INTRODUCTION
Among infectious diseases, diarrhoeal diseases rank as the third leading cause of mortality and morbidity in low- and middle-income countries [Reference Lopez1]. It is estimated that diarrhoeal diseases account for 1·78 million deaths per year and 58·7 million disability-adjusted life years. Cholera, a rapidly dehydrating diarrhoeal disease, is estimated to cause the death of 100 000–130 000 persons and account for 3–5 million cases per year [Reference Zuckerman, Rombo and Fisch2]. Kenya suffers from a high burden of cholera, having reported 11 425 cases and 264 deaths in 2009 [3].
Cholera transmission is closely associated with environmental conditions, spread by faecal contamination of water and food [4]. Access to safe water and adequate sanitation are fundamentals of cholera prevention. In many countries, however, implementing relevant measures has proved difficult and slow [Reference Zuckerman, Rombo and Fisch2, 5]. Furthermore, improvements may not reach the most vulnerable populations, such as those living in slums and remote rural areas, in the near future [5]. Vaccines may therefore have a critical role as a provisional public health tool in cholera control in these communities. In 2005, the World Health Organization (WHO) first suggested oral cholera vaccines (OCVs) be used in cholera-endemic areas as a supplementary control strategy [6] and they strengthened that recommendation in 2010 [4].
Two OCVs are currently pre-qualified by the WHO for international use. Both Dukoral® (Crucell, The Netherlands), containing recombinant cholera toxin B subunit and killed whole-cell V. cholerae O1, and Shanchol™ (Shantha Biotechnics Ltd, India), containing killed V. cholerae O1 and O139, have been shown to be efficacious in endemic settings [Reference Lucas7, Reference Sur8]. Although safety, efficacy and an efficient health system to distribute the vaccine are critical, understanding cultural preferences and the willingness of communities to accept the vaccine are also essential. Assessing socio-cultural features of the illness and willingness to accept a vaccine indicate perceived need, demand and cultural barriers that may reduce coverage in a vaccine campaign. Notwithstanding recognized value of such research [Reference Larson9, Reference Stanton10], studies have been largely confined to high-income countries [Reference Raude, Caille-Brillet and Setbon11, Reference Mortensen12]. Cholera vaccine acceptance studies focus mainly on socio-demographics and willingness to pay [Reference Islam13–Reference Lucas15], while studies that have considered socio-cultural aspects of cholera have concentrated on Asia [Reference DeRoeck16, Reference Ali17]. Research is lacking on cultural dimensions and social determinants of cholera vaccine acceptance in Kenya.
This study was conducted in Nyanza province of Western Kenya due to the disproportionately high number of cholera cases reported there compared to the rest of Kenya [Reference Feikin, Tabu and Gichuki18]. Two large cholera outbreaks occurred there in 1997–1998 and 2008 that accounted for 43–47% and 72%, respectively, of all cholera cases in Kenya [Reference Shapiro19, Reference Shikanga20]. Urban and rural sites were chosen because they differ significantly in terms of environmental conditions, population density, residents' income and occupation; the implication being that the findings from one setting may not be attributable to the other. Cultural epidemiological methods [Reference Weiss21] were employed to understand community experience, meaning and behaviour with a cholera-like illness. The objectives of this paper are to (a) assess community willingness to accept an OCV in urban and rural populations in Western Kenya, (b) analyse socio-cultural determinants of anticipated OCV acceptance and (c) clarify the role of socio-cultural features of illness in explaining anticipated OCV acceptance by comparing models that consider socio-cultural determinants with exclusively socio-demographic models.
METHODS
Setting
This study was conducted at both urban and rural sites in Nyanza province, Western Kenya, where cholera is considered endemic. The urban site at Nyalenda A, Winam division, Kisumu district is a heavily populated informal settlement and the rural site is comprised of villages at Kakum Kombewa sub-location, Boro division, Siaya district.
