrum β-lactamase-producing enterobacteria.
** P < 10−7.
Species and ESBL enzyme distribution
Bacterial species distribution was not statistically different between the two hospitals (P = 0·06), CTX-M was the most frequent ESBL identified in ESBL-producing E. coli and Klebsiella spp. isolates detected in the gut of the studied patients. PCR and the check-point system showed that the CTX-M-1 group was the most prevalent enzyme produced by ESBL-E in both TBRI and Bj (59% and 79%, respectively). No significant difference was found in the relative distribution of CTX-M-1 and SHV groups between the two populations studied, but CTX-M-9 group enzymes were significantly more common in TBRI than in Bj (P = 0·027) whereas TEM-derived ESBLs were significantly more common in Bj than in TRBI (P = 0·047) (Table 2).
Table 2. Species and molecular type distribution of ESBLs in TBRI and Beaujon hospitals
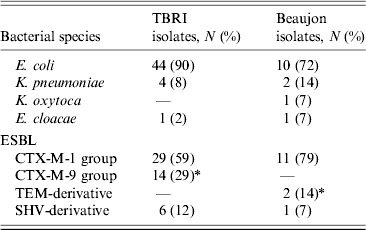
ESBL, Extended spectrum β-lactamase.
* P ⩽ 0.05.
Antibiotic susceptibility of ESBL-producing E. coli in TBRI and Bj
ESBL-producing E. coli isolates had high resistance rates to tested antibiotics. The resistance rates for amikacin were the lowest in both hospitals (18% and 10%). Statistical analysis of the antibiotic susceptibility showed no significant difference regarding the global sensitivity of E. coli isolates to antibiotics between the two hospitals (Table 3).
Table 3. Antibiotic susceptibility of ESBL-producing E. coli in TBRI and Beaujon hospitals
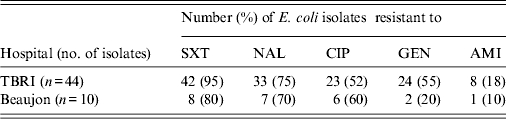
ESBL, Extended spectrum β-lactamase.
The sensitivity of the isolates in TBRI to augmentin (AMC), cotrimoxazole (SXT), nalidixic acid (NAL), gentamicin (GEN) and amikacin (AMI) was not significantly affected by the molecular type of the ESBL. However, a significant difference was observed for ciprofloxacin (CIP) (P = 0·005) between E. coli isolates producing CTX-M-1 group and SHV enzymes in comparison to E. coli isolates producing CTX-M-9 group enzymes (Table 4). No statistical significance was observed in ESBL-E in Bj in relation to the type of ESBL produced.
Table 4. Antibiotic resistance of ESBL-producing enterobacteria in relation to ESBL type in TBRI
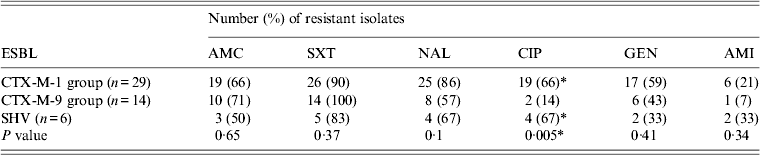
ESBL, Extended spectrum β-lactamase.
* P ⩽ 0.05.
Molecular analysis of ESBL-producing E. coli isolates
Molecular analysis of all isolates was performed in the microbiology laboratory of Bj. The genetic background of 44 E. coli isolates from TBRI showed that 59% (26/44) belonged to phylogenetic group A, 27% (12/44) to group D, 7% (3/44) to group B1 and 7% (3/44) to group B2. Among the 10 E. coli isolated in Bj, 40% belonged to group A, 40% to group B1 and 20% (2/10) to group D.
Globally, there was a significant difference between the phylogenetic group distribution of the E. coli isolates from TBRI and Bj (P < 0·05). Moreover, group B1 E. coli isolated at Bj (40%) were significantly higher than those isolated at TBRI (7%) (P = 0·017). Molecular typing by ERIC PCR of E. coli isolates from Egypt showed that for 26 group A isolates, two had a profile identical to that of ST709, four had an identical profile (Pf1) to each other and to one isolate from France, two had another identical profile (Pf2), four had an identical profile to ST167 and one had an identical profile to ST10; while 13 isolates each had a unique profile (Fig. 1). Therefore, 50% of the E. coli isolates of group A had clonal relationship to seven representatives of the clonal complex ST10 including ST167, ST709 and ST10. Three of the four E. coli isolates from France belonging to group A showed a unique profile. The two French strains of group D had a unique profile, while in the 12 Egyptian isolates of this group, three had the same profile PFA, two had the same profile PFB, and two had the same profile PFC. The five remaining strains each had a unique profile. In total 58% of the Egyptian strains of group D were clonally related. For the isolates of group B1, unique profiles were observed for three of the four French isolates, and for all of the Egyptian isolates. All three Egyptian isolates belonging to group B2 had a profile identical to the representative of ST131. This was confirmed by the amplification of pabB recovered from those three isolates.

