The early weaning process is commonly associated with intestinal barrier dysfunction and mucosal inflammation in weaned piglets, which are responsible for the stunted growth and diarrhoea observed in the first 2 weeks after weaning(Reference Pié, Lallès and Blazy1–Reference Kim, Hansen and Mullana3). Zn is involved in anti-inflammation, anti-diarrhoea and restoration of mucosal barrier integrity(Reference Sargeant, Miller and Shaw4, Reference Patel, Mamtani and Dibley5). Pharmacological addition of Zn (2000–4000 mg/kg of Zn as ZnO) in diets for weaned pigs is widely used in the pig industry worldwide due to its proven effects on alleviating post-weaning diarrhoea and improving performance(Reference Kim, Hansen and Mullana3, Reference Hahn and Baker6). However, high levels of ZnO in diets for weaner pigs has been criticised or banned because increased Zn excretion causes environmental pollution(Reference Kim, Hansen and Mullana3, Reference Poulsen and Larsen7). If the inclusion of ZnO is dramatically decreased and if the same effect as that of high levels of ZnO is achieved, then the Zn excretion will decrease and will be beneficial for sustaining swine production.
Diosmectite (DS) is an aluminosilicate clay mineral, which is composed of two outer tetrahedral silicon layers sandwiching an inner octahedral aluminum layer. Naturally occurring cations (i.e. Na+) reside between the sheets to balance the overall negative surface charge of DS(Reference Vaiana, Leonard and Drummy8). The ion exchange capacity of DS enables replacement of Na+ with other organic and inorganic cations to add functionality, spurring research into the use of DS as a controlled-release carrier for various active ingredients in drug delivery systems(Reference Vaiana, Leonard and Drummy8–Reference Joshi, Patel and Bajaj10). DS intercalated by drug molecules delays and/or targets drug release, increases drug stability and modifies drug delivery patterns(Reference Aguzzi, Cerezo and Viseras11). On the other hand, DS can reinforce the intestinal mucosal barrier and epithelial regeneration(Reference Droy-Lefaix, Drouet and Schatz12). It has anti-inflammatory and anti-diarrhoeal effects in rats, human subjects and pigs(Reference Gonzalez, Sanchez de Medina and Martinez-Augustin13–Reference Song, Liu and Soares15).
ZnO acts largely within the gastrointestinal tract, as the majority of ingested Zn is excreted in faeces(Reference Sargeant, McDowall and Miller16). Controlling the delivery and release of ZnO in the gastrointestinal tract may improve its effectiveness(Reference Kim, Hansen and Mullana3). The ion exchange nature and biocompatibility of DS makes it a potential controlled-release carrier for ZnO delivery. DS–ZnO composite (DS-ZnO) has recently been synthesised by a sol–gel intercalation reaction(Reference Khaorapapong, Khumchoo and Ogawa17, Reference Hu, Song and You18). Our previous dose–response test has shown that supplementation with DS-ZnO at 500 mg Zn/kg alleviated post-weaning diarrhoea and improved intestinal mucosal integrity(Reference Hu, Song and You18). In the present experiment, we evaluated the efficacy of DS-ZnO as a substitute for high concentration of ZnO (2250 mg Zn/kg). Additionally, a treatment of supplementing the mixture of 2·0 g DS/kg and 500 mg Zn/kg as ZnO (equal amount of DS and ZnO in the DS-ZnO treatment) was used to compare the DS-ZnO's effects. The growth performance, diarrhoea and intestinal morphology of weanling pigs were determined. Intestinal barrier function was assessed by transepithelial electrical resistance (TER) and paracellular flux of fluorescein isothiocyanate dextran 4 kDa (FD4) across the epithelium by the Ussing chamber technique. The expression of pro-inflammatory cytokines and tight junction (TJ) protein was also determined.
Materials and methods
Materials
DS was obtained from Chifeng WHTB Mining Company Limited. The DS content was 99·2 %. The cation exchange capacity was 141 mmol/100 g DS. The DS-ZnO was synthesised using a sol–gel intercalation between the colloidal suspension of exfoliated DS nanosheets and the sol solution of ZnO reactants(Reference Khaorapapong, Khumchoo and Ogawa17–Reference Hur, Kim and Hwang19). The aqueous solution of zinc chloride was mixed with NaOH solution at the molar ratio of Zn2+:OH− of 1:15 and vigorously stirred at 70°C for 24 h. Then, the aqueous solutions of zinc chloride and NaOH were added into the colloidal suspension of exfoliated DS with continuous stirring at 70°C and reacted for 24 h. The DS-ZnO were separated by centrifugation at a speed of 10 000 g for about 15 min, washed three times with distilled water and dried at 150°C. The Zn content in DS-ZnO was 250 g/kg.
