Introduction
The taeniid cestode Echinococcus multilocularis is the causative agent of human alveolar echinococcosis (AE), a potentially lethal helminthic zoonosis (Eckert & Deplazes, Reference Eckert and Deplazes2004). Although AE is a rare disease within the distribution range of the parasite, several endemic areas have been reported in North America, Europe and Asia (Vuitton et al., Reference Vuitton, Zhou, Bresson-Hadni, Wang, Piarroux, Raoul and Giraudoux2003). Echinococcus multilocularis has a complex life cycle that involves carnivores (principally foxes) as definitive hosts, and cricetid rodents (e.g. Microtus spp.) or lagomorphs (e.g. Ochotona spp.) as intermediate hosts. Dogs are also good definitive hosts. The assemblage of wildlife host communities varies according to ecological features on multiple spatial scales (Giraudoux et al., Reference Giraudoux, Pleydell, Raoul, Quere, Wang, Yang, Vuitton, Qiu, Yang and Craig2006). From a genetic point of view, E. multilocularis appears as an organism with low polymorphism (Haag et al., Reference Haag, Zaha, Araújo and Gottstein1997; Eckert et al., Reference Eckert, Gemmel, Meslin and Pawlowski2001). However, distinct European, Asian and North American genotypes have been described (Bretagne et al., Reference Bretagne, Assouline, Vidaud, Houin and Vidaud1996; Bart et al., 2006) and the geographical location of the transitional zone between Asian and European genotypes, somewhere between eastern Europe and western China, is currently unknown. Furthermore, a tandemly repeated microsatellite, EmsB, has been used to describe the relative diversity of parasite genetic profiles on both regional and local scales (Knapp et al., Reference Knapp, Bart, Glowatzki, Ito, Gerard, Maillard, Piarroux and Gottstein2007, Reference Knapp, Guislain, Bart, Raoul, Gottstein, Giraudoux and Piarroux2008, Reference Knapp, Bart, Giraudoux, Glowatzki, Breyer, Raoul, Deplazes, Duscher, Martinek, Dubinsky, Guislain, Cliquet, Romig, Malczewski, Gottstein and Piarroux2009).
Kyrgyzstan is one of the five republics of central Asia that, with northern Iran, eastern Turkey and Caucasia, provides the geographical link between the transmission foci of Asia and continental Europe. However, nothing is known about the genotypes of E. multilocularis circulating in the area, which theoretically may belong either to the Asian or the European clades, or both. In Kyrgyzstan, cystic echinococcosis caused by E. granulosus, is a national public health concern across the whole country (Torgerson et al., Reference Torgerson, Oguljahan, Muminov, Karaeva, Kuttubaev, Aminjanov and Shaikenov2006). The highest incidences of human alveolar echinococcosis, however, are currently recorded in the sub-national administrative regions of Issyk-kul, Naryn and Osh, the latter including the Alay valley (Usubalieva et al., Reference Usubalieva, Minbaeva, Ziadinov, Deplazes and Torgerson2013). In the Alay valley (altitude 2900–3500 m) land cover is mostly Alpine grassland. Echinococcus multilocularis definitive hosts are the red fox (Vulpes vulpes) and domestic dogs (Ziadinov et al., Reference Ziadinov, Mathis, Trachsel, Rysmukhambetova, Abdyjaparov, Kuttubaev, Deplazes and Torgerson2008, Reference Ziadinov, Deplazes, Mathis, Mutunova, Abdykerimov, Nurgaziev