Fig. 1. Molecular typing by ERIC-2 PCR of E. coli isolates of phylogenetic group A.
Demographic features of patients colonized by ESBL-E in the two hospitals
No significant difference was found between the two study populations concerning the mean age (P = 0·21) or in the distribution of sexes (P = 0·75). Previous history of hospitalization was more common in patients harbouring ESBL-E in Bj (92%) compared to those from TBRI (40%) (P = 0·007). Regarding the associated pathology in patients colonized by ESBL-E, the two studied populations showed significant difference (P < 0·05). Chronic HCV infection was significantly higher in TBRI patients, found in 35/45 patients (78%) compared to 4/13 patients (4%) in Bj. Notably 57% of patients affected by chronic HCV were previously treated by parenteral antischistosomal therapy during the campaign of eradication of this endemic parasitic disease in Egypt (n = 20), this phenomenon was not observed in France (P = 0·002). No cases of alcoholic hepatitis were found in TBRI; however, chronic alcoholism represent an important part (31%, n = 4) of the aetiology of chronic hepatitis in France (P = 0·002). No significant difference was seen in the distribution of cirrhotic and non-cirrhotic patients between Bj and TBRI. Statistical analysis of gravity score (model for end-stage liver disease; MELD) and different biological parameters (prothrombin, creatinine, international normalized ratio, aspartate transaminase, alanine transaminase, total bilirubin) did not show significant difference between the two populations (Table 5).
Table 5. Comparison of demographic and biological characteristics of patients colonized by ESBL-E in TBRI and Beaujon
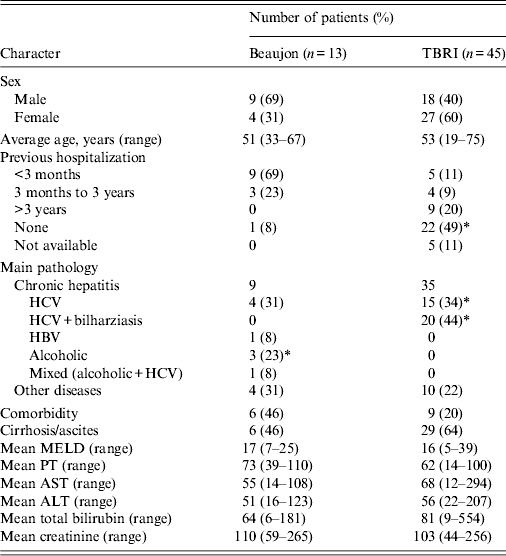
MELD, Model for end-stage liver disease; PT, prothrombin time; AST, aspartate transaminase; ALT, alanine transaminase.
ESBL-E, Extended spectrum β-lactamase-producing enterobacteria.
* P ⩽ 0.05.
Clonal relationship of strains and previous hospitalizations
The majority of patients harbouring clonal strains in TRBI had no history of hospitalization. This is the case of the four patients harbouring strains of group A which had an identical profile (PF1), of the two patients harbouring the three strains of group D that had an identical profile (PFA), and the two patients harbouring strains of group D having the same profile (PFB). Two of the three Egyptian patients harbouring strains of group B2 related to clone ST131 had a previous history of hospitalization, one in 2002 and the other one in 1995.
DISCUSSION
This comparative epidemiological study demonstrated a very high prevalence (77·6%) of faecal colonization with ESBL-E in liver disease patients at the time of their admission to the TBRI Hepatology unit. As patients presenting to the two hospitals in our study are liver disease patients so high ESBL faecal carriage rate could be expected as liver disease has been described previously among the risk factors for colonization with ESBL [Reference Ben-Ami14]. Moreover the treatment for complications of liver cirrhosis such as hepatic encephalopathy, spontaneous bacterial peritonitis and variceal bleeding with antibiotics is believed to impact directly or indirectly on the composition of gut microbiota which may enhance faecal carriage of ESBL [Reference Bajaj, Hylemon and Younossi15]. Prevalence in TBRI was 10 times higher than that found in patients presenting in the same period to Bj (6·5%). Gender difference can be a further contributing factor to high ESBL carriage rate. It is of note that 27 (60%) TBRI patients were female. Female sex has been described as one of the multivariate risk factors for rectal carriage of ESBL-producing enterobacteria at hospital admission [Reference Friedmann16]. Our results are also consistent with the findings of a study performed in Sweden that investigated the occurrence of ESBL-E in patients with traveller's diarrhoea. They reported that out of the patients who had travelled in Europe, 3% (2/63) were found to be ESBL-E carriers in comparison to 36% (50/138) of those who had travelled outside Europe. ESBL-producing E. coli was especially common in patients returning from India (11/14, 79%), Egypt (19/38, 50%) and Thailand (8/38, 22%) [Reference Tham17]. Similarly Wickramasinghe et al. found a statistically significant difference between ESBL-producing faecal E. coli carriers from Europe (8·1%) and the Middle East/South Asia (22·8%) with a higher rate of CTX-M-15-producing E. coli carriage in the latter group [Reference Wickramasinghe18].
