The glycaemic index (GI) was introduced in 1981 as a means of classifying carbohydrate-rich foods according to their effect on glucose levels( Reference Jenkins, Wolever and Taylor 1 ). A food’s glycaemic effect is derived from the body’s glucose response to the food. As such, the GI is a property of foods that is measured in humans( Reference Wolever 2 ). In the total diet, the importance of an individual food’s GI is quantified by its overall contribution to carbohydrate intake. The glycaemic load (GL) expresses the amount of carbohydrates consumed multiplied by its GI( Reference Brouns, Bjorck and Frayn 3 ). In epidemiological studies, diets high in GI and/or GL have been associated with an increased risk of developing type 2 diabetes( Reference Wolever 2 , Reference Brand-Miller and Buyken 4 ). However, the association appears to be more pronounced in women than in men( Reference Livesey, Taylor and Livesey 5 ).
When studying GI and GL at a population level, it is important to know which food items contribute to the total GI and GL of the diet. Food items that contribute most to the absolute dietary GI are those that have a high GI and/or foods that contribute a greater proportion of the total amount of carbohydrates eaten – that is, are eaten most often and/or in larger amounts( Reference Schulz, Liese and Mayer-Davis 6 ). Furthermore, food items that capture most inter-individual variation are able to rank persons according to their dietary GI or GL. These foods do not necessarily have a high GI, but may also be markers of a high- or low-GI diet. Several studies have identified food items contributing to the absolute dietary GI and GL and inter-individual variation in GI and GL. Across several countries and populations, these foods mainly include carbohydrate-rich foods – for example, bread, potatoes, pasta, rice, cereals, dairy products, fruits, vegetables and sugar-containing foods and drinks( Reference Du, van der and van Bakel 7 – Reference van Woudenbergh, Kuijsten and Sijbrands 11 ).
Two studies have shown that beer consumption contributed to the inter-individual variation in dietary GI. Together with dairy products, potatoes, bread, cereals and fruits, beer contributed the most to the inter-individual variation in GI of 1071 US adults. Beer contributed to 30 % of the variation in men and to 5 % in women( Reference Schulz, Liese and Mayer-Davis 6 ). Beer and milk captured most between-individual variation of GI in 25 943 male participants from a large Finnish intervention study. In this study, beer contributed to 41 % of the inter-individual variation( Reference Simila, Valsta and Kontto 12 ). This may be due to the higher GI of beer, but it may also be that persons with a higher beer intake also have in general a higher consumption of high-GI foods. Thus, in the Netherlands, it has previously been observed that persons who preferred beer also consumed more high-GI foods such as potatoes and bread( Reference Sluik, van Lee and Geelen 13 ).
Despite the potential substantial contribution of beer to dietary GI and GL, a valid GI value for beer is still lacking in the scientific literature. Several GI values have been reported, ranging from 0 to 119. A GI of 119 is the only published value determined following the standard research methodology( Reference Hätönen, Virtamo and Eriksson 14 ). In this study, the GI of Nikolai Lager (4·5 % alcohol by volume) was determined. In the Netherlands, beer is a regularly consumed alcoholic beverage, of which pilsner is the most consumed type( Reference van Rossum, Fransen and Verkaik-Kloosterman 15 ). In 2015, 67 % of the Dutch adult men and 28 % of the Dutch adult women reported to consume beer at least once a month( Reference de Jongh and van Teeffelen 16 ). Therefore, the objective of this study was to provide a reliable estimate of the GI of pilsner beer. Moreover, our aim was to estimate the contribution of beer and all other foods to the absolute level of dietary GL and to the inter-individual variation of dietary GI and GL in the Dutch diet.
Methods
Glycaemic index testing of pilsner beer
The GI testing was performed by the Sydney University’s Glycemic Index Research Service (SUGiRS) and conducted according to the internationally recognised GI methodology( 17 , Reference Wolever, Gibbs and Spolar 18 ). The experimental procedures were in accordance with international standards for conducting ethical research with humans and were approved by the Human Research Ethics Committee of Sydney University. The GI of Pilsner Urquell beer (4·4 % alcohol by volume; Plzeňský Prazdroj, A.S.) was determined using pure glucose sugar (Glucodin® powder; Boots Health Care Company) as the reference food. The brand of Pilsner Urquell was chosen because of its availability in Australia and the Netherlands. It was assumed that the nutritional composition between different brands of pilsner beers do not differ significantly.