and Torgerson2010). In terms of potential prey biomass, the three dominant species in local small mammal assemblages are: Microtus gregalis (the narrow-headed vole), Cricetulus migratorius (the grey dwarf hamster) and Ellobius tancrei, (the Eastern mole vole) (Giraudoux et al., Reference Giraudoux, Raoul, Afonso, Ziadinov, Yang, Li, Li, Quéré, Feng, Wang, Wen, Ito and Craig2013 and unpublished). Although, historically, M. gregalis and E. tancrei have been found to be infected naturally in Kyrgyzstan (Gagarin et al., Reference Gagarin, Steshenko and Tokobaev1957; Tokobaev, Reference Tokobaev1959), their relative contribution to E. multilocularis transmission is still unknown. Ellobius tancrei has a wide distribution range, stretching from north-eastern Turkmenistan and eastern Uzbekistan through China and Mongolia (Batsaikhan & Tinnin, Reference Batsaikhan and Tinnin2008). More than 50 years ago this species was already recorded as being infected naturally with E. multilocularis in Kyrgyzstan (Tokobaev, Reference Tokobaev1959), but in the original paper it was likely confused with E. talpinus, the Northern mole vole, which actually is not present in Kyrgyztan. No other mention since then of E. tancrei voles infected by E. multilocularis could be found in the literature. However, population surges of this species have been observed regularly, for instance in the Alay valley, the Tien Shan (Narati area, Xinjiang, China) and the Altai Mountains (Giraudoux et al., Reference Giraudoux, Zhou, Quéré, Raoul, Delattre, Volobouev, Déforêt, Ito, Mamuti, Scheifler and Craig2008, Reference Giraudoux, Raoul, Afonso, Ziadinov, Yang, Li, Li, Quéré, Feng, Wang, Wen, Ito and Craig2013 and unpublished).
Here we report infection of E. tancrei in Sary Mogol village (39°40′33.06″N, 72°53′02.06″E) (fig. 1). Furthermore, dog faeces were sampled and tested for E. multilocularis in the same area, and one of them was used to compare genetic profiles. Those genotypic profiles were then compared to other E. multilocularis isolates from Eurasia and North America.

Fig. 1 Map of Kyrgyzstan to show the study site (circled) in the Alay valley.
Materials and methods
In May 2012, a total of 42 Ellobius specimens were trapped within the periphery of Sary Mogol village using tong traps, in an area of about 0.53 ha (72°53′27.78″E, 39°40′50.952″N) at an altitude of 3000 m. As in every other household of this area, the hamlet was surrounded by Alpine grassland and farmland (fig. 2a). Eastern mole voles were identified to the specific level using conspicuous and typical morphometric criteria (short and soft fur, small eyes, long and straight incisors extending far forward of the nasal cavities; fig. 2c). All animals were weighed, measured and sexed in a field laboratory. Rodent eyeballs were collected to assess their relative age by using their dry crystalline weight, and were preserved in 5% formalin (Kozakiewicz, Reference Kozakiewicz1976). At necropsy, the liver and lungs were examined macroscopically for any lesions. When lesions were found, samples were collected and stored in a 90% alcohol solution. The presence of protoscoleces was assessed under microscopy after a puncture into the lesion with a syringe. Rodent carcases were preserved in 10% formalin for reference collection.