Two studies from Egypt have also reported a high rate of intestinal colonization with ESBL-E in healthy carriers (63·3% and 22·6%) [Reference Abdul Rahman and El-Sherif19, Reference Al-Agamy20]. The prevalence rates reported from Egypt are comparable with the prevalence rate of 65·7% of CTX-M-type ESBL-E found in asymptomatic rural Thai people [Reference Luvsansharav21], but higher than that recorded in other countries in the Middle East region: 16% in Lebanon [Reference Moubareck22], 26·1% of patients hospitalized with diarrhoea in Saudia Arabia [Reference Kader23] and 12·7% of healthy people studied in Saudi Arabia [Reference Kader and Kamath3].
A factor contributing to this high prevalence may be the high population density in Cairo (38 221 inhabitants/km2) which may facilitate transmission of ESBL-E in the general population. Easy access to antibiotics in Egypt and their availability without medical prescription may also contribute to this high rate of faecal colonization. [Reference Fam8, Reference Al-Agamy20, Reference El Kholy24]. The colonized non-hospitalized patients may be considered as community reservoirs that play a role in the epidemiology of ESBL-E infections [Reference Bert25].
Although the prevalence (6·6%) of patients harbouring ESBL-E in Bj seems negligible in comparison to that of TBRI, and less than that observed by Valverde et al. (11·8%) in patients admitted in their hospital in Spain in 2003 [Reference Valverde26], it is higher than the rate observed in the general ICU of Bj during their admission (2·6%) at the same time of the study but it is very close to that observed in patients admitted to the ICU of Hepato-gastro-enterology (7·5%) (M.-H. Nicolas Chanoine, personal communication). This is not surprising as the latter ICU receives critical patients with liver transplantation that are followed from the Hepatology department in Bj. This is a serious issue as a 10-year study conducted in Bj reported that pre-transplant carriage of ESBL-E was an independent risk factor for infection after liver transplantation. Interestingly, a significant association between faecal colonization status and occurrence of subsequent infection has been suggested. In post-transplant patients, ESBL-E infection developed in ≈45% of carriers and molecular typing showed that the infecting isolate was identical to the isolate from the pre-transplant faecal swab for most of these patients [Reference Bert25].
CTX-M enzymes are the most prevalent ESBL types worldwide in both hospital and community settings [Reference El Kholy24, Reference Bert25, Reference Bonnet27, Reference Paterson and Bonomo28], so it was expected to be predominant in both study groups (88% in TBRI and 79% in Bj). Group-specific PCR typing showed that the majority (79%) of ESBL-positive isolates from Bj belonged to group CTX-M-1. It has been reported that CTX-M-15, which belongs to CTX-M-1 cluster, is the most widely distributed type in Western Europe [Reference Valverde26, Reference Bonnet27, Reference Livermore and Hawkey29]. Similarly studies from Egypt reported that CTX-M-15 was also the predominant ESBL type [Reference Fam8, Reference Al-Agamy20, Reference Peirano and Pitout30–Reference Khalaf, Eletreby and Hanson33]. In this study we found that the CTX-M-1 group represented 59% of ESBL-positive isolates from TBRI and 29% belonged to CTX-M-9. CTX-M-14, a member of the CTX-M-9 group, has been reported in Egypt in E. coli and K. pneumoniae and Enterobacter cloacae isolates [Reference Al-Agamy, Ashour and Wiegand31, Reference Khalaf, Eletreby and Hanson33]. However DNA sequencing was not performed on our isolates to confirm the presence of this enzyme type.