A group of ten healthy, non-smoking persons, aged between 18 and 65 years, were recruited from the staff and student population of the University of Sydney. People volunteering to participate in the study were excluded if they were overweight or underweight, were dieting, had impaired glucose tolerance, were suffering from any illness or food allergy or were regularly taking prescription medication other than standard contraceptive medication.
A total of three men and seven women, Caucasian, with a mean age of 31·7 years and mean BMI of 23·7 kg/m2 (range 21·4–25·0 kg/m2) were included in the study. The reference food and the pilsner beer were served to the subjects in fixed test portions containing 25 g of digestible carbohydrate. A dose of 25·7 g pure glucose sugar dissolved in 250 g water was consumed by each subject on three separate occasions. Subjects consumed 554·3 g of the test beer on one occasion only. This amount was calculated according to the total carbohydrate content as displayed on the food label. During these occasions, two fasting finger-prick blood samples were obtained following an overnight fast. After the second fasting sample (0 min) was obtained, subjects were given the fixed portion of the test or reference food, which they consumed with 250 ml of water within 12 min. Additional blood samples were collected at 15, 30, 45, 60, 90 and 120 min after consumption. Glucose concentration of each subject’s eight plasma samples was analysed in duplicate using a glucose hexokinase enzymatic assay (Roche Diagnostic Systems).
A 2-h plasma glucose response curve was constructed for each subject’s test sessions using the average glucose concentrations for each of their plasma samples. The incremental area under the 2-h plasma glucose curve (iAUC) was calculated. A GI value for the Pilsner Urquell beer was then calculated for each subject by dividing their 2-h glucose iAUC value for the test product by their average 2-h plasma glucose iAUC value for the reference food and multiplying it by 100.
Glycaemic index and glycaemic load food composition table
On the basis of the six-step methodology for assigning GI values as suggested by Louie et al. ( Reference Louie, Flood and Atkinson 19 ), GI values were assigned to 2556 food items in the 2006 and 2011 Dutch food composition database( 20 ). All GI values were expressed on the glucose scale. In Step 1, foods with <2·5 g available carbohydrates/100 g were assigned a GI of 0. In Step 2, GI values were assigned for foods with a direct match in the databases consulted in the following order: (1) the ‘International table of glycemic index and glycemic load values’( Reference Atkinson, Foster-Powell and Brand-Miller 21 ), (2) the SUGiRS online database( 22 ), (3) the Dutch GI table as prepared within the DiOGenes project( Reference Du, van der and van Bakel 7 , Reference van Bakel, Kaaks and Feskens 23 ) and (4) a study of Chinese foods by Chen et al. ( Reference Chen, Sun and Wong 24 ). In this step, all beers were assigned a GI value of 89. When there was no direct match, GI values of closely related food items were assigned in Step 3. In Step 4, the weighted average GI values of the components of mixed meals were assigned regardless of the availability( Reference Wolever 2 ). In Step 5, the median GI of the corresponding food group was assigned. Finally, in Step 6, foods that were not significant sources of carbohydrates in portions normally consumed in diets were assigned a GI of 0. In this study, all herbs and spices, miscellaneous and dietetic products were assigned a GI of 0. Moreover, unprocessed flours and powders were assigned a GI value of 0. The GI of these products – for instance, rice flour or custard flour – has not been tested. These food codes were not consumed as such, and the GI value would be largely determined by its processed form. These food items were not used in Step 4. The number of products assigned GI values according to this stepwise methodology as well as the median GI and GL of the food group are shown in Table 1.
Table 1 Foods with glycaemic index (GI) and glycaemic load (GL) assigned at each stepFootnote * (Numbers, medians and 25th–75th percentiles (P25–P75))

* Step 1: foods with <2·5 g available carbohydrates/100 g were assigned a GI of 0; Step 2: direct match in one of the four consulted databases; Step 3: GI value of a closely related food item; Step 4: for mixed meals, the weighted average GI of the components were assigned; Step 5: the median GI of the corresponding food group was assigned; Step 6: foods that were not significant sources of carbohydrates were assigned a GI of 0.
