The metabolic syndrome is tightly associated with hyperuricaemiaReference Zavaroni, Mazza, Fantuzzi, Dall'Aglio, Bonora, Delsignore, Passeri and Reaven1–Reference Culleton, Larson, Kannel and Levy3. TAG levels are directly associated with uric acid levels and these associations seem to be also related to several genetic polymorphismsReference Cardona, Tinahones, Collantes, Escudero, Garcia-Fuentes and Soriguer4–Reference Tinahones, Pérez-Lindon, C-Soriguer, Pareja, Sánchez-Guijo and Collantes7. We have also reported that hyperuricaemic-hypertriacylglycerolaemic patients have high levels of VLDL components and a reduced fractionated excretion of uric acidReference Tinahones, Pérez-Lindon, C-Soriguer, Pareja, Sánchez-Guijo and Collantes7.
The association between serum uric acid and the metabolic syndrome seems to be related to insulin resistance. Insulin acts in the proximal tubules and interferes with excretion of Na and uric acid under physiological states. Hyperinsulinaemia has been associated with decreased urate and Na excretion and thus with hypertension and hyperuricaemia. It seems that higher insulin resistance is also associated with higher serum uric acid levels, although most studies reporting this association were done in healthy subjects and under fasting or baseline conditionsReference Facchini, Chen, Hollenbeck and Reaven8, Reference Quinones Galvan, Natali, Baldi, Frascerra, Sanna, Ciociaro and Ferrannini9.
Oxidative stress has recently been shown to play an important role in the genesis of the metabolic syndromeReference Cardona and Tinahones10. The metabolization of purins has a paradoxical effect on oxidative stress. On the one hand, the action of xanthine oxidase on xanthine produces uric acid and superoxide radicals that are derived from reactive oxygen speciesReference Berry and Hare11, and on the other hand is the antioxidant effect of uric acid itself. This paradox has resulted in some studies showing that the administration of allopurinol, which is hypouricaemic, reduces reactive oxygen speciesReference Farquharson, Butler, Hill, Belch and Struthers12, whilst others show that the rise in uric acid concentrations increases the antioxidant capacity of plasmaReference Waring, Webb and Maxwell13, Reference Waring, Convery, Mishra, Shenkin, Webb and Maxwell14.
An oral glucose tolerance test (OGTT) causes an increase in insulin levels and oxidative stress, both in persons with diabetes and in healthy personsReference Ceriello15, Reference Ceriello, Bortolotti, Motz, Crescentini, Lizzio, Russo, Tonutti and Taboga16. Recent studies have shown that hyperglycaemia induces an overproduction of superoxide by the mitochondrial electron-transport chainReference Brownlee17. Superoxide overproduction is accompanied by increased NO generation, due to endothelial NO synthase and inducible NO synthase uncoupled state, a phenomenon favouring the formation of the strong oxidant peroxynitrite. Additionally, during an oral glucose challenge, a reduction in the antioxidant defences is observedReference Ceriello, Bortolotti, Crescentini, Motz, Lizzio, Russo, Ezsol, Tonutti and Taboga18–Reference Zou, Shi and Cohen21.
Considering that uric acid may play a role in oxidative stress and that it has a direct relation with insulin levels, and aware that an oral glucose challenge increases oxidative stress and insulin levels, we sought to determine whether an OGTT was able to modify plasma levels of uric acid. Furthermore, we attempted to determine the possible association between this hypothetical change in uric acid levels and the levels of TAG, which is the variable in the metabolic syndrome that is most closely related with hyperuricaemia.
Subjects and methods
A total of 656 persons aged 18–65 years, selected randomly from the municipal census, were included in the present study. The general characteristics of the study have been reported elsewhereReference Rojo-Martinez, Esteva, Ruiz de Adana, Garcia-Almeida, Tinahones, Cardona, Morcillo, Garcia-Escobar, Garcia-Fuentes and Soriguer22. Pregnant women, hospitalized patients or those who lived in health institutions, as well as those with severe psychological disorders, were excluded. The subjects were requested by mail to attend their local health centre for a medical examination. Those who failed to attend their first appointment were sent a second letter giving them another appointment and all those still not attending were visited at home in order to ascertain the reason. The final sample distribution by age and sex was not significantly different from the population distribution23.
Procedures
All subjects were interviewed and given a standardized clinical examination based on standard procedures performed by the same researchers. The clinical data included weight, height, BMI (weight (kg)/height (m)2), hip circumference, abdominal circumference and waist:hip ratio, all measured as usual. An OGTT was given to all the participants. Blood samples were taken from all the subjects at baseline and 120 min after the OGTT. The plasma was separated and frozen at − 70°C for later analysis.