The urban site covers an area of 2·8 km2, has 23 731 residents and a population density of 8475 persons/km2 [22]. The majority of residents do not have access to piped water and largely rely on shallow wells that are subject to a high level of contamination due to the predominance of pit latrines [22, Reference Olago23]. There are no government health facilities in Nyalenda A and private health services involve higher costs to be borne.
The rural site is comprised of nine villages at Kakum Kombewa with a population density of around 270 individuals/km2 [24]. Main sources of water in this region are untreated streams and boreholes. The majority (73%) of the population have access to latrines; however, over 24% of these are in a poor state and hence not used [24]. Lack of public transport makes access to Siaya district hospital, which is located about 15 km away, difficult.
Study design and sampling
This cross-sectional study required a minimum sample size of 328 to allow for cross-site comparisons with 95% significance and 80% power [Reference Schaetti25]. Men and women from the general population between the ages of 18 and mid-60s were included.
At the urban site, only an estimate of the population size was obtainable, hence, systematic probability sampling was done. The area was divided into seven roughly equal segments and every fifth household was approached to get a total of 28 households per segment. At the rural site, detailed household lists were accessible through community health workers. A specific number of households per village, proportional to the total number of households in that village, which had been identified in advance through random selection, were approached. At both sites, one willing adult of the household was interviewed; selection was made to maintain a roughly equal balance between men and women. When more than one eligible adult was available, we asked them to decide whom we should interview. If a household had no suitable, willing candidate, the neighbouring household was approached.
Instrument and data collection
This study used a semi-structured explanatory model interview based on the framework of the Explanatory Model Interview Catalogue (EMIC) for cultural epidemiology [Reference Weiss26]. It was developed for the study of cholera to assess locally valid features of illness-related experience, meaning and behaviour from the perspective of community residents [Reference Schaetti27]. The illness was introduced to participants using a clinical vignette that described a person with physical symptoms of cholera. Respondents were asked what they would call such an illness and the term for the illness provided by the respondent was used when asking further questions. In addition to questions on socio-cultural features of illness (i.e. physical symptoms, social impact, perceived causes, help-seeking behaviour), the interview also included questions on respondents' socio-demographic characteristics and their ideas on general vaccination. Quantitative and qualitative data were both collected.
Respondents were also asked if they would be willing to take a vaccine that is swallowed to prevent cholera. Details of efficacy and duration of protection were not discussed. OCV acceptance questions were posed at four different prices: ‘high’, based on estimated full production cost recovery for manufacture of two doses of Dukoral (KES 650/US$ 8·4)Footnote †; ‘medium’, which is half the high price (KES 325/US$ 4·2); ‘low’, close to the US$ 1 price that is considered a realistic vaccine price for low- and middle-income countries (KES 65/US$ 0·8) [Reference DeRoeck16] and ‘free’, fully subsidized as in the case of many immunization campaigns.
Interviews were conducted between March and May 2010, in Kiswahili, Dholuo and English. Interviewers received extensive training in sampling procedures, interviewing and obtaining informed consent. The interviewers were science or social science graduates from Maseno University and introduced themselves accordingly. Interviews were voice-recorded with permission.
Data management and analysis
EMIC interview data were double-entered using Epi Info software version 3.5.1 (Centers for Disease Control and Prevention, USA), programmed with logic and range checks. For analysis of socio-cultural features of illness, prominence of categories was calculated based on whether a response was reported spontaneously (assigned value of 2) or after probing (assigned value of 1). When a category was identified as most important among all others, it was assigned an additional value of 3. A mean prominence was then calculated for each category. Through this method of prominence calculation, categories were evaluated based on relative importance ascribed to them by local cultural ideas.
Logistic regression analyses were done to empirically identify socio-cultural determinants (i.e. socio-cultural features of illness and socio-demographic characteristics) associated with anticipated OCV acceptance at various prices. Dichotomized anticipated OCV acceptance variables, reflecting vaccine acceptance or non-acceptance, were used as outcome variables. Separate regression analyses were performed for anticipated OCV acceptance at the medium price and at the high price, but not for the low price or no-cost models as acceptance rates over 95% did not allow for it.