Experimental design and sample collection
All procedures were approved by the Zhejiang University Animal Care and Use Committee. A total of 144 early weaned piglets (Duroc × Landrace × Yorkshire), with an average weight of 5·9 (sem 0·4) kg and weaned at an age of 21 ± 1 d, were allocated to four treatment groups. Each treatment group had six pens of six piglets. The dietary treatments were as follows: (1) control; (2) DS-ZnO, 500 mg Zn/kg diet; (3) ZnO (feed-grade sources of ZnO containing 78 % Zn), 2250 mg Zn/kg diet; and (4) mixture of 2·0 g DS/kg diet and 500 mg Zn/kg diet from ZnO (equal amount of DS and ZnO in the DS-ZnO treatment). Diets were formulated to meet or exceed requirements suggested by the National Research Council(20) (Table 1). No antibiotic was included in the diets. The piglets were given ad libitum access to feed and water. The feeding experiment lasted for 14 d. Average daily gain, average daily feed intake and gain:feed ratio were calculated from days 0 to 7 post-weaning and days 7 to 14 post-weaning.
Table 1 Ingredients and chemical composition of the basal diet on an as-fed basis
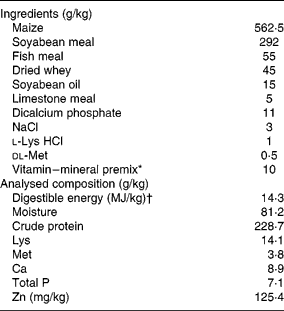
* Provided per kg of diet: vitamin A, 5000 IU (1720 μg); vitamin E, 30 IU (30 mg); vitamin D3, 400 IU (20 μg); vitamin K3, 1·0 mg; biotin, 0·10 mg; riboflavin, 5·0 mg; thiamine, 2·0 mg; niacin, 30 mg; pantothenic acid, 20 mg; pyridoxine, 3·0 mg; folic acid, 0·6 mg; vitamin B12, 0·03 mg; choline, 800 mg; Cu, 16 mg (CuSO4.5H2O); Fe, 125 mg (FeSO4); Zn, 100 mg (ZnSO4); Mn, 15 mg (MnSO4.H2O); Se, 0·3 mg (Na2SeO3); and I, 0·2 mg (KI).
† Digestible energy was calculated from data provided by Feed Database in China(39).
Post-weaning scour score was monitored according to the previous faecal scoring system from 1 to 5(Reference Hu, Song and You18): 1 = hard faeces (rarely seen), 2 = normal consistency of faeces formed (no diarrhoea), 3 = soft, partially formed faeces (mild diarrhoea), 4 = loose, semi-liquid faeces (moderate diarrhoea) and 5 = watery faeces (severe diarrhoea). The faecal score of each piglet in a pen was recorded individually every day and the average scour scores per pen were calculated daily.
At 7 or 14 d after weaning, six piglets from each treatment (one pig from every pen) were killed based on average diarrhoea score. The gastrointestinal tract was quickly removed. Segments (20 cm) of the proximal jejunum were removed, immediately placed into bicarbonate Ringer's solution (145 mm-Na+, 6·3 mm-K+, 2 mm-Ca2+, 1 mm-Mg2+, 25 mm-HCO3−, 128 mm-Cl−, 0·32 mm-PO43 − and 1 mm-SO42 −; pH 7·4) and mounted in the Ussing chambers(Reference Hamard, Mazurais and Boudry21). Adjacent specimens were fixed in 10 % formalin for morphology measurements. Mucosal scrapings from the remaining jejunum were collected, rapidly frozen in liquid N2 and stored at − 80°C until expression analysis of pro-inflammatory cytokines and TJ protein.
Intestinal morphology
Three cross-sections for each jejunal sample were stained with haematoxylin and eosin using standard paraffin embedding procedures. Crypt depth and villus height were measured in at least ten well-oriented crypt–villus units using image analysis (Leica Imaging Systems Limited) and averaged for each sample.