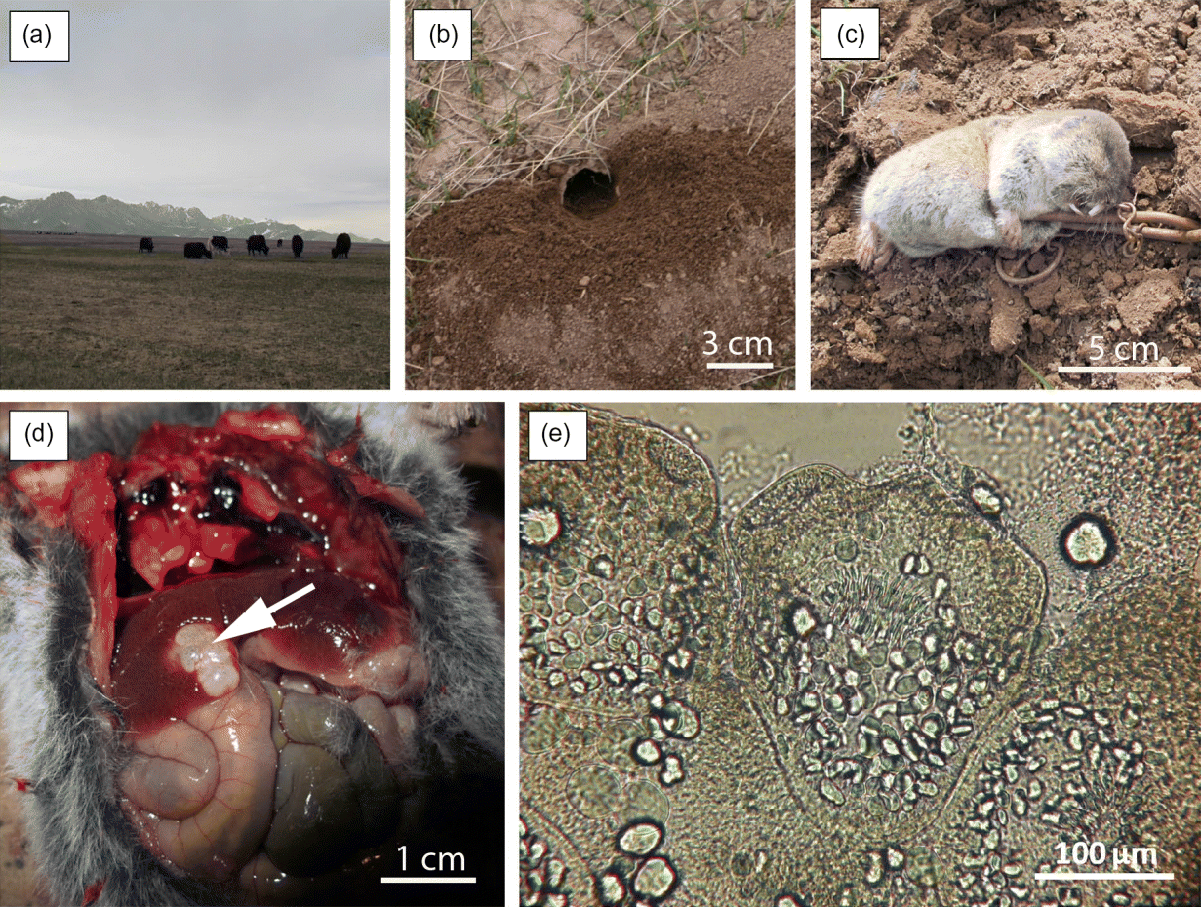
Fig. 2 (a) The landscape of the Alay valley with (b) a burrow entrance of Ellobius tancrei; (c) an entire specimen of E. tancrei caught in a tong trap; (d) liver lesion (arrowed) caused by Echinococcus multilocularis; (e) invaginated protoscolex of E. multilocularis.
Dog faeces were sampled in Sary Mogol and other villages over the same period. Echinococcus multilocularis DNA was amplified from dog faeces found in Taldy Suu village (72°58′15.75″E, 39°42′24.41″N) situated 7.4 km from the small mammal sampling spot (see van Kesteren et al., Reference van Kesteren, Mastin, Mytynova, Ziadinov, Boufana, Torgerson, Rogan and Craig2013).
Total genomic DNA from the rodent liver lesion was extracted by using the High Pure PCR Template Preparation kit (Roche Diagnostics, Mannheim, Germany), as recommended by the manufacturer. The Echinococcus species determination was done with DNA amplification by polymerase chain reaction (PCR) and sequencing of the mitochondrial DNA (mtDNA) fragment of the nd1 gene (primers ND1_Fwd: 5′-AGATTCGTAAGGGGCCTAATA-3′ and ND1_Rev: 5′-ACCACTAACTAATTCACTTTC-3′; Bowles & McManus, Reference Bowles and McManus1993) and compared to the GenBank database. Sequencing using the Sanger method was performed from the two ND1 primers, in order to obtain a consensus sequence. For the dog faecal sample, DNA was extracted using a Qiagen stool mini kit (Qiagen, Hilden, Germany) following the manufacturer's instructions but using 1 g of faeces. The positive dog faecal sample from Taldy Suu was also amplified for the nd1 gene.