The E. coli species have been classified into four main phylogenetic groups (A, B1, D, B2), with the commensal strains belonging mainly to A and B1 phylogenetic groups whereas extraintestinal pathogenic strains are essentially from the B2 and D groups [Reference Picard34]. Phylogenetic analysis of ESBL-producing E. coli isolates from both hospitals in our study showed that group A was the major group detected in TBRI (59%), whereas group A (40%) and group B1 (40%) represented the majority in Bj. However, groups D and B2 were also detected in faecal carriers in our study (B2: 7% in TBRI) and (D: 27% in TBRI; 20% in Bj) as well as in other studies [Reference Luvsansharav21, Reference Matsumura35]. ERIC PCR analysis showed that four E. coli strains belonging to group A from Bj had a unique profile and were not related to the profile of the known sequence type, whereas 50% of the Egyptian E. coli strains of same group A had clonal relatedness to seven strains of the clonal complex ST10 which includes ST10, ST167 and ST709 [Reference Leflon-Guibout36]. This was similar to what was previously found by Fam et al.; i.e. 40% of group A E. coli clinical isolates belonged to clonal complex ST10 [Reference Fam8]. One strain from Bj (2F), and four other strains from TBRI had a profile identical to clone ST167. In addition two isolates (39E and 40E) from TBRI had a similar profile to a French isolate (PF2). These data may suggest clonal propagation of sequence type complexes worldwide. The E. coli O25b-ST131 clone is a highly resistant and virulent clone that has been reported worldwide and represents a major public health concern [Reference Peirano and Pitout30, Reference Coque37, Reference Suzuki38]. The rapid intercontinental spread of this clone was mainly in the form of isolates producing CTX-M-15, but has also been found in isolates free of CTX-M enzymes in 10% of healthy subjects in Paris [Reference Nicolas-Chanoine12, Reference Wickramasinghe18, Reference Leflon-Guibout36, Reference Oteo, Perez-Vazquez and Campos39]. In the present study, PCR amplification of the pabB gene that is specifically found in isolates belonging to the E. coli O25b-ST131 clone [Reference Clermont13] confirmed the presence of this clone in three ESBL-producing E. coli faecal carriers from Egypt. The three isolates carried three different enzyme groups, i.e. CTX-M-1, CTX-M-9 and SHV. This clone had been detected in our previous study where seven CTX-M-15-producing E. coli isolates were found causing urinary tract infections in both community and hospital settings [Reference Fam8]. The presence of this clone with ESBL enzymes in potentially pathogenic E. coli (group B2) strains colonizing the lower gastrointestinal tract could represent a potential source for infection with drug-resistant bacteria. So the possibility of infections due to of extended-spectrum cephalosporin-resistant isolates must be taken into consideration in the empirical treatment given to those patients particularly in areas with known high prevalence rates of ESBL in the community. Concerning the adjacent pathology of patients colonized by ESBL-E, the two studied populations showed a significant difference (P = 0·0003). The proportion of patients affected by chronic HCV was significantly higher in TBRI (78%) than in Bj (30%), whereas chronic alcoholism represented an important part (31%) of the aetiology of chronic hepatitis in France. That was expected as Egypt has the largest epidemic of HCV in the world [Reference Guerra40]. The recently released Egyptian Demographic Health Survey (EDHS) that tested a representative sample of the entire country for HCV antibody reported that percentage of people positive for antibody to HCV was 14·7%, of which 10% were positive for HCV RNA [Reference El-Zanaty and Way41]. The samples included both urban and rural populations, including all 27 governorates of Egypt and over 11 000 individuals were tested.
As 44% of TBRI patients had associated HCV and history of previous antischistosomal therapy, further studies are needed to assess possible correlation between these factors and the change in faecal flora.
It is noteworthy to mention that some limitations in our study exist, such as the possible effect of the shortened enrolment period of patients to 1 month in TBRI (initially designed for 2 months). However this change in the plan of study duration is not likely to affect the conclusion, as the prevalence of ESBL-E carriers was significantly higher than in Bj. Moreover, the study only covered two hepatology centres, so further multicentre studies are required to apply in other settings.
CONCLUSION
The prevalence of faecal colonization with ESBL-E in non-hospitalized liver disease patients in TBRI is one of the highest reported worldwide. This raises concern that empirical therapy of liver disease patients presenting with serious Gram-negative infections as spontaneous bacterial peritonitis may need to be modified. The presence of faecal carriage of the E. coli B2 ST131 clone in ESBL-producing E. coli confirms its pervasiveness in the community
ACKNOWLEDGMENTS
This work was supported by research project number 79D of the Microbiology Department, TBRI Cairo, Egypt and THE Microbiology Department, Beaujon Hospital AP HP, Clichy, Paris.
DECLARATION OF INTEREST
None.