Ex vivo Ussing chamber to measure intestinal barrier function
Ussing chamber procedures were performed as described by Hamard et al. (Reference Hamard, Mazurais and Boudry21). Briefly, segments of jejunum were stripped from the seromuscular layer in oxygenated (95 % O2/5 % CO2) Ringer's solution and then mounted in the EasyMount Ussing chamber system with a multi-channel voltage–current clamp (model VCC MC6; Physiologic Instruments). They were bathed on each side with a bicarbonate Ringer's solution, with 10 mm-glucose and 10 mm-mannitol on the serosal and mucosal sides, respectively, and maintained at 37°C. The clamps were connected to Acquire and Analyse software (Physiologic Instruments) for automatic data collection. After a 30-min equilibration period on the Ussing chambers, TER (Ω cm2) and short-circuit current (μA/cm2) were recorded at 15-min intervals over a 2-h period and then averaged to derive the TER values for a given pig. FD4 (Sigma-Aldrich) was added on the mucosal side at a final concentration of 0·375 mg/ml. Mucosal-to-serosal flux of FD4 (ng/cm2 per h) was monitored by sampling 100 μl of solution from the serosal side at 30-min intervals for 120 min. The concentrations of FD4 in the serosal side were measured by fluorescence microplate reader (FLx800; Bio-Tek Instruments, Inc.). The flux over the 2-h period was calculated.
Pro-inflammatory cytokine mRNA by real-time PCR
Relative mRNA abundance of TNF-α, IL-6 and interferon-γ (IFN-γ) in jejunal mucosa was determined by real-time PCR, as described by Liu et al. (Reference Liu, Huang and Hou22). Briefly, total RNA was extracted using the TRIzol reagent (Invitrogen) and treated with DNase I (Invitrogen Life Technologies) following the manufacturer's guidelines. To amplify complementary DNA fragments, the following sequences of PCR primer were used: forward 5′-ATGGTGAAGGTCGGAGTGAAC-3′, reverse 5′-CTCGCTCCTGGAAGATGGT-3′ for glyceraldehyde-3-phosphate dehydrogenase (235 bp); forward 5′- CATCGCCGTCTCCTACCA-3′, reverse 5′-CCCAGATTCAGCAAAGTCCA-3′ for TNF-α (199 bp); forward 5′-GAGCCAAATTGTCTCCTTCTAC-3′, reverse 5′-CGAAGTCATTCAGTTTCCCAG-3′ for IFN-γ (140 bp); and forward 5′-CCTGTCCACTGGGCACATAAC-3′, reverse 5′-CAAGAAACAACCTGGCTCTGAAAC-3′ for IL-6 (253 bp). Quantitative real-time RT-PCR was performed on a StepOne Plus real-time PCR system (Applied Biosystems) using a SYBR Green Master mix (Promega), according to the kit's instructions. Gene-specific amplification was determined by melting curve analysis and agarose gel electrophoresis. Each sample was run in triplicate. The average C t was calculated for pro-inflammatory cytokine and GAPDH and the ΔC t (C t, cytokine-C t, GAPDH) was determined, where C t is the number of cycles required to reach an arbitrary threshold. The 2− ΔΔC t method(Reference Livak and Schmittgen23) was used to analyse the relative expression (fold changes), calculated relative to the control group on day 7 post-weaning.
Tight junction protein immunoblot analysis
TJ protein expressions of zonula occludens-1 (ZO-1), occludin and claudin-1 were measured by Western blotting, as described by Ewaschuk et al. (Reference Ewaschuk, Murdoch and Johnson24). Briefly, the total protein was extracted as directed by the instructions of a total protein extraction kit (Keygen Biotech). Equal amounts of protein from each treatment (20 μg) were separated by SDS-PAGE on 7·5 % (ZO-1) and 10 % (claudin-1 and occludin) polyacrylamide gels. Proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Millipore). The following primary antibodies were used: rabbit polyclonal anti-claudin-1 (1:300; Santa Cruz Biotechnology), rabbit polyclonal anti-occludin (1:450; Santa Cruz Biotechnology) and rabbit polyclonal anti-ZO-1 (1:400; Santa Cruz Biotechnology). Blots were also probed with anti-actin antibodies. The secondary antibody was horseradish peroxidase-conjugated anti-rabbit antibody (Santa Cruz Biotechnology). An enhanced chemiluminescence detection kit (Amersham) was used to detect the positive bands. Densitometric analysis for protein bands were carried out using ImageQuant software (Molecular Dynamics). The value of protein expression was the ratio of the densitometry units of TJ protein and β-actin. The control sample on day 7 post-weaning was used as the reference sample. The protein expression of all samples was expressed as fold changes, calculated relative to the control group on day 7 post-weaning.