Glycaemia, uric acid and plasma proteins were measured at baseline and 120 min after the OGTT (Dimension autoanalyzer; Dade Behring Inc., Deerfield, IL, USA). Measurements were also made in the baseline sample of total cholesterol, TAG and HDL-cholesterol by enzymatic methods in an autoanalyzer (Dimension; Dade Behring Inc.). Baseline insulin levels were measured by RIA administered by BioSource S.A. (Biosource Europe SA, Nivelles, Belgium) and insulin resistance was calculated from the homeostasis model assessment (HOMA) with the formula: insulin resistance = [fasting serum insulin (μU/ml) × fasting blood glucose (mmol/l)]/22·5Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner24 and HOMA-IS = (fasting insulin (μU/ml) × 20)/(fasting glucose (mmol/l) − 3.5).
Statistical analysis
The data are presented as means and standard deviations. The 75th percentile (δP75) of the frequency distribution of the difference between uric acid at baseline and after the OGTT was used as the cut-off point for changes in the levels of uric acid during the OGTT. The percentiles for the uric acid difference were: 25–18·230; 50 8·862; 75 40·874 μmol/l.
Means contrast was done with the Student t test and bivariate correlations by calculating the linear correlation coefficient (Pearson r). The contribution of different variables to the variance in uric acid level change between baseline and 120 min was calculated by multiple regression models. In all cases the rejection level for a null hypothesis was set at α = 0·05. The statistical analysis was done with SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). The study was approved by the Ethics and Clinical Investigation Committee of Carlos Haya Hospital.
Results
Table 1 shows the age, sex and the baseline and 120-min values for uric acid, total proteins, glucose and insulin, as well as the TAG level, BMI, waist:hip ratio, Homeostasis model assessment-insulin resistance (HOMA-IR), HOMA-insulin secretion (IS), HDL-cholesterol and LDL-cholesterol in the study population. The levels of uric acid were significantly lower after the OGTT (281·93 (sd 92·19) v. 267·48 (sd 90·40) μmol/l; P < 0·0001).
Table 1 Biological characteristics of the study population*
(Mean values and standard deviations)
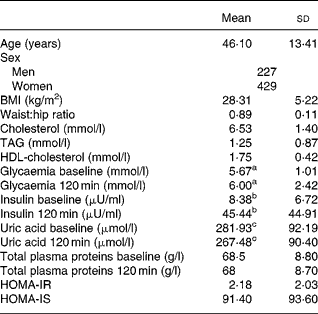
* For details of subjects and procedures, see Materials and methods.
a,b,c Mean values were significantly different between before and after oral glucose tolerance test (P < 0·0001).
HOMA-IR, homeostasis model assessment insulin resistance; HOMA-IS, homeostasis model assessment insulin secretion.
The subjects with the greatest differences between uric acid values at baseline and after 120 min (δP75) also had the highest levels of TAG (P < 0·001), baseline insulinaemia (P < 0·02), HOMA-IS (P < 0·023) and HOMA-IR (P < 0·034). Women with a difference in plasma uric acid levels above the 75th percentile had higher baseline insulin levels (P = 0·019), higher concentrations of plasma TAG (P = 0·0001) and a greater insulin resistance index (P = 0·029), whereas the men only had a significant difference in TAG levels (Table 2).
Table 2 Distribution of the biological variables studied according to the 75th percentile (δP75) of the difference in plasma uric acid levels before and after oral glucose tolerance test and grouped according to sex (Student t test)*
(Mean values and standard deviations)
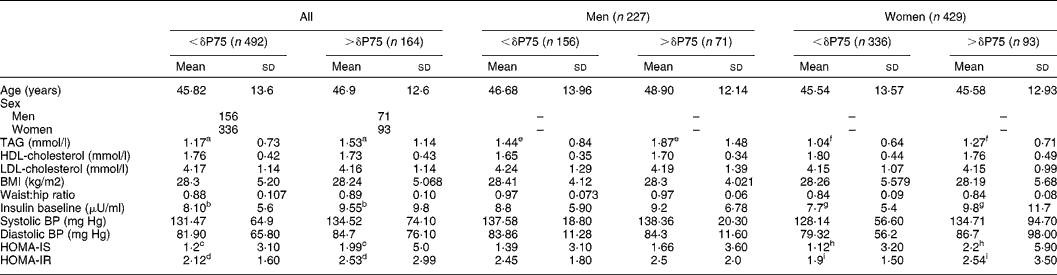
* For details of subjects and procedures, see Materials and methods.
P < 0·05 significant differences: a,e,fP < 0·0001; b,g,hP = 0·02; c,d,iP < 0·05. No significant differences were detected according to sex.