Genotyping of parasite samples was performed by amplification of the tandemly repeated microsatellite EmsB as described previously (Knapp et al., Reference Knapp, Bart, Glowatzki, Ito, Gerard, Maillard, Piarroux and Gottstein2007) and modified (Umhang et al., Reference Umhang, Knapp, Hormaz, Raoul and Boué2014). Briefly, the reaction was performed in a 25 μl reaction mixture, containing 200 μm of each deoxynucleoside triphosphate (dNTP), 0.4 μm fluorescent forward primer EmsB A (5′FAM-GTGTGGATGAGTGTGCCATC-3’), 0.7 μm classical reverse primer EmsB C (5′-CCACCTTCCCTACTGCAATC-3′) and 0.5 U of Platinum Taq DNA polymerase enzyme (Life Technologies, Foster City, California, USA), with the addition of Platinum 1 × PCR buffer (Life Technologies). The amplification reaction was performed in a Veriti thermocycler (Life Technologies), under the following conditions: a pre-amplification step of 94°C for 2 min; followed by 45 cycles with a denaturing step at 94°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 1 min; with a final elongation at 72°C for 45 min. The PCR products were analysed by fragment size analysis using an ABI Prism 310 apparatus and the GeneMapper 4.1 software (Life Technologies, Carlsbad, California, USA). The Kyrgyz sample isolated from E. tancrei was compared to a database composed of 1084 genotyped samples from Europe (France, n= 537; Germany, n= 88; Switzerland, n= 109; Austria, n= 99; Slovakia, n= 63; Czech Republic, n= 66; and Poland, n= 94), from Asia (Tibetan plateau in China, n= 5; Hokkaido in Japan, n= 6) and from North America (Canada, n= 1; Alaska, n= 13). The Kyrgyz positive dog faecal sample contaminated by E. multilocularis (n= 1) was included, and a sample of E. granulosus sensu stricto as an outgroup (n= 2). The genetic distance amongst samples was assessed by Euclidean distance between EmsB profiles. As described previously, two samples were considered as identical when the genetic distance was below 0.08 (Knapp et al., Reference Knapp, Bart, Glowatzki, Ito, Gerard, Maillard, Piarroux and Gottstein2007).
Results
Among the 42 individuals, 15 were females and 27 males. The body weight ranged from 47 to 77 g and crystalline dry mass from 0.45 to 3.8 mg. One Ellobius specimen, an adult male, was caught by hand and brought by children from the hamlet. Its body weight was 62 g and crystalline dry mass 1.1 mg. This specimen was the only individual that presented larval cysts of E. multilocularis. It showed two liver lesions (12–18 mm in diameter; fig. 2d). Protoscoleces were found after examining cyst vesicle fluid under a light microscope (fig. 2e). For both the Ellobius specimen and the dog faecal sample, the amplification of the mtDNA fragment of the nd1 gene allowed us to generate a 400-bp consensus sequence. The two isolates had 100% identity with each other and presented 99% identity with the nd1 sequence from the complete mitochondrial genome (AB018440.2). One mutation was observed (position 8012 G/A mutation) in the referenced sequence in both the forward and reverse sequences, in comparison to the other sequences referenced in the GenBank database for the E. tancrei sample and the dog faeces extract (see sequences in fig. 3). This mutation was observed amongst, for example, a Polish sample (GenBank reference: AJ132908.1) and Chinese samples (Xinjiang sample: EU704124.1 and Sichuan: EU704123.1), these reference samples having the nucleotide A at the position 8012 in the nd1 gene, and the Kyrgyz samples a nucleotide G. The presence of the mutation was confirmed by performing the sequencing twice. In comparison to the EmsB database (n= 1084 samples) no identical samples ( < 0.08 of genetic distance) were clustered with the Kyrgyz sequences (from E. tancrei and the dog faecal samples), but the two Kyrgyz sequences were clustered together with a genetic distance of 0.12. They can subsequently been considered as similar strains but not identical, perhaps due to poor DNA quality (fig. 4). Moreover, the two samples were linked with Tibetan (China) and Hokkaido (Japan) samples, and one Alaskan sample, with a genetic distance ranging from 0.17 to 0.24 (fig. 4), but with neither the European nor American isolates.