Dutch National Food Consumption Survey 2007–2010
The Dutch National Food Consumption Survey 2007–2010 was conducted among children and adults aged 7–69 years in the Netherlands and aimed to gain insight into the Dutch diet( Reference van Rossum, Fransen and Verkaik-Kloosterman 15 ). Data were collected from March 2007 to April 2010 by means of age-specific general questionnaires and two non-consecutive 24-h dietary recalls. The survey population was representative with respect to age and sex and within each age group, region, degree of urbanisation and educational level. All persons living in the Netherlands were eligible for inclusion, except for pregnant and lactating women, institutionalised persons or persons with insufficient knowledge of the Dutch language. In total, 5502 people were invited, of which 3819 agreed to participate. For the present study, 2106 adults aged 19 years and older were included.
The two 24-h recalls were obtained within an interval of 2–6 weeks; the recalls were spread equally over days of the week and seasons. Food consumption from Sunday to Friday was recalled the next day, and consumption on a Saturday was recalled on the following Monday. The recalls were conducted by telephone using the computer-directed programme EPIC-SOFT( Reference Slimani, Deharveng and Charrondière 25 , Reference Slimani, Ferrari and Ocke 26 ). Consumption data were linked to the GI food composition table. Dietary GL was calculated by multiplying the carbohydrate content of the reported food by the consumed quantity per day and its GI. The GL was summed for all reported foods, after which dietary GI was calculated as the dietary GL divided by the total carbohydrate intake.
Statistical analysis
All statistical analyses were performed with SAS, version 9.3, software (SAS Institute Inc.). For GI testing, ANOVA and the Fisher’s protected least significant differences post hoc test for multiple comparisons were used to determine whether there was a difference between the GI values of the test beverage and the reference food.
In contrast to nutrients, the GI of foods does not vary with the consumed quantity. Therefore, it was not possible to derive the absolute contribution of all food items to dietary GI. The GL does take into account the consumed amount of carbohydrates; therefore, the GL was used for calculating the percentage absolute contribution of all 2556 food items to dietary GL. Second, stepwise linear regression was used to estimate the contribution of the food items to the inter-individual variation in dietary GI and GL. Two regression models, one for GI and one for GL, were specified, where dietary GI or GL was the dependent variable and all food items were independent variables. These analyses were not adjusted for covariates.
Dietary GI and GL, including and excluding beer, were calculated across categories of alcoholic beverage preference. A person was classified as having a beer, wine or spirit preference when the average number of glasses of the respective drink comprised 70 % or more of the total number of glasses. When the average number of glasses of beer, wine or spirits did not add up to 70 %, the person was classified as having no preference( Reference Sluik, van Lee and Geelen 13 ). Dietary GI and GL were adjusted for age, sex, BMI, smoking status (never, former, current), educational level (low, medium, high), physical activity, energy, total alcohol consumption (average number of glasses per day) and drinking frequency and weighted for demographic factors, seasons and day of the week.
Results
Glycaemic index and glycaemic load of pilsner beer
Fig. 1 displays the average plasma glucose response curves for the equal carbohydrate portions of both the reference food and the pilsner beer. There was normal variation in glucose response and resulting GI values among the subjects, with no outliers. The final mean GI value for Pilsner Urquell was 89 (sd 5). The reference food’s GI value was significantly greater than the average GI value produced by Pilsner Urquell (P<0·05). According to the Dutch food composition table, 100 ml of pilsner beer contains 3·1 g of digestible carbohydrate, resulting in a GL of (3·1×89)/100=3 g/100 ml and 9 g/330 ml serving.

Fig. 1 Average plasma glucose response curves for the equal carbohydrate portions of the reference food and the pilsner beer, shown as change in plasma glucose from fasting baseline level. ![]() , Pilsner Urquell;
, Pilsner Urquell; ![]() , glucose.
, glucose.
Absolute contribution of pilsner beer to dietary glycaemic load
Foods with the highest absolute contribution (%) to the dietary GL among 2106 adults from the Dutch National Food Consumption Survey 2007–2010 included the following: potatoes (8·5 %), wheat bread (7·9 %), white bread (5·4 %), whole-grain bread (4·2 %), table sugar (3·1 %), multigrain bread (2·8 %), white rice (2·5 %), cookies (2·5 %), beer (2·4 %) and white pasta (2·4 %). Results were similar for men and women, except that beer and cola contributed more to the dietary GL of men and bananas had a higher contribution among women.