HOMA, homeostasis model assessment; IR insulin resistance; IS insulin secretion; BP blood pressure.
The baseline uric acid level correlated negatively with HDL-cholesterol and positively with cholesterol, TAG, age, BMI, baseline and 120-min insulinaemia and glycaemia, HOMA-IR and HOMA-IS. The difference in the uric acid levels between baseline and 120 min after the OGTT was significantly associated with plasma TAG, cholesterol and glycaemia at 120 min (r 0·23, P < 0·001, r 0·10, P = 0·006, r 0·092 and P = 0·022, respectively) (Table 3). Separate correlation analyses according to sex showed no differences (data not shown).
Table 3 Correlation analysis of the biological variables studied (Pearson r)†
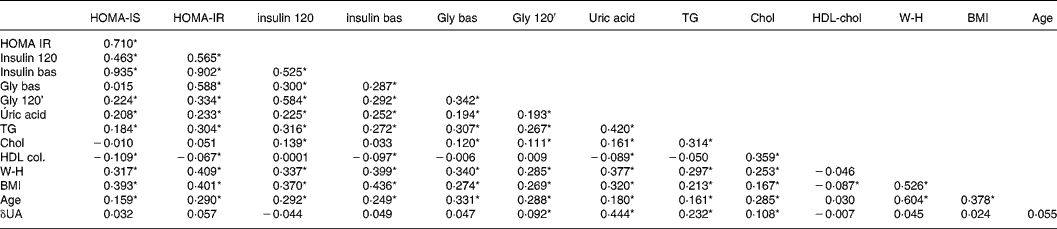
Statistical significance: *P < 0·05. No significant differences were detected according to sex.
† For details of subjects and procedures, see Materials and methods.
δUA, difference uric acid; Chol, cholesterol; insulin bas, insulin baseline; HOMA, homeostasis model assessment; IR, insulin resistance; IS, insulin secretion; W-H, waist:hip ratio; Gly bas, glycaemia basal; Gly 120, glycaemia 120.
Multiple regression analysis with the difference in the uric acid levels between baseline and 120 min after the OGTT as the dependent variable showed that the TAG levels, insulin 120 min, glycaemia 120 min and waist:hip ratio significantly predicted the variance in the uric acid difference (r 2 0·077) (Table 4). The following variables failed to enter the model: baseline insulin; baseline and 120-min glycaemia; HDL-cholesterol; cholesterol; HOMA-IS; HOMA-IR. Separate analysis showed no difference according to sex.
Table 4 Multiple regression analysis*
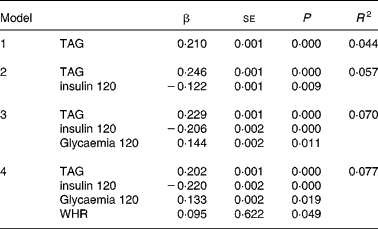
* For details of subjects and procedures, see Materials and methods.
Dependent variable: difference uric acid (δUA); independent variables: cholesterol, uric acid, age, BMI, LDL-cholesterol, homeostasis model assessment insulin resistance (HOMA-IR), homeostasis model assessment insulin secretion (HOMA-IS), insulin, insulin 120 min, glucose, glucose 120 min.
Statistical significance: P < 0·05.
Variables not entering the model: baseline insulin, baseline glycaemia, HDL-cholesterol, cholesterol, BMI, age, HOMA-IS and HOMA-IR. No significant differences were detected according to sex.
WHR, waist:hip ratio.
Discussion
The main finding in the present study was that uric acid levels drop after a glucose challenge and this change is dependent on TAG levels and insulin resistance. The reasons for this reduction may include reduced synthesis of uric acid after a glucose challenge; increasing renal excretion of urates, although this is physiologically unlikely as uric acid excretion in 2 h is not enough to produce a drop in plasma levels of uric acid; a reduction due to the effect of dilution in serum after the glucose challenge, which again is unlikely as no change was seen in levels of total plasma proteins. A final possibility is that this reduction is due to the consumption of uric acid as it carries out its antioxidant function. In line with our hypothesis, the latter option is most likely. The reduction in uric acid levels after an OGTT with 75 g glucose may have an important biological meaning, since it highlights the role of uric acid as an antioxidant in plasma in a situation that increases oxidative stress, as is a glucose challenge.
