Starch, serving as the most popular energy-yielding part of the diet, is the major carbohydrate in monogastric mammalian nutrition( Reference Yin, Deng and Huang 1 , Reference Regmi, van Kempen and Matte 2 ). The critical physiological function of starch is to release the glucose and serve as a source of energy for the body with the timeline of digestion( Reference Wiseman 3 ). Starch not only supplies acetyl-CoA for the tricarboxylic acid cycle and lipid metabolism regulation but also provides α-keto acids as carbon skeleton for non-essential amino acid (AA) synthesis. These coordinated-regulation metabolic processes cause effective utilisation of dietary starches, and key enzymes and genes involved in these metabolisms are closely regulated by dietary nutrients.
A previous study had found that starch could be classified into three types, according to the different digestibility depending upon their polymer structures in the intestine of monogastric mammals, followed as rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch( Reference Englyst, Kingman and Cummings 4 ). The metabolic reactions could vary greatly because of the widely variable digestibility of starches from different types( Reference Stevnebø, Sahlströ and Svihus 5 ). Starch with low amylose:amylopectin ratio is quickly digested, which could lead to a critical increase in plasma glucose and stronger insulinaemic responses( Reference Englyst, Veenstra and Hudson 6 – Reference Yin, Yin and Zhang 8 ), and then promote up-regulation of lipogenesis in liver( Reference Foufelle, Gouhot and Pégorier 9 – Reference He, Chen and Zhang 11 ). However, starch with high amylose:amylopectin ratio results in moderate glycaemic and insulinaemic responses( Reference Regmi, van Kempen and Matte 2 , Reference Bird, Brown and Topping 12 , Reference He, Chen and Yu 13 ). Diet with different starch types has a different influence on the digestion of protein and absorption of AA in the jejunum of pigs. In weaned and growing pigs, a diet with SDS significant increased the appearance of AA in the portal circulation and improved N utilisation( Reference Li, Huang and Wu 14 , Reference Li, Dai and Yin 15 ). However, the latest research from Yin et al.( Reference Yin, Zhang and Huang 16 ) found that a diet with RDS could regulate AA metabolism and increase the dietary AA into the systemic circulation of weaned pigs. In the meantime, a previous study in our laboratory proved that a diet with pea starch (PS) increased the average weight gain, loin eye area and decreased the feed:gain ratio and back fat of finishing pigs compared with the waxy maize starch (WMS) group( Reference Li, Li and Zhang 17 ). However, the effect of dietary starch types on the mechanisms behind these metabolic responses is unknown.
We hypothesised that the PS diet increased the growth performance and reduced lipid deposition by varying the plasma glucose and insulin levels, and then altered nutrient metabolism in liver. Therefore, the objective of this study was to examine the effect of dietary starch types on liver nutrient metabolism in finishing pigs.
Methods
Animal and diets
All procedures including animal care and experimental treatments in the present study were approved by the Animal Care and Use Committee of Nanjing Agricultural University. In all, ninety barrows (Yorkshire×Landrace×Meishan) with an average initial body weight of 68 (se 2·0) kg were selected and randomly divided into three dietary treatments with five pens per treatment and six pigs per pen.
All experimental diets were isoenergetic and isonitrogenous and formulated on the basis of nutrient requirements of the National Research Council for 75–100 kg pigs( 18 ) (Table 1). The nutrient composition was calculated according to the recommendations of ‘Chinese Feed Database’( Reference Xiong, Pang and Luo 19 ). Purified WMS, non-waxy maize starch (NMS) and PS were, respectively, used as the only dietary energy sources. The amylose:amylopectin ratios of diets were 0·07, 0·19 and 0·28, respectively, measured by a Megazyme Amylose/Amylopectin assay kit (K-AMYL 09/14; Megazyme Int.). The experiment was carried out at Jiangsu Academy of Agricultural Sciences. Pigs were afforded ad libitum with water and feed throughout 28 d experimental period.
Table 1 Composition and nutrient levels of diets for finishing pigs

WMS, waxy maize starch; NMS, non-waxy maize starch; PS, pea starch.