Contribution to inter-individual variation in dietary glycaemic index and glycaemic load
Foods that contributed to ≥1 % of the total inter-individual variation in dietary GI and GL, as revealed from a stepwise linear regression, are listed in Tables 2 and 3, respectively. Foods that contributed most to the inter-individual variation in GI included potatoes, beer, wheat bread, white bread, wine, and coffee and tea (Table 2). In men, beer contributed more to the variation than in women (9·9 v. 3·2 %), whereas in women wine contributed more to the variation compared with men (8·0 v. 2·3 %). With respect to the contribution to GL, beer took the fourth place with an explained variance of 5·3 %, after sugar, cola and wheat bread (Table 3). Sex-specific stepwise linear regression showed that sugar, wheat bread and beer explained more inter-individual variation in men than in women. In women, fruit drinks and sport drinks contributed more to the variation in dietary GL than their counterparts.
Table 2 Foods that contributed to ≥1·0 % of the inter-individual variation in dietary glycaemic index (GI) in 2106 men and women from the Dutch National Food Consumption Survey 2007–2010

Table 3 Foods that contributed to >1·0 % of the inter-individual variation in dietary glycaemic load (GL) in 2106 men and women from the Dutch National Food Consumption Survey 2007–2010

Alcoholic beverage preference and dietary glycaemic index and glycaemic load
Table 4 shows the mean dietary GI and GL across categories of alcoholic beverage preference, including and excluding beer from the measurement. Persons with a beer preference had the highest dietary GI compared with the other preference categories, after adjustment for socio-demographic and lifestyle factors as well. When beer was excluded from the GI measurement, those with a beer or wine preference had the lowest dietary GI. Differences in dietary GL between the alcoholic beverage preference groups were smaller than that for dietary GI. Crude means and differences were similar to the adjusted values (data not shown).
Table 4 Dietary glycaemic index (GI) and glycaemic load (GL) in 2106 adults from the Dutch National Food Consumption Survey 2007–2010 according to alcoholic beverage preferenceFootnote † (Numbers and percentages; mean values with their standard errors)
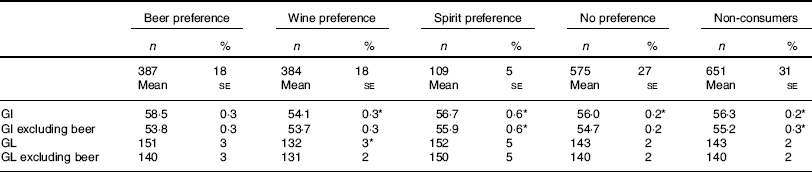
* P<0·05 compared with beer preference.
† Intakes are adjusted for age, sex, BMI, smoking status, educational level, physical activity, energy, total alcohol consumption (average number of glasses per day) and drinking frequency and weighted for demographic factors, seasons and day of the week.
Discussion
The GI value of pilsner beer, as determined according to the internationally recognised GI methodology, was 89 (sd 5). Although the GI has been commonly regarded as a property of high carbohydrate foods, beer can be categorised as a high-GI food despite its relatively low carbohydrate content/100 ml. Within the Dutch diet, beer had a considerable contribution both to the absolute GL and to the inter-individual variation in GI and GL, especially in men. The high dietary GI and GL of beer consumers was mostly driven by the GI of beer, indicating that it was not simply a marker of a high-GI diet. Other important contributors to dietary GI and GL were potatoes, bread, sugar, soft drinks and candy.
Foods that contributed to the absolute dietary GI included carbohydrate-rich foods, but also products with a relatively low GI that are consumed in high amounts, such as semi-skimmed milk. Next to starchy foods, foods high in sugar such as sweets and sugar-sweetened beverages contributed to the inter-individual variation in dietary GI and GL. Furthermore, low-GI foods including wine, coffee and tea also contributed to the inter-individual variation in dietary GI. This confirms that foods that capture most inter-individual variation do not necessarily have a high GI, but may also be markers of a high- or low-GI diet. Therefore, we have additionally calculated dietary GI and GL, across categories of alcoholic beverage preference, including and excluding beer from the calculations. The results indicated that the dietary GI of persons with a beer preference is indeed mostly driven by the consumption of beer rather than other high-GI foods. The differences in dietary GL between the alcoholic beverage preference groups were smaller than that for dietary GI, which was probably due to the low carbohydrate content of beer.