An increase in oxidative stress occurs after an OGTT in persons with and without diabetesReference Ceriello15, Reference Ceriello, Bortolotti, Motz, Crescentini, Lizzio, Russo, Tonutti and Taboga16. Several studies in patients with type 2 diabetes have reported the generation of oxidative stress during the postprandial period. These observations suggest that control of glucose excursions, and accompanying oxidative stress, during the postprandial period may be important in the prevention of potential long-term diabetes complicationsReference Ceriello, Bortolotti, Motz, Crescentini, Lizzio, Russo, Tonutti and Taboga16, Reference Ceriello, Bortolotti, Motz, Pieri, Marra, Tonutti, Lizzio, Feletto, Catone and Taboga25, Reference Ceriello26. This increase potentially influences the urate levels by two different mechanisms: 1) the reduction of the plasma level of uric acid after a glucose challenge is due to the consumption of uric acid after its sudden participation in antioxidant reactions. Allantoin is formed from the non-enzymatic oxidation of urate and it is a marker of the antioxidant effect of uric acidReference Benzie, Chung and Tomlinson27. Thus, the reduction in plasma levels of uric acid may be at the expense of increased levels of allantoin, after carrying out its antioxidant function; 2) experimental and clinical studies have shown that uric acid levels rise in response to chronic oxidative stressReference Ozguner, Armagan, Koyu, Caliskan and Koylu28, Reference Elmas, Aslan, Caglar, Derin, Agar, Aliciguzel and Yargicoglu29.
The drop in plasma levels of uric acid may be due to less synthesis, as a result of the inhibition of xanthine oxidase by the high degree of postprandial oxidative stress as a consequence of high levels of free radicalsReference McNally, Saxena, Cai, Dikalov and Harrison30, Reference Mueller, Laude, McNally and Harrison31. We therefore propose a hypothesis according to which situations of acute stress produce a small decrease in the levels of uric acid, due to the consumption of uric acid as it carries out its antioxidant function and conversely that chronic stress would induce a permanent increase in uric acid levels.
The association between hyperuricaemia and hypertriacylglycerolaemia has been known for some time. Two different phenotypes exist, pure hyperuricaemic persons and persons with both hyperuricaemia and hypertriacylglycerolaemiaReference Collantes, Tinahones, Cisnal, Anon and Sanchez-Guijo32. Fox et al. found that environmental factors such as diet were responsible for this association of hypertriacylglycerolaemia and hyperuricaemiaReference Fox, John, DeBruyne, Dwosh and Marliss33. Furthermore, our group has detected this association of hypertriacylglycerolaemia and hyperuricaemia even in the absence of these environmental factorsReference Collantes Estevez, Pineda Priego, Anon Barbudo and Sanchez Guijo34 and evidence exists for genetic associations between these two metabolic disorders and several genesReference Cardona, Tinahones, Collantes, Escudero, Garcia-Fuentes and Soriguer4–Reference Cardona, Tinahones, Collantes, Garcia-Fuentes, Escudero and Soriguer6. The results of the present study show that the decrease in uric acid levels after a glucose challenge is closely associated with baseline TAG levels and with variables associated with insulin resistance in the general population and support the association between hypertriacylglycerolaemia and hyperuricaemia found previouslyReference Tinahones, Pérez-Lindon, C-Soriguer, Pareja, Sánchez-Guijo and Collantes7. A possible explanation for this is that persons with hypertriacylglycerolaemia and insulin resistance have greater oxidative stress, with the consequent greater consumption of uric acid. Hypertriacylglycerolaemia produces a rise in oxidative stress via an increase in free radicals, particularly in superoxide anions proceeding from the increased proton gradient in the mitochondria as a result of excess acetyl CoA after β-oxidation of NEFA derived from the TAG; an increase that is reverted with antioxidantsReference Ceriello, Assaloni, Da Ros, Maier, Piconi, Quagliaro, Esposito and Giugliano35, Reference Ceriello and Motz36.
In conclusion, this study showed that in a situation that increases oxidative stress, as is an OGTT, there is an acute drop in levels of uric acid and that those persons who experience the greatest reduction have greater insulin resistance and higher TAG levels. Whether this reduction in uric acid concentration is associated with the antioxidant effect of uric acid remains to be shown.
Acknowledgements
The authors wish to thank all the subjects for their collaboration and IMABIS. We also gratefully acknowledge the help of Ian Johnstone for his expertise in preparing this manuscript. This study was undertaken with finance from the Fondo de Investigación Sanitaria ‘Centros de Investigación En Red’ (CIBER, CB06/03/0018) of the ‘Instituto de Salud Carlos III’, SAF 2006/12 894 of the MCYT and FIS 05/1307 of the ‘Instituto de Salud Carlos III’, Madrid, Spain. The investigation group belongs to the ‘Red de Diabetes y Metabolismo’ (RD06/0015/0008) of the Instituto de Salud Carlos III.