Statistical analysis
Data were analysed using the SAS statistical package (version 8.1; SAS Institue)(25). A pen of pigs served as the experimental unit for all data. Differences among means were tested using Duncan's multiple range tests. Differences were considered significant at P< 0·05.
Results
Growth performance and post-weaning scour scores
Table 2 shows the growth performance and post-weaning scour scores of newly weaned pigs. As compared with the control, dietary addition of DS-ZnO at 500 mg Zn/kg or ZnO at 2250 mg Zn/kg improved (P< 0·05) average daily gain and average daily feed intake and decreased (P< 0·05) faecal scores on both days 0–7 post-weaning and days 7–14 post-weaning. The weaned piglets fed DS-ZnO at 500 mg Zn/kg did not differ in average daily gain, average daily feed intake and post-weaning scour scores from those fed ZnO at 2250 mg Zn/kg (P>0·05), while they had better performance than those fed the mixture of equal amount of DS and ZnO. Supplementation with the mixture of DS and ZnO had no effect on post-weaning scour scores of piglets.
Table 2 Efficacy of diosmectite–zinc oxide composite (DS-ZnO) on growth performance and post-weaning scour scores of weanling pigs (Mean values with their standard errors)
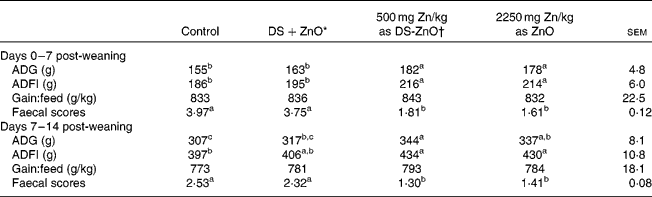
ADG, average daily gain; ADFI, average daily feed intake.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Supplemental mixture of 2·0 g DS/kg diet and 500 mg Zn/kg diet from ZnO (equal amount of DS and ZnO in the DS-ZnO group).
† DS-ZnO, DS–ZnO composite, containing 250 g Zn/kg DS-ZnO.
Jejunal morphology of weaned piglets
Table 3 shows jejunal morphology of piglets on days 7 and 14 post-weaning. Supplementation with DS-ZnO at 500 mg Zn/kg or ZnO at 2250 mg Zn/kg had both higher (P< 0·05) villus height and the ratio of villus height and crypt depth at the jejunal mucosa, as compared with the control. The weaned piglets fed DS-ZnO at 500 mg Zn/kg did not differ in jejunal morphology from those fed ZnO at 2250 mg Zn/kg (P>0·05), while they had higher (P< 0·05) villus height and the ratio of villus height and crypt depth on day 7 post-weaning than those fed the mixture of DS and ZnO. Supplemental mixture of DS and ZnO had no (P>0·05) effect on jejunal morphology.
Table 3 Effects of diosmectite–zinc oxide (DS-ZnO) on jejunal morphology of weanling pigs (Mean values with their standard errors)
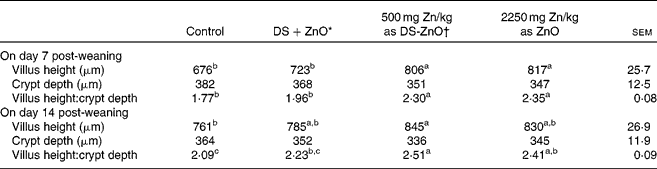
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Supplemental mixture of 2·0 g DS/kg diet and 500 mg Zn/kg diet from ZnO (equal amount of DS and ZnO in the DS-ZnO group).
† DS-ZnO, DS–ZnO composite, containing 250 g Zn/kg DS-ZnO.