In crude analysis, associations between OCV acceptance and explanatory variables that were reported by 5–95% of respondents were analysed. Variables with P < 0·2 were considered for multivariate analysis. ‘Focal’ models of socio-cultural features of illness for specific groups of variables (i.e. related to physical symptoms, social impact, perceived causes, help-seeking), adjusted for socio-demographic variables, were run. Focal models for socio-demographic factors alone were also considered. Interaction of site with each of the variables was tested and site-interaction terms with P < 0·1 were included. To estimate the combined influence of all categories identified in the focal models on anticipated OCV acceptance, a ‘comprehensive’ model was calculated using variables with P < 0·2 and site-interaction terms with P < 0·1 from focal models. Corrected Akaike's Information Criterion (AICc) values were computed to compare relative goodness of fit in various focal and comprehensive regression models. Δ(AICc) which represents the difference in AICc between each model and the model with the lowest AICc, was used to make this comparison. Models with lower Δ(AICc) values are considered better in explaining OCV acceptance than those with higher values. Quantitative analysis was done with SAS version 9.2 (SAS Institute Inc., USA).
Narrative accounts were translated into English and entered in word processor software. Additional detail from key questions of the interview was added by transcribing relevant voice records. Typed data were then imported into MAXQDA version 10 (VERBI Software, Germany) for qualitative data management and analysis. Text segments were thematically coded based on the interview structure. Variables were imported into MAXQDA to enable the selection of narrative records of interest based on results from the quantitative analysis. This approach enabled integrated analysis of quantitative and qualitative data.
Ethical considerations
The study protocol received ethical approval from the Kenya Medical Research Institute and the WHO Research Ethics Review Committee. Interviews were conducted after obtaining written informed consent. No financial or other incentives were provided to respondents. Data collected in this study was maintained with utmost confidentiality and anonymized for reporting.
RESULTS
Sample characteristics
Of 379 respondents interviewed, 50% were from the urban site and 51% were female (Table 1). All respondents at the rural site and 96·8% at the urban site identified Christianity as their religion. The median personal monthly income was KES 2500 (US$ 32) at the urban site and KES 1000 (US$ 13) at the rural site (P = 0·01). Significantly more respondents at the urban site reported a dependable source of income. Self-employment (e.g. petty trading and skilled labour) was the most frequently mentioned primary occupation at the urban site; and agriculture at the rural site. Urban respondents were better educated: more in the urban sample had a secondary education or higher; more in the rural sample had no education. The rural site had significantly more individuals living within a household than at the urban site.
Table 1. Socio-demographic characteristics of study respondents
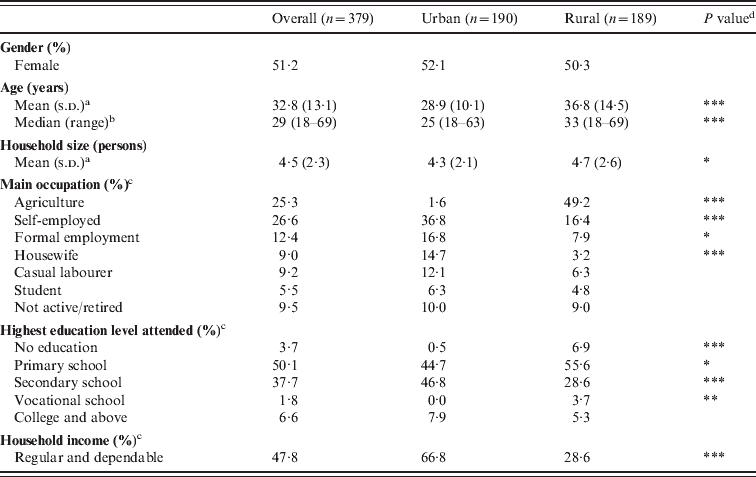
s.d., Standard deviation.
a t test.
b Wilcoxon test.
c Fisher's exact test. Only categories with overall reported percentages >1·5% are displayed.
d P value obtained from a comparison between the urban and rural site; * P ⩽ 0·05; ** P ⩽ 0·01; *** P ⩽ 0·001.