* The premix provided per kg of diet: 100 mg of Fe as iron sulphate, 100 mg of Zn as zinc oxide, 30 mg of Mn as manganous oxide, 20 mg of Cu as copper sulphate, 0·3 mg of Se as sodium selenite, 0·5 mg of iodine as calcium iodate, 1720 μg of retinyl acetate, 25 μg of cholecalciferol, 8·0 mg of dl-α-tocopheryl acetate, 3·0 mg of menadione sodium bisulphite, 2·0 mg of thiamin mononitrate, 6·0 mg of riboflavin, 3·0 mg of pyridoxine hydrochloride, 30 mg of nicotinic acid, 30 mg of calcium pantothenate, 1·0 mg of folic acid, 20 μg of cyanocobalamin, 300 mg of choline.
† Calculated according to ‘Chinese Feed Database’.
Sample collection
After 28 d of the feeding trial, thirty pigs in total (ten per treatment, two per pen close to the average body weight of the pen) were selected, weighed and transferred to the slaughterhouse. After a 12-h fast, the thirty pigs were electrically stunned and slaughtered by exsanguination. The samples of liver tissue were collected and quickly frozen in liquid N2 for subsequent analysis.
Biochemical analysis
Frozen samples of liver were thawed, homogenised and centrifuged to gain supernatants for the following biochemical analysis, and the protein concentration of the supernatants was assayed by using the bicinchoninic acid protein assay kit (Sangon Biotec.). The activities of some glucose-metabolism-related enzymes in liver including glycogen synthase (GCS), glycogen phosphorylase a (GPa), hexokinase (HK), glucose-6-phosphatase (G-6-P), pyruvate carboxylase (PK) and phosphoenolpyruvate carboxykinase (PEPCK) were assayed in accordance with the manufacturer’s instructions (Keming Bioengineer Company). The concentrations of TAG, total cholesterol (TC) and the activity of hepatic lipase (HL) in liver were determined by using the commercially available kit from Nanjing Jiancheng Bioengineering Institute, and the concentrations of acyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) were measured by the ELISA (Angle Gene Bioengineer Institute). The alanine transaminase (ALT) and aspartate transaminase (AST) activities were measured according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute).
Total RNA was extracted from frozen samples of liver with RNAiso Plus reagent (TaKaRa Biotechnology Co. Ltd). The purity and concentration of total RNA were analysed at the OD260:OD280 ratio with a NanoDrop ND-1000 UV spectrophotometer (Thermo Scientific). All the OD260:OD280 ratios of RNA samples were between 1·8 and 2·0. Thereafter, DNA was removed from total RNA by DNase I (TaKaRa Biotechnology Co. Ltd). The 500-ng RNA was reverse-transcribed to complementary DNA (cDNA) with a PrimeScript RTTM Master Mix kit (TaKaRa Biotechnology Co. Ltd) according to the manufacturer’ instructions. Real-time PCR was performed on an ABI 7500 Real-Time PCR System (Applied Biosystems) with SYBR Premix Ex Taq Kits (Takara Biotechnology Co. Ltd). The target genes and the housekeeping gene were synthesised by Sangon and used for real-time PCR (Table 2). The PCR system comprised 10 μl of SYBR Premix Ex Taq, 0·4 μl of ROX Reference Dye II, 2 μl of cDNA, 6·8 μl of double-distilled water and 0·4 μl of each primer pair (10 μmol/l) in a total volume of 20 μl. The PCR protocol was as follows: 95°C for 30 s, then forty cycles at 95°C for 5 s and 60°C for 34 s. The β-actin that served as the housekeeping gene was applied to normalise the expression of the target genes. Relative gene expression was computed using the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method according to a previously described method(
Reference Livak and Schmittgen
20
). All samples were repeated in triplicate.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method according to a previously described method(
Reference Livak and Schmittgen
20
). All samples were repeated in triplicate.
Table 2 Primer sequence of target and reference genes

PCK1, phosphoenolpyruvate carboxykinase 1; G6PC, glucose-6-phosphatase, catalytic subunit; FOXO1, forkhead box protein O1; SREBP-1c, sterol regulatory element binding protein-1c; LXRa, liver X receptor; ChREBP, carbohydrate-responsive element-binding protein; GA, glutaminase; GDH, glutamate dehydrogenase; CPS-1, carbamoyl phosphate synthetase 1; INR, insulin receptor; Akt, protein kinase B, also named PKB; mTOR1, mammalian target of rapamycin complex 1; 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; S6K1, ribosomal protein S6 kinase β-1.