As a reliable GI value for beer has been lacking in scientific literature, several studies have assigned different GI values to beer. In DiOGenes, a GI value of 61 was assigned to beer, liquors and cocktails, which is similar to that of sucrose, and a value of 0 to other alcoholic beverages( Reference Du, van der and van Bakel 27 ). In unpublished observations of SUGiRS, a GI value of 66 for Toohey New Draft (4·6 % alcohol by volume) was found, using a non-standard portion of 10 g carbohydrates( Reference Brand-Miller, Fatema and Middlemiss 28 ). In contrast, Schulz et al. ( Reference Schulz, Liese and Mayer-Davis 6 ) assigned a value of 95 to beer because of its starch content and a value of 61 to wine and other alcoholic beverages. Simila et al. ( Reference Simila, Valsta and Kontto 12 ) also assigned a high value to beer. The only reliable GI value that is published is a GI of 119 for Nikolai Lager (4·5 % alcohol by volume) as tested by Hätönen et al. ( Reference Hätönen, Virtamo and Eriksson 14 ). Compared with this study, the present study tested three glucose solutions instead of two to minimise within-person variability. However, as the GI cannot easily be predicted and variations within food groups may exist( Reference Wolever 2 ), the difference in GI might be due to the nutritional composition of different types of beer. Nikolai Lager contained slightly more carbohydrates than pilsner beer – that is, 4·9 g/100 ml v. 4·5 g/100 ml. However, this should not necessarily explain the higher GI value, as the test portion takes into account differences in available carbohydrates. Nikolai Lager may contain different types of carbohydrates or small differences in alcohol percentage or pH that may influence the GI. Nevertheless, both Hätönen et al.( Reference Hätönen, Virtamo and Eriksson 14 ) and the present study classify beer as a high-GI food.
Several experimental studies have shown that ethanol acutely impairs insulin sensitivity due to delayed gastric emptying( Reference Avogaro, Fontana and Valerio 29 ). Hätönen et al.( Reference Hätönen, Virtamo and Eriksson 14 ) observed an 18 % increase in 2-h glucose after a glucose solution with alcohol; 2-h glucose was lower after non-alcoholic beer, but consumption of beer and alcohol in the glucose solution gave similar responses( Reference Hätönen, Virtamo and Eriksson 14 ). It appears that alcohol may catalyse the breakdown of the complex carbohydrates, causing the high GI of beer. Brand-Miller et al. ( Reference Brand-Miller, Fatema and Middlemiss 28 ) confirmed these results and showed that beer produced higher glucose scores than wine and gin compared with white bread.
The high GI of beer might be caused by its complex carbohydrate content, rather than due to monosaccharides or disaccharides. In the brewing process, a mixture of amylases breaks down starch to glucose, maltose, maltotriose and higher saccharides including maltodextrins. Yeast ferments glucose and maltose to ethanol, but cannot break down all maltodextrins. Thus, beer does not contain any significant amounts of maltose or other sugars, but it can contain maltodextrins( Reference Whelan 30 ). Hätönen et al.( Reference Hätönen, Virtamo and Eriksson 14 ) also tested the nutritional composition of their beer: a portion of 25 g contained 16 g starch, 0·03 g maltose and 9 g maltooligosaccharides( Reference Hätönen, Virtamo and Eriksson 14 ). According to the Dutch food composition table, pilsner beer does not contain any maltose or other monosaccharides and disaccharides, but it does contain polysaccharides including starch( 20 ). In contrast, different types of beer including stout, ale and lager were assigned maltose values ranging from 0 to 6·10 g/100 g in the food composition tables of McCance and Widdowson’s (UK) and the food composition table from Finland( 31 , 32 ). In addition, these beers contained only small amounts of glucose, fructose and sucrose and did not contain any starch. It remains unclear whether the soluble starch molecules or maltodextrins are responsible for the high GI of beer. Moreover, it is not known whether other types of beer with different carbohydrate composition will have the same GI, even though all might be classified as high-GI foods.