Past experience with vaccination and general ideas on vaccines
Two-thirds (66·8%) of all respondents reported having personally received a vaccination in the past. Fewer respondents reported prior vaccination experience at the rural (51·3%) than at the urban (82·1%, P < 0·001) site.
All but four respondents (98·9%) stated that in their experience, vaccines were helpful. The idea that vaccines were beneficial in preventing disease was reported pervasively. More knowledgeable respondents provided accounts with a scientific basis, such as, ‘Vaccines are helpful; they boost the immune system and prevent future infections’ (urban woman, 22 years). There also seemed to be a high level of confidence in the protective effect of vaccines, as seen in this narrative, ‘I rarely get sick because I was vaccinated’ (urban man, 30 years).
When asked whether some vaccines were also likely to cause problems, 27·7% of the respondents agreed; pain at the injection site, infection/abscess, fever and disability were frequently cited problems. Of respondents who believed that vaccines were not likely to cause problems, there was a significant difference in terms of site (P = 0·036) and gender (P = 0·007), with more women and more urban respondents espousing this view.
Anticipated OCV acceptance
Almost all respondents (98·7%) reported an interest in accepting an OCV if it were to be made available free of charge (Fig. 1). At the low price, 95·3% respondents were willing to accept the vaccine. At the medium and high prices, 72·8% and 58·8% respondents, respectively, were interested in the vaccine. More urban than rural respondents were willing to accept an OCV at the medium (P = 0·008) and high (P = 0·002) prices. Anticipated OCV acceptance rates between men and women were similar.

Fig. 1. Anticipated oral cholera vaccine (OCV) acceptance at different prices among urban and rural residents of Western Kenya. OCV price mentioned in Kenya shillings (KES) to respondents: low (KES 65/US$ 0·8), medium (KES 325/US$ 4·2), and high (KES 650/US$ 8·4) (exchange rate: KES 1 = US$ 0·01287). Y axis denotes percentage of respondents who provided a favourable response when questioned on whether they were likely to buy the vaccine at the stated price. Fisher's exact test was used for comparison of percentages between the two sites. ** P ⩽ 0·01.
Narrative accounts demonstrate an active demand for cholera vaccines. A sense of urgency in obtaining cholera vaccines was communicated as follows:
We have a water problem because there is a lot of pollution in the water and water points are scarce. We are also far from the hospital in Siaya. So I ask, when will this vaccine come? Or will you just disappear after the research? We really need the vaccine (rural man, 32 years).
A highly mentioned reason for willingness to purchase an OCV was that it would be more cost-effective than spending money on cholera treatment in the future.
Vaccine cost was a critical point of consideration for many respondents. While requesting a free vaccine, a 35-year-old rural woman explained:
If a vaccine is introduced, let it be free of charge so that it can help everyone. If it is brought with a price, others will die if they cannot afford it.
However, demand for a vaccine was high enough for respondents to offer suggestions that could enable vaccine purchase, even if it could not be availed for free.
If you bring the vaccine, tell us in advance so that we have enough time to collect money to pay for it. If you come without notice, we may not have the money ready (rural woman, 26 years).
The idea that health was more important than money was widespread and many stated: ‘You cannot compare your life to money.’
Determinants of anticipated OCV acceptance at the medium price and high price
Focal regression models considered specific groups of explanatory variables in explaining anticipated OCV acceptance at the medium and high prices (Tables 2 and 3). As per their Δ(AICc) values, at the medium price, ‘somatic symptoms’ and ‘self-treatment at home’ models explained acceptance better than the focal model with only socio-demographic characteristics. At the high price, all socio-cultural focal models were better than the exclusively socio-demographic model. Comprehensive models, which combined significant variables from all focal models, explained OCV acceptance best.