Statistical analysis
Data analysis was carried out by ANOVA with the SPSS (version 16.0; SPSS Inc.). The data were analysed with the pen as the experimental unit (n 5; the means of two pigs per pen were applied to represent pens). The significant difference among treatments was verified separately by the least significant difference test. The results were provided as mean with their standard errors. P<0·05 was used to indicate statistical significance.
Results
Activities of some glucose-metabolism-related enzymes in liver
The enzyme activities of GCS, HK, GPa, G-6-P, PK and PEPCK in liver of finishing pigs are shown in Table 3. There was no significant difference in the activities of GCS, HK, G-6-P and PK among the three groups (P>0·05), whereas PS and NMS diets significantly decreased the GPa activity in liver compared with the WMS diet (P<0·05). In addition, the activity of PEPCK was lower in the PS group than that in the WMS group (P<0·05).
Table 3 Effect of dietary starch types on the activities of some glucose-metabolism-related enzymes in liver of finishing pigs (nmol/min per mg of protein) (n 5)
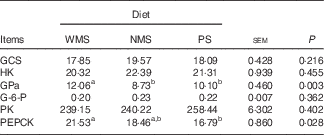
WMS, waxy maize starch; NMS, non-waxy maize starch; PS, pea starch; GCS, glycogen synthase; HK, hexokinase; GPa, glycogen phosphorylase a; G-6-P, glucose-6-phosphatase; PK, pyruvate kinase; PEPCK, phosphoenolpyruvate carboxykinase.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Relative mRNA expression of some glucose-metabolism-related genes in liver
The abundances of Forkhead box protein O1 (FOXO1), phosphoenolpyruvate carboxykinase 1 (PCK-1), glucose-6-phosphatase C subunit (G6PC) and GLUT4 in liver of finishing pigs are reported in Fig. 1. Compared with the WMS diet, PS and NMS diets significantly reduced the mRNA expression of PCK1 in the liver (P<0·05). However, no significant difference in the mRNA expressions of FOXO1, G6PC and GLUT4 was observed among groups (P>0·05).

Fig. 1 Effect of dietary starch types on the relative mRNA abundance of some glucose-metabolism-related genes in liver of finishing pigs. Values are means (n 5), with standard errors represented by vertical bars. ![]() , Waxy maize starch;
, Waxy maize starch; ![]() , non-waxy maize starch;
, non-waxy maize starch; ![]() , pea starch; FOXO1, forkhead box protein O1; PCK1, phosphoenolpyruvate carboxykinase 1; G6PC, glucose-6-phosphatase C subunit. a,b Mean values with unlike letters were significantly different (P<0·05).
, pea starch; FOXO1, forkhead box protein O1; PCK1, phosphoenolpyruvate carboxykinase 1; G6PC, glucose-6-phosphatase C subunit. a,b Mean values with unlike letters were significantly different (P<0·05).
Lipid contents and some lipid-metabolism-related enzymes in liver
The concentrations of TAG and TC were lower in the liver of the PS and NMS groups compared with those of the WMS group (P<0·05) (Table 4). In contrast, the starch types did not affect the activity of HL among groups (P>0·05). The concentrations of ACC and FAS in liver of PS and NMS groups were lower than those of the WMS group (P<0·05).
Table 4 Effect of dietary starch types on lipid contents and some lipid-metabolism-related enzyme activities or concentrations in liver of finishing pigs (n 5)
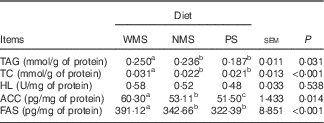
WMS, waxy maize starch; NMS, non-waxy maize starch; PS, pea starch; TC, total cholesterol; HL, hepatic lipase; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Relative mRNA expression of some lipid-metabolism-related genes in liver
As shown in Fig. 2, the present results indicated that finishing pigs fed PS and NMS diets had lower mRNA abundance of sterol regulatory element binding protein-1c (SREBP-1c) in liver compared with those fed the WMS diet. However, no effect was observed in the expressions of carbohydrate-responsive element-binding protein (ChREBP), PPAR alpha (PPARα) and liver X receptor (LXR) among groups (P>0·05).