Although diets high in GI and GL have been associated with increased type 2 diabetes risk( Reference Livesey, Taylor and Livesey 5 , Reference Bhupathiraju, Tobias and Malik 33 ), moderate alcohol consumption is related to a lower risk of diabetes( Reference Baliunas, Taylor and Irving 34 ). Some studies have found differential effects for the type of alcoholic beverage, leaning towards a more favourable association for wine consumption on diabetes risk( Reference Beulens, van der Schouw and Bergmann 35 – Reference Hodge, English and O’Dea 37 ). In contrast, several other large observational studies have observed no beneficial effect of wine over beer or spirit consumption( Reference Conigrave, Hu and Camargo 38 – Reference Wannamethee, Camargo and Manson 40 ). It has been argued that the largest part of the beneficial effect of moderate alcohol consumption on diabetes risk is most likely due to ethanol itself and that any beverage-specific effects are due to (residual) confounding( Reference Beulens, van der Schouw and Bergmann 35 ). Furthermore, Brand-Miller et al. ( Reference Brand-Miller, Fatema and Middlemiss 28 ) observed that when consumed with or before a carbohydrate meal, beer tended to reduce postprandial glycaemia. Therefore, the difference between GI and glycaemic response of beer may also play a role( Reference Wolever 2 ). As alcoholic beverages are often consumed together with a meal, the glycaemic response of beer consumed together with a meal might be more important to consider for diabetes risk than the GI itself.
Next to beer, other products that contributed to the absolute dietary GL and the inter-individual variation in dietary GI and GL included carbohydrate-rich foods such as potatoes, breads, rice, pasta, sweets and sugar-sweetened beverages. These findings are in line with other epidemiological studies assessing the contribution of foods to GI and GL. Several investigations from different countries in Europe, USA, Australia and Asia identified cereals, breads, pasta, rice, potatoes, dairy products, fruits, juices, vegetables, sugar and sugar-containing foods as main contributors to the dietary GI and GL( Reference Patel, McCullough and Pavluck 8 , Reference Sluijs, van der Schouw and van der A 10 , Reference van Woudenbergh, Kuijsten and Sijbrands 11 , Reference Hodge, English and O’Dea 41 – Reference Salmeron, Manson and Stampfer 43 ). Most of these observational studies have calculated dietary GI and GL with a general FFQ, which might not be able to specify between all low- and high-GI food items within the same food group. To estimate the absolute contribution as well as the contribution to inter-individual variation is a common method to select informative foods for a FFQ( Reference Mark, Thomas and Decarli 44 ). Indeed, the food items that have been identified in the present study will be used to develop a dedicated FFQ to assess dietary GI and GL. This FFQ will be developed and validated within the framework of the PREVention of diabetes through lifestyle intervention and population studies in Europe and around the World (PREVIEW) project.
In conclusion, the present study provides a reliable GI value for pilsner beer and describes the development of a GI table for the Netherlands according to a standardised methodology. Next to potatoes, bread, sugar-sweetened beverages, candy, wine, and coffee and tea, beer captured a large proportion of between-person variability in GI and GL in the Dutch diet. Thus, beer consumption should be considered when studying dietary GI and GL.
Acknowledgements
This study was performed with a grant from The Dutch Beer Institute. The sponsor played no role in the study design, collection, analysis and interpretation of the data, writing of the report or in the decision to submit the paper for publication. This study was performed within the framework of the PREVIEW study. PREVIEW is the acronym of the project PREVention of diabetes through lifestyle intervention and population studies in Europe and around the World (PREVIEW) project. The research described here received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant no. 312057.
D. S. and E. J. M. F. designed the study and formulated the research questions; D. S. and F. S. A. carried out the study and analysed the data. D. S. drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved of the final version to be published.
J. C. B.-M. is President of the Glycemic Index Foundation, a not-for-profit food endorsement programme, manager of a GI testing service at the University of Sydney and the co-author of books about the GI of foods. All other authors declare no conflicts of interest.