Table 2. Multivariate analysis (focal models) of socio-cultural determinants of anticipated oral cholera vaccine acceptance at the medium price (US$ 4·2) and assessment of models

a Each of the four focal models (somatic symptoms, social impact, perceived causes, self-treatment at home) were adjusted for socio-demographic characteristics.
b Logistic regression coefficient with 95% confidence interval.
c Bold values indicate P ⩽ 0·05.
d Interaction with site: refers to rural compared to urban site, with urban site as the baseline. Site-specific effects on variables considered only if P < 0·1 for site-interaction term. * P ⩽ 0·05, ** P ⩽ 0·01.
e Difference in corrected Akaike's Information Criterion [Δ(AICc)] between each model and the model with the lowest AICc. Comprehensive model (Table 4) had the lowest AICc and was assigned a value of zero. Models with lower Δ(AICc) values are considered better fitted than those with higher values. Bold values indicate models that are better than the model containing only socio-demographic characteristics.
f ‘Other causes’ refers to responses that could not be coded within designated categories of the interview. The variety of responses coded under ‘other causes’ included contact with infected persons, unprotected sexual intercourse, cold weather, mosquitoes, breathing in contaminated air and eating cold food.
g Variables with which each focal model was adjusted.
h Compared with the occupation of agriculture.
Table 3. Multivariate analysis (focal models) of socio-cultural determinants of anticipated oral cholera vaccine acceptance at the high price (US$ 8·4) and assessment of models

a Each of the three focal models (somatic symptoms, perceived causes, self-treatment at home) were adjusted for socio-demographic characteristics.
b Logistic regression coefficient with 95% confidence interval.
c Bold values indicate P ⩽ 0·05.
d Interaction with site: refers to rural compared to urban site, with urban site as the baseline. Site-specific effects on variables considered only if P < 0·1 for site-interaction term. † P < 0·1, ** P ⩽ 0·01.
e Difference in corrected Akaike's Information Criterion [Δ(AICc)] between each model and the model with the lowest AICc. Comprehensive model (Table 5) had the lowest AICc and was assigned a value of zero. Models with lower Δ(AICc) values are considered better fitted than those with higher values. Bold values indicate models that are better than the model containing only socio-demographic characteristics.
f ‘Other causes’ refers to responses that could not be coded within designated categories of the interview. The variety of responses coded under ‘other causes’ included contact with infected persons, unprotected sexual intercourse, cold weather, mosquitoes, breathing in contaminated air and eating cold food.
g Variables with which each focal model was adjusted.
h Compared with the occupation of agriculture.
Most variables that were significant in the focal models remained so in the comprehensive models. Socio-cultural determinants identified in the comprehensive models that were associated with anticipated OCV acceptance at the medium price and the high price are shown in Tables 4 and 5, respectively. Although different explanatory variables were identified in the analyses at the two price levels, they refer to coherent themes explaining anticipated OCV acceptance: specificity of symptoms for cholera, level of education, restricted preference for treatment and financial viability.
Table 4. Multivariate analysis (comprehensive model) of socio-cultural determinants of anticipated oral cholera vaccine acceptance at the medium price (US$ 4·2)

a Logistic regression coefficient with 95% confidence interval.
b Bold values indicate P ⩽ 0·05.
c ‘Other causes’ refers to responses that could not be coded within designated categories of the interview. The variety of responses coded under ‘other causes’ were contact with infected persons, unprotected sexual intercourse, cold weather, mosquitoes, breathing in contaminated air and eating cold food.
Table 5. Multivariate analysis (comprehensive model) of socio-cultural determinants of anticipated oral cholera vaccine acceptance at the high price (US$ 8·4)

a Logistic regression coefficient with 95% confidence interval.
b Bold values indicate P ⩽ 0·05.
c Interaction with site: refers to rural compared to urban site, with urban site as the baseline. Site-specific effects on variables considered only if P < 0·1 for site-interaction term. **P ⩽ 0·01.
Identification of physical symptoms that were unrelated to cholera such as bloody stool, and non-specific for cholera such as loss of appetite and confusion, were negatively associated with OCV acceptance. A cholera-specific symptom of being ‘very thirsty’ was positively associated.
Having attended secondary school was positively associated with OCV acceptance at the medium price. However, this did not remain significant at the high price.