Fig. 2 Effect of dietary starch types on the relative mRNA abundance of some lipid-metabolism-related genes in liver of finishing pigs. Values are means (n 5), with standard errors represented by vertical bars. ![]() , Waxy maize starch;
, Waxy maize starch; ![]() , non-waxy maize starch;
, non-waxy maize starch; ![]() , pea starch; SREBP-1c, sterol regulatory element binding protein-1c; LXR, liver X receptor; ChREBP, carbohydrate-responsive element-binding protein. a,b Mean values with unlike letters were significantly different (P<0·05).
, pea starch; SREBP-1c, sterol regulatory element binding protein-1c; LXR, liver X receptor; ChREBP, carbohydrate-responsive element-binding protein. a,b Mean values with unlike letters were significantly different (P<0·05).
Some nitrogen-metabolism-related enzymes and genes in liver
As shown in Fig. 3, no effect was observed in AST and ALT activities in the liver (P>0·05). Compared with the WMS diet, PS and NMS diets reduced the mRNA abundance of glutamate dehydrogenase (GDH) and carbamoyl phosphate synthetase 1 (CPS-1) in the liver (P<0·05; Fig. 4). Nevertheless, there was no significant difference in the expression of glutaminase in liver among groups (P>0·05).

Fig. 3 Effect of dietary starch types on alanine aminotransferase (ALT) and aspirate aminotransferase (AST) activities in liver of finishing pigs. Values are means (n 5), with standard errors represented by vertical bars. ![]() , Waxy maize starch;
, Waxy maize starch; ![]() , non-waxy maize starch;
, non-waxy maize starch; ![]() , pea starch.
, pea starch.

Fig. 4 Effect of dietary starch types on the relative mRNA abundance of some nitrogen-metabolism-related enzyme genes in liver of finishing pigs. Values are means (n 5), with standard errors represented by vertical bars. ![]() , Waxy maize starch;
, Waxy maize starch; ![]() , non-waxy maize starch;
, non-waxy maize starch; ![]() , pea starch; GA, glutaminase; GDH, glutamate dehydrogenase; CPS-1, carbamoyl phosphate synthetase 1. a,b Mean values with unlike letters were significantly different (P<0·05).
, pea starch; GA, glutaminase; GDH, glutamate dehydrogenase; CPS-1, carbamoyl phosphate synthetase 1. a,b Mean values with unlike letters were significantly different (P<0·05).
mRNA abundance of the target genes involved in the insulin/protein kinase B/mammalian target of rapamycin complex 1 signalling pathway in liver
Compared with the WMS diet, PS diet critically increased insulin/protein kinase B (Akt)/mammalian target of rapamycin complex 1 (mTOR1) signalling-related gene expression such as mTOR1 and ribosomal protein S6 kinase β-1 (S6K1) (P<0·05), whereas it reduced the expression of the insulin receptor (INR) (Fig. 5), and the expression of S6K1 is higher in the PS diet than that in the NMS diet (P<0·05). In contrast, there was no significant difference in the expressions of Akt and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) among groups (P>0·05).

Fig. 5 Effect of dietary starch types on the relative mRNA abundance of the insulin/protein kinase B (Akt, also named PKB)/mammalian target of rapamycin complex 1 (mTOR1) signalling pathway and the target genes in liver of finishing pigs. Values are means (n 5), with standard errors represented by vertical bars. ![]() , Waxy maize starch;
, Waxy maize starch; ![]() , non-waxy maize starch;
, non-waxy maize starch; ![]() , pea starch; INR, insulin receptor; 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; S6K1, ribosomal protein S6 kinase β-1. a,b,c Mean values with unlike letters were significantly different (P<0·05).
, pea starch; INR, insulin receptor; 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; S6K1, ribosomal protein S6 kinase β-1. a,b,c Mean values with unlike letters were significantly different (P<0·05).