Intestinal barrier function
Table 4 shows the intestinal barrier function of piglets on days 7 and 14 post-weaning, which was reflected by TER and paracellular flux of FD4 measured in the Ussing chambers. As compared with the control, dietary addition of DS-ZnO at 500 mg Zn/kg or ZnO at 2250 mg Zn/kg decreased (P< 0·05) FD4 flux on both days 7 and 14 post-weaning. The piglets fed DS-ZnO at 500 mg Zn/kg did not differ in FD4 flux from those fed ZnO at 2250 mg Zn/kg (P>0·05), while they had lower (P< 0·05) FD4 flux than those fed the mixture of DS and ZnO. Supplemental mixture of DS and ZnO had no (P>0·05) effect on jejunal barrier function of weaned pigs. The TER and short-circuit current were not affected by dietary treatments (P>0·05).
Table 4 Effect of diosmectite–zinc oxide (DS-ZnO) on jejunal barrier function of weanling pigs (Mean values with their standard errors)
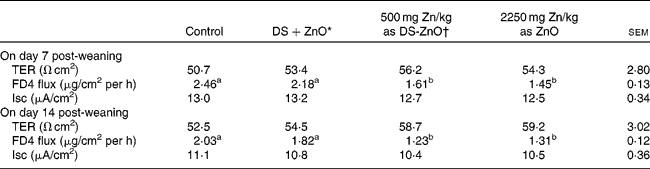
TER, transepithelial electrical resistance; FD4, fluorescein isothiocyanate dextran 4 kDa; Isc, short-circuit current.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Supplemental mixture of 2·0 g DS/kg diet and 500 mg Zn/kg diet from ZnO (equal amount of DS and ZnO in the DS-ZnO group).
† DS-ZnO, DS–ZnO composite, containing 250 g Zn/kg DS-ZnO.
Pro-inflammatory cytokine mRNA
The mRNA levels of TNF-α, IL-6 and IFN-γ in jejunal mucosa on days 7 and 14 post-weaning are presented in Table 5. As compared with control, supplementation with DS-ZnO at 500 mg Zn/kg or ZnO at 2250 mg Zn/kg both decreased (P< 0·05) mucosal mRNA of TNF-α, IL-6 and IFN-γ on day 7 post-weaning. The piglets fed DS-ZnO at 500 mg Zn/kg did not differ in cytokine mRNA from those fed ZnO at 2250 mg Zn/kg (P>0·05), while they had lower (P< 0·05) mRNA levels of TNF-α and IL-6 on day 7 post-weaning compared with those fed the mixture of DS and ZnO. The mRNA levels of TNF-α, IL-6 and IFN-γ on day 14 post-weaning were not affected by dietary treatments.
Table 5 Relative mRNA levels of pro-inflammatory cytokines in jejunal mucosa of piglets* (Mean values with their standard errors)
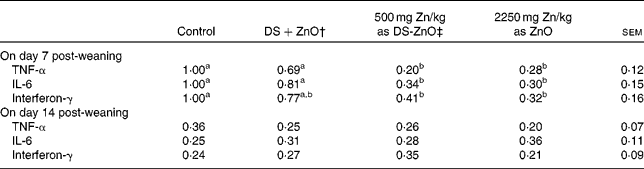
DS-ZnO, diosmectite–ZnO composite.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* The 2− ΔΔC t method was used to analyse the relative expression (fold changes), calculated relative to the control group on day 7 post-weaning. Data are means of six pigs, each in triplicate.
† Supplemental mixture of 2·0 g DS/kg diet and 500 mg Zn/kg diet from ZnO (equal amount of DS and ZnO in the DS-ZnO group).
‡ DS-ZnO, DS–ZnO composite, containing 250 g Zn/kg DS-ZnO.
Tight junction protein expression
Fig. 1 shows the protein expression levels of occludin, claudin-1 and ZO-1 in jejunal mucosa on days 7 and 14 post-weaning. As compared with control, supplementation with DS-ZnO at 500 mg Zn/kg or ZnO at 2250 mg Zn/kg both increased (P< 0·05) protein expression levels of occludin, claudin-1 and ZO-1 on day 7 post-weaning, and protein expression of occludin and ZO-1 on day 14 post-weaning. The piglets fed DS-ZnO at 500 mg Zn/kg did not differ in TJ proteins expression from those fed ZnO at 2250 mg Zn/kg (P>0·05), while they had higher (P< 0·05) protein expression levels of occluding and ZO-1 compared with those fed the mixture of DS and ZnO. Supplemental mixture of DS and ZnO had no (P>0·05) effect on TJ proteins' expression.