With an increase in price of the vaccine from medium to high, the reporting of herbal treatment as a home remedy became significantly negatively associated with acceptance. In contrast, the reporting of prayer as a form of self-treatment at home was positively associated with OCV acceptance at both prices. An analysis of qualitative accounts revealed that prayer and medical interventions are considered complementary forms of treatment, carried out in parallel. Prayer and medicine are believed to have different, but non-conflicting roles, in treatment. ‘Prayer must be conducted to have God's intervention while pharmacy drugs help in controlling the situation’ (urban woman, 22 years). However, medical help was often implicitly assigned a greater priority while prayer was recommended in addition. Even a respondent who stated, ‘Prayer helps because God is above everything, even above drugs’, further mentioned that she would first give the patient water and drugs to combat diarrhoea, and thereafter pray.
Household income and household size significantly influenced OCV acceptance. The former was positively associated while an increasing household size was negatively associated.
Site-specific interactions were not observed for any variables at the high price and were present for just one variable at the medium price.
DISCUSSION
Findings from this study indicate high levels of acceptance for an OCV among urban and rural residents in Western Kenya. Quantitative and narrative analysis showed that respondents perceive a general benefit from immunization. The extensive interest and demand for OCVs indicates a likelihood of good coverage during mass vaccination initiatives. The study also showed that 91·3% of the respondents considered the illness as very serious and 96·3% believed that it had life-threatening consequences. The prospect of an effective vaccine campaign is further supported by this data given the widely acknowledged observation that perceived severity of a disease is closely associated with likelihood of vaccine uptake [Reference Stanton10, Reference Teitler-Regev, Shahrabani and Benzion28].
This study demonstrated that socio-cultural determinants explained anticipated vaccine acceptance better than socio-demographic factors alone. Identification of such socio-cultural determinants of OCV acceptance provides data relevant to ensure better coverage in an actual campaign. Past cholera control campaigns that have faced severe community resistance [Reference Nations and Monte29], and free treatment initiatives for other diseases that were rejected [Reference Parker, Allen and Hastings30], further underscores the importance of paying attention to local socio-cultural environments prior to interventions. Community studies are necessary to plan and prepare for vaccine campaigns [Reference Kaljee31]. This study provides an approach to integrate qualitative and quantitative empirical data, explain local cultural concepts of illness and guide disease control.
Several determinants of anticipated OCV acceptance were notable. Thirst, a cholera-specific symptom, was associated positively with acceptance. Non-specific physical symptoms for cholera were associated with a lower priority for the vaccine. Bloody stool, a characteristic symptom of other diarrhoeal illnesses, such as shigellosis, amoebic dysentery, campylobacteriosis, etc., was also negatively associated with OCV acceptance. The ability to discern cholera symptoms from symptoms of other diarrhoeal illnesses argues for a high level of awareness in the community. These findings also indicate that efforts to promote community awareness during control interventions need to highlight cholera-specific symptoms. Furthermore, given the definitive ideas of cholera possessed by the community, non-specific reference to diarrhoeal illness may lead to unreasonable expectations that the OCV will prevent all diarrhoea, leading to disappointment and possible discrediting of a useful vaccine.
Education was a predictor of vaccine acceptance. Interestingly, health education was reported as the most useful method of preventing cholera by the majority of respondents, and was frequently requested. A similar finding was reported from a study in Pakistan where education and knowledge about vaccines were associated with vaccine uptake [Reference Mitchell32]. These findings highlight the value of education and promoting health awareness in cholera control. However, at the high price, secondary school education was no longer significantly associated with OCV acceptance, indicating that education too has the ability to influence acceptance only to a certain extent. Above a certain price, economic factors may play a more prominent role in influencing OCV acceptance.
At the high price, self-help with herbal treatment was a significant negative determinant of acceptance. It appears that higher cost of the vaccine makes alternative, less expensive forms of treatment preferable. This finding is consistent with other literature in Kenya noting that the high cost of conventional Western drugs often makes them inaccessible, thereby promoting reliance on traditional remedies [Reference Matu and vanStaden33]. Pluralistic health-seeking practices, including traditional remedies, are widely used in other African countries [Reference Cassell34]; however, in our study they appear to compete with biomedical interventions, especially when the higher cost of vaccine becomes a barrier.