Discussion
Starch is the main source of dietary energy for monogastric mammals. However, the metabolic responses may be widely variable depending on starch types with different digestibility( Reference Stevnebø, Sahlströ and Svihus 5 ). As we know, starch with high amylopectin content is more easily digested, whereas starch with a low amount of amylopectin could act as a source of SDS and cause moderate glycaemic and insulinaemic responses to reduce type-II diabetes induced by carbohydrate metabolism( Reference Bird, Brown and Topping 12 , Reference He, Chen and Yu 13 , Reference Ells, Seal and Bal 21 – Reference Birt, Boylston and Hendrich 23 ). In this study, PS diet significantly decreased the activities of GPa and PEPCK in the liver compared with the WMS diet (Table 3). This may partly attribute to the slower digestion and absorption of PS than WMS so that the level of plasma glucose was moderate( Reference Deng, Wu and Bin 7 ). Although plasma glucose level of pigs fed WMS diet declined too fast, the liver started to maintain the balance of plasma glucose level by increasing the activities of GPa and PEPCK to strengthen glycogenolysis and gluconeogenesis, respectively( Reference Berg, Tymoczko and Stryer 24 ). No significant difference was observed in the activities of GCS, HK and PK, which indicated that the balance of plasma glucose level was independent of glycogen synthesis and glycolysis. Simultaneously, we found that the mRNA expression level of PCK1 was significantly lower in the PS and NMS groups compared with that in the WMS group (Fig. 1). The real-time PCR result was consistent with the enzyme activity produced in liver. The over-expression of PCK-1 leads to hyperglycaemia( Reference Valera, Pujol and Pelegrin 25 ). In contrast, the defect of PCK-1 causes marked hypoglycaemia, liver steatosis and early postnatal death( Reference Hakimi, Johnson and Yang 26 ). As a transcription factor, FOXO1 plays an important role in regulating PEPCK and G-6-P expressions and activating the glucose production via insulin signalling( Reference Nakae, Biggs and Kitamura 27 ). G-6-P is a rate-controlling enzyme in glycogenolysis. In the present study, no significant difference was observed in the activity of G-6-P, the mRNA expression levels of FOXO1 and G-6-P among groups.
The liver plays a key role in whole-body energy balance. The results of this study showed that PS and NMS diets induced fewer lipid contents stored in liver (Table 4). A previous study also reported similar result in weaned pigs, which indicated that a diet with high amylose content might induce a down-regulation of lipogenesis in liver( Reference He, Chen and Zhang 11 ). In contrast, the increased liver lipid contents in the WMS group may be induced by rapidly increased insulin concentration that could stimulate the transcriptions of lipogenic enzymes( Reference Uyeda and Repa 28 , Reference Ferré and Foufelle 29 ). Therefore, we measured some lipid-metabolism-related enzymes in liver to verify the hypothesis. The most important function of ACC is to provide the malonyl-CoA substrate for the biosynthesis of fatty acids( Reference Tong 30 ); in the meantime, FAS is the rate-controlling enzyme in fatty acid synthesis( Reference Smith, Witkowski and Joshi 31 ). The present results showed that the concentrations of ACC and FAS were down-regulated in the PS and NMS groups. However, the activity of HL in liver was not affected by different dietary starch types.
We measured the transcription levels of some key genes involved in lipid metabolism to explore the mechanisms behind the metabolic responses. PS and NMS diets significantly decreased the transcription of SREBP-1c (Fig. 2). SREBP-1c promotes the expression of genes, such as FAS and ACC( Reference Foufelle and Ferré 32 ). In the present study, the real-time PCR result was in keeping with the enzyme concentrations produced in liver (Table 4). In addition, SREBP-1c transcription is regulated by insulin via a PI(3)-kinase-dependent pathway and the activation of LXR( Reference Janowski, Grogan and Jones 33 ). We found that the transcription of LXR was not affected by diet treatment. On the other hand, ChREBP is up-regulated in response to high glucose and induces the expression of genes involved in the metabolic conversion of glucose into fat( Reference Uyeda, Yamashita and Kawaguchi 34 ). In the present study, no effect was observed in the transcription of ChREBP. The results of this study suggested that diet with lower amylose content may increase the transcription of SREBP-1c via an insulin-PI(3)-kinase pathway, up-regulate the lipogenic enzymes and increase the liver lipid contents( Reference Fleischmann and Iynedjian 35 ). The protein encoded by PPARα is a major regulator of lipid metabolism in liver, which promotes fatty acid catabolism by up-regulating family genes involved in lipolysis. In the present study, no significant difference was observed in the transcription of PPARα among three groups. The above results indicated that liver lipid contents of finishing pigs fed PS diet were lower than those of other groups. This might be due to diet with high amylose content, which down-regulated lipogenic enzyme concentrations by a moderate plasma glucose level. Therefore, a diet with high amylose content might decrease the conversion of glucose into fatty acid in liver, which supports more glucose for transporting to other organs for utilisation or transform into other nutrition substrates for accumulation.