Fig. 1 Relative tight junction protein expressions in jejunal mucosa of piglets. (A) and (C) are representative blots of occludin, claudin, zonula occludens-1 (ZO-1) and β-actin on days 7 and 14 post-weaning, respectively. (B) and (D) show relative tight junction proteins expression on days 7 and 14 post-weaning, respectively. Values are means and standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). DS-ZnO, diosmectite–zinc oxide composite, containing 250 g zinc/kg DS-ZnO. DS+ZnO, mixture of 2·0 g DS/kg diet and 500 mg zinc/kg diet from zinc oxide (equal amount of DS and zinc oxide in the DS-ZnO group). The value of protein expression was the ratio of the densitometry units of tight junction protein and β-actin. The control sample on day 7 post-weaning was used as the reference sample. The protein expression of all samples was expressed as fold changes, calculated relative to the control group on day 7 post-weaning. □, Control; ![]() , DS+ZnO;
, DS+ZnO; ![]() , DS-ZnO; ■, ZnO.
, DS-ZnO; ■, ZnO.
Discussion
Early weaned piglets frequently encounter growth impairment and post-weaning diarrhoea, which induce major economic losses for pig producers(Reference Wijtten, Meulen and Verstegen2, Reference Kim, Hansen and Mullana3). The importance of Zn in the treatment of acute and persistent diarrhoea is well-known(Reference Patel, Mamtani and Dibley5). The addition of a high concentration of ZnO to the weaning diet (between 2000 and 4000 mg/kg of Zn as ZnO) alleviates post-weaning diarrhoea and increases growth, although the mechanisms underlying these effects have not been fully illuminated(Reference Kim, Hansen and Mullana3, Reference Hahn and Baker6, Reference Sargeant, McDowall and Miller16). In the present study, supplementation with ZnO at 2250 mg Zn/kg in weaned pigs improved feed intake and growth and decreased post-weaning diarrhoea, which is consistent with previous reports. The increased feed intake might have beneficial effects on the barrier function and inflammation, as weaning anorexia may contribute to local inflammation in the piglet small intestine(Reference McCracken, Spurlock and Roos26).
In the present experiment, a supplemental mixture of 2·0 g DS/kg and 500 mg Zn/kg had no influence on post-weaning diarrhoea, inflammation and intestinal barrier function. In contrast to our findings, it was reported that DS was effective in anti-diarrhoea, anti-inflammation and intestinal mucosal restoration in human subjects and pigs(Reference Gonzalez, Sanchez de Medina and Martinez-Augustin13–Reference Song, Liu and Soares15). DS may adhere to gastrointestinal mucous membranes and reinforce the physical mucous barrier(Reference Droy-Lefaix, Drouet and Schatz12). Its anti-diarrhoeal mechanisms involve adsorption of toxins and bacteria, modifications of mucus rheological properties and modulatory action of cytokine production(Reference Gonzalez, Sanchez de Medina and Martinez-Augustin13, Reference Dupont and Vernisse14). The possible reason for the ineffectiveness of DS in the present experiment may be that the inclusion of DS (2·0 g/kg) was too low. The dosage of DS in anti-diarrhoeal remedy was suggested to be 20–30 g/kg(Reference Song, Liu and Soares15). Gonzalez et al. (Reference Gonzalez, Sanchez de Medina and Martinez-Augustin13) found that the anti-inflammatory effect of DS was dose-dependent. The present result, that supplementation with ZnO at 500 mg Zn/kg had no influence on growth performance and post-weaning diarrhoeal of piglets, was consistent with previous research. Davis et al. (Reference Davis, Brown and Maxwell27) found that supplementing ZnO to provide 500 parts per million Zn in weanling pig diets did not result in any benefit. Hollis et al. (Reference Hollis, Carter and Cline28) reported no growth improvement when supplementing ZnO at levels less than 1000 mg/kg in weanling pigs.