The priority of prayer, on the other hand, was complementary to vaccine interventions – an additional, rather than alternative source of help. Other studies suggest religious beliefs may be antagonistic to vaccine intervention [Reference Ruijs35, Reference Jegede36]. A study in Benin found that vaccination was rejected in some religious communities because they believed that they ‘require only prayer to protect and heal them in times of illness’ [Reference Fourn37]. The finding in Kenya, that reporting self-help with prayer was significantly associated with OCV acceptance, suggests a possible role played by religious institutions in encouraging the use of biomedicine. Although religious sectarian differences may influence the perceived benefits of medical interventions, we found no differences in anticipated OCV acceptance in members of the Legio Maria church, which some studies suggest may promote faith healing and reject biomedicine [Reference Prince38], and members of other church groups.
Financial viability, based on reporting a regular household income and a smaller household size, influenced OCV acceptance. While this finding is what would be expected for relatively high-priced vaccines, it underscores the importance of keeping costs reasonable. Furthermore, as the price of OCV was increased, determinants that had influenced vaccine acceptance at a lower price, such as education, were no longer relevant. The increasing price levels of OCV were introduced to provide an indication of priority and demand for the vaccine. Findings suggest that regardless of priorities and commitment to obtain an OCV, above a certain price it was simply beyond the means of many. In this study, the high price was the threshold. Hence, a full-cost recovery model with Dukoral may not be considered in this setting. However, it may be considered for OCVs that can be produced at a far lower cost, such as Shanchol [39].
Analysis of OCV acceptance did not reveal significant site-specific determinants; socio-cultural factors influencing OCV acceptance for urban and rural residents were similar. However, enthusiasm for an OCV was significantly higher in urban than rural respondents at the medium and high prices. This may be explained by the presence of greater disposable income and better education as has been observed in another vaccine study [Reference Mitchell32] or by higher perceived risk and vulnerability to the disease. In this study, both hypotheses remain plausible, as the urban respondents have better incomes and education than their rural counterparts. They also may attach a greater priority to receiving an OCV given the more crowded and unsanitary conditions that they have to contend with.
The main limitation of this study is the ability to relate anticipated acceptance with actual acceptance in the context of a vaccine campaign. Recognizing that there is a difference between what people say and what they actually do [Reference Mpazi and Mnyika40] anticipated acceptance may not perfectly guide actual acceptance. Inasmuch as this study provided a community assessment of vaccine demand and findings on predictors of OCV acceptance which support reasonable expectations (e.g. secondary school education was a predictor for OCV acceptance), further research addressing the nature of the relationship between anticipated and actual acceptance is needed. It also remains to be seen whether the predictors of anticipated OCV acceptance would remain significant in the context of an actual mass vaccination campaign.
Further research could include an assessment of whether findings from this study may be generalized across other settings. At some level we expect broad similarities in factors influencing OCV acceptance; however, particular priorities may be culture-specific. It would be fruitful to develop a framework for vaccine acceptance by conducting more such studies in different settings to explain common features and context-specific differences.
In conclusion, this study found high levels of interest for an OCV in community residents in Western Kenya, although vaccine cost was revealed as a critical consideration. Socio-cultural factors played an important role in anticipated OCV acceptance and specific determinants were identified. This research also provides an approach for the study of socio-cultural determinants and barriers to vaccine acceptance in other settings.
ACKNOWLEDGEMENTS
We gratefully acknowledge the participation of the study population and the commitment of research assistants who conducted interviews. We thank public health officials from Siaya and Kisumu districts for providing cholera incidence data and other essential information. We thank Leticia Grize and Christian Schindler for valuable statistical support. This study was supported by funds from the Bill and Melinda Gates Foundation that is also gratefully acknowledged.
C.-L. Chaignat and R. Hutubessy are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
DECLARATION OF INTEREST
None.