The liver is the centre of the N metabolism in mammals, which could not only compound protein by using AA for maintaining and promoting cell function but also eliminate excessive AA in the form of urea. In this study, no effect was observed in the activities of AST and ALT among groups, probably because of the reversible reactions of AST and ALT. Compared with the WMS diet, PS diet significantly decreased the transcriptions of GDH and CPS-1. The protein encoded by GDH produced ammonia by using Glu, which served as a substrate for the urea cycle, and the CPS-1-encoded protein was the first committed step in the urea cycle( Reference Holden, Thoden and Raushel 36 ). The present results suggested that the PS diet acutely decreased the N emissions, namely increased N deposition in finishing pigs.
To explore the mechanisms behind the nutrient metabolic responses in liver, we measured the transcription abundance of some key genes involved in the insulin/PI3K/Akt signalling pathway. We found that the PS diet significantly decreased the transcription of INR compared with the WMS diet (Fig. 5), which should be owed to higher glycaemic and stronger insulinaemic responses induced by the diet with higher amylopectin content. Activation of Akt regulated the phosphorylation of FOXO1; however, no significant difference was observed in the transcriptions of Akt and FOXO1 among three groups.
Activated PI3K also leads to activation of SREBP-1c. The present results agreed with the above hypothesis; PS diet led to fewer lipid contents stored in liver through the insulin/PI3K/Akt immediate down-regulation of the transcription of SREBP-1c. In addition, Akt leads to the activation of mTOR1, which plays an important role in the regulation of protein synthesis through its downstream targets S6K1 and 4EBP1, and is controlled by the presence of growth factors and energy status to regulate the biological process( Reference Asnaghi, Bruno and Priulla 37 , Reference Yang, Yang and Farberman 38 ). In this study, we found that the PS diet acutely increased the transcriptions of mTOR1 and S6K1 compared with the WMS diet, which was probably due to moderate and longer glycaemic and insulinaemic responses induced by diet with higher amylose content. Similarly, Deng et al.( Reference Deng, Wu and Bin 7 ) reported that the diet with the suitable amylose:amylopectin ratio had a better effect on the protein deposition through the insulin stimulates protein synthesis in splanchnic tissues. In contrast, no significant difference was observed in the transcription of 4EBP1 among groups, which was likely because of the interaction of 4EBP1-encoded protein with eukaryotic translation initiation factor 4E inhibiting complex assembly and repressing translation. In this study, the results indicated that dietary starch with higher amylose content may lengthen moderate glycaemic and insulinaemic responses, which could stimulate proliferation and protein synthesis via the insulin/PI3K/Akt/mTOR signalling pathway, and then improved the production performance of finishing pigs to some degree( Reference Li, Li and Zhang 17 ).
In summary, the PS diet with high amylose content could decrease liver lipid contents, the enzyme activities of gluconeogenesis and concentrations of lipid-related synthetase. Furthermore, dietary starch with higher amylose:amylopectin ratio could induce a down-regulation of lipogenesis and up-regulation of protein deposition in liver of finishing pigs by altering the insulin/PI3K/Akt signalling pathway.
Acknowledgements
The authors would like to acknowledge the personnel of all teams for their participation and contribution to this work.
This research was supported by the National Key Basic Research Program of China (2013CB127306) and the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period of China (2012BAD28B03). None of these funders had a role in the design, analysis or writing of this article.
The authors’ contributions are as follows: C. X., Y. L. and F. G. conceived and designed the experiments; C. X., Y. L., L. Z. and J. L. performed the experiments; C. X. and Y. L. analysed the data; C. X., F. G. and G. Z. contributed reagents/materials/analysis tools; C. X. and F. G. were responsible for the writing of the paper. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.