To decrease inclusion of ZnO and to achieve the same effect of high levels of ZnO, we used DS as a controlled-release carrier for ZnO delivery. DS has been researched as an effective drug delivery carrier for controlled-release of bioactive molecules, drugs and nutrients, such as epidermal growth factor (EGF)(Reference Vaiana, Leonard and Drummy8), ibuprofen(Reference Zheng, Luan and Wang9) and vitamin B6(Reference Joshi, Patel and Bajaj10). In the present study, we functionalised DS with ZnO via ion exchange reaction to create a composite (DS-ZnO). Khaorapapong et al. (Reference Khaorapapong, Khumchoo and Ogawa17) found that ZnO was located between the interlayer space and on the surface of DS. The present experiment demonstrated that the growth performance and post-weaning diarrhoea of weaned piglets fed DS-ZnO at 500 mg Zn/kg was comparable with those fed ZnO at 2250 mg Zn/kg, while significantly better than those fed the equivalent amount of the mixture of DS and ZnO. Zheng et al. (Reference Zheng, Luan and Wang9) intercalated ibuprofen into DS to synthesise an ibuprofen/DS composite as a drug release system. The in vitro release experiment showed that the release of ibuprofen from the ibuprofen/DS composite in simulated intestinal fluid was markedly higher than that in simulated gastric fluid. Vaiana et al. (Reference Vaiana, Leonard and Drummy8) used DS as a delivery mechanism for EGF and found that the DS-EGF composite enhanced both the haemostatic and the proliferative stages of epithelial wound healing. They observed a strong bond between EGF and DS after several days of incubation in complete media, suggesting that DS-EGF remains largely intact. Controlling the release of ZnO in the gastrointestinal tract may improve its effectiveness(Reference Kim, Hansen and Mullana3). A lipid-coated ZnO has been claimed to prevent the absorption or chemical change of ZnO in the stomach, allowing it to enter the upper intestine where the lipid coating is broken down by lipase enzymes(Reference Kim, Hansen and Mullana3). This releases the ZnO in the critical area of the gastrointestinal tract for maximum effectiveness(Reference Kim, Hansen and Mullana3). DS served as a controlled-release carrier that might modify the rate and site of ZnO release. However, a better understanding of the binding and release properties of DS-ZnO is needed to fully characterise the mechanism of DS-ZnO.
Compromising alterations in villus–crypt structure are common in weaned pigs. Weaning is associated with villus atrophy and crypt hyperplasia(Reference Kim, Hansen and Mullana3, Reference Montagne, Boudry and Favier29). The shorter villus and deeper crypt observed in the control treatment verified the deterioration of intestinal morphology induced by weaning. Dietary inclusion of DS-ZnO at 500 mg Zn/kg or ZnO at 2250 mg Zn/kg reduced the weaning-associated damage to small intestinal morphology. The small-intestinal epithelium serves as a barrier against noxious antigens and pathogens. Impaired intestinal barrier function may increase the translocation of intestinal bacteria and the entering of toxic or allergenic substances from the gut into the body(Reference Wijtten, Meulen and Verstegen2). It has been reported that the early weaning process is associated with intestinal barrier dysfunction of piglets(Reference Wijtten, Meulen and Verstegen2, Reference Kim, Hansen and Mullana3, Reference Smith, Clark and Overman30). Zn supplementation improved mucosal repair in rats with experimental colitis(Reference Sturniolo, Fries and Mazzon31), and reduced intestinal permeability in children with acute diarrhoea and persistent diarrhoea syndromes(Reference Patel, Mamtani and Dibley5). However, little data are available regarding the addition of pharmacological doses of ZnO on intestinal permeability in weaned pigs. In the present experiment, the ex vivo Ussing chamber was used to monitor intestinal permeability. The flux of intact FD4 across the intestinal epithelium occurs mainly through paracellular pathways(Reference Hamard, Mazurais and Boudry21). An increased flux of FD4 reflects an impaired intestinal barrier. Supplementation with DS-ZnO at 500 mg Zn/kg or ZnO at 2250 mg Zn/kg reduced the paracellular flux of FD4 across the epithelium, indicating that the intestinal barrier function of weaned pigs was improved. Our findings are similar to another study(Reference Zhang and Guo32), which used urinary recovery of lactulose and mannitol to assess paracellular permeability and reported that addition of ZnO at 2000 mg Zn/kg decreased intestinal permeability of weaned piglets.
Weaning-associated intestinal inflammation and activation of the mucosal immune system have been described in the weanling rat(Reference Cummins, Steele and LaBrooy33). Pié et al. (Reference Pié, Lallès and Blazy1) also reported the up-regulated expression of pro-inflammatory cytokines in the intestine in newly weaned pigs. Over-production of pro-inflammatory cytokines has an adverse effect on intestinal mucosal integrity(Reference Liu, Huang and Hou22). Recent studies have indicated that most pro-inflammatory cytokines, such as TNF-α, IFN-γ, IL-1β and IL-6, induce a pathologic opening of the intestinal TJ barrier and increase intestinal epithelial permeability(Reference Al-Sadi, Boivin and Ma34). The cytokine TNF-α can act synergistically with INF-γ to induce structural changes in TJ(Reference Roselli, Finamore and Garaguso35). Controlling the release of intestinal pro-inflammatory cytokines may have potential benefits in alleviating gut disorders induced by weaning stress(Reference Liu, Huang and Hou22). In vitro, ZnO reduced inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic Escherichia coli infection(Reference Sargeant, Miller and Shaw4), and counteracted the up-regulated expression of the inflammatory IL-8, TNF-α mRNA levels caused by E. coli K88 using Caco-2 enterocytes(Reference Roselli, Finamore and Garaguso35). In the present study, dietary addition of DS-ZnO at 500 mg Zn/kg or ZnO at 2250 mg Zn/kg decreased mucosal mRNA of TNF-α, IL-6 and IFN-γ on day 7 post-weaning, while it did not affect pro-inflammatory cytokine mRNA on day 14 post-weaning. It has been demonstrated that weaning is associated with a transient up-regulation of inflammatory cytokine mRNA content on days 3 to 4 post-weaning, and that the levels of most cytokines rapidly return to pre-weaning values after day 9 post-weaning(Reference Pié, Lallès and Blazy1). This might be why the mRNA levels of TNF-α, IL-6 and IFN-γ on day 14 post-weaning were not affected by the dietary treatments in the present study. The down-regulation of pro-inflammatory cytokine on day 7 post-weaning in the presence of DS-ZnO indicated that weaning-induced inflammation was minimised. It has also been shown that activation of mast cells and release of mast cell proteases play a major role in the intestinal barrier dysfunction during the post-weaning period(Reference Moeser, Ryan and Nighot36). Furthermore, Ou et al. (Reference Ou, Li and Cao37) suggest a link between ZnO and mast cells in the porcine gut.
The intestinal barrier is mainly formed by a layer of epithelial cells joined together by TJ(Reference Li, Akhtar and Choudhry38). TJ consist mainly of the transmembrane protein complexes (e.g. claudins and occludins) and the cytosolic proteins ZO (e.g. junctional adhesion molecule, ZO-1, ZO-2 and ZO-3)(Reference Kim, Hansen and Mullana3). These proteins form a structure at the boundary of two adjacent cells, working as a barrier within the epithelial cell space. The TJ proteins are the rate-limiting step in the paracellular pathway and form a selectively permeable barrier(Reference Li, Akhtar and Choudhry38). The early weaning-induced up-regulation of pro-inflammatory cytokine might elicit alterations of TJ protein expression, because pro-inflammatory cytokine have been shown to down-regulate TJ protein expression(Reference Al-Sadi, Boivin and Ma34). The present study indicated that supplementation with DS-ZnO increased the protein expression of ZO-1, claudin-1 and occludin.
Overall, the results demonstrated that the weaned piglets fed DS-ZnO at 500 mg Zn/kg did not differ in growth performance, post-weaning diarrhoea and intestinal barrier function from those fed ZnO at 2250 mg Zn/kg, while they had better performance than those fed the equivalent amount of mixture of DS and ZnO. Dietary inclusion of DS-ZnO at 500 mg Zn/kg may alleviate diarrhoea and improve intestinal barrier function by down-regulation of pro-inflammatory cytokines and up-regulation of TJ proteins.
Acknowledgements
This research was jointly supported by the National Natural Science Foundation of China (grant no. 31072039), the Zhejiang Provincial Natural Science Foundation (no. Z3100071), the ‘948’ Project from the Ministry of Agriculture (no. 2011-G7), the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT1040). C. H. designed research; C. H., J. S., Y. L., Z. L. and K. Z. conducted the experiment and analysed the data; C. H. and J. S. wrote the paper. C. H. had primary responsibility for the final content. There are no financial or personal conflicts of interest to report.