Fig. 3 Part of the nd1 gene sequenced from the Ellobius tancrei liver lesion and from the positive dog faecal sample contaminated by Echinococcus multilocularis. The underlined nucleotide corresponds to the mutation position in comparison to the AB018440.2 complete mitochondrial genome referenced.

Fig. 4 EmsB profiles of Echinococcus multilocularis from samples in (A) Alaska; (B) dog faeces, Kyrgyzstan; (C) liver of Ellobius tancrei, Kyrgyztan; (D) liver of Microtus limnophilus, Siqhu, Tibetan plateau, Sichuan, China; (E) fox intestine, Hokkaido, Japan; and (F, G) fox intestine, Europe. The dendrogram represents the similarities between samples, with bootstrap values (B = 1000) at each node and the limit of high similarity being 0.08 (Knapp et al., Reference Knapp, Bart, Glowatzki, Ito, Gerard, Maillard, Piarroux and Gottstein2007).
Discussion
The current results add further information about the natural infection of the Eastern vole mole, E. tancrei, with E. multilocularis, first discovered more than 50 years ago. These findings based on EmsB genotyping indicate, first, that the two isolates (vole and dog) found in our study belong to the Asian strain of E. multilocularis, hence extending the western limit of the known distribution range of this genotype in central Asia. The Pamir Mountain range is situated in altitudinal continuity with the Tibetan plateau but, due to its complex high-altitude ranges, might have been considered a biogeographical barrier to the spread of the eastern Asian strain of E. multilocularis to the central Asian republics – a hypothesis that is refuted here. Second, very similar strains were found in dog faeces and the E. tancrei specimen in the study area, and the common mutation first described in the present study emphasized, as a fingerprint, the involvement of E. tancrei and dogs in the local parasite cycle. The occurrence of this mutation amongst Asian E. multilocularis isolates needs further studies to be understood. Associated with the fact that E. tancrei could be trapped at less than 10 m from house walls, and all of them at less than 100 m, this indicates that a synanthropic cycle involving dogs and the Eastern mole vole may exist, not excluding the contribution of other small mammal potential host species (e.g. M. gregalis, C. migratorius) that were also observed not only in habitats remote from villages but also in the close vicinity of houses, where Mus musculus was also captured. Large population densities of both dogs and E. tancrei were observed in the Alay valley. Ellobius tancrei abundance has been shown to increase with grassland vegetation biomass (Giraudoux et al., Reference Giraudoux, Raoul, Afonso, Ziadinov, Yang, Li, Li, Quéré, Feng, Wang, Wen, Ito and Craig2013). This leads to the maintenance of larger vole populations in farmland that surrounds villages, where barley is grown, and in hay fields close to villages, with vole population spillover into villages. Moreover, 38–74% of households have at least one dog in the villages studied in the Alay Valley (van Kesteren et al., Reference van Kesteren, Mastin, Mytynova, Ziadinov, Boufana, Torgerson, Rogan and Craig2013), which leads to a high concentration of potentially infective dog faeces. This should be added to a large red fox population in the area, with tens of fox dens found at less than 1–2 km from villages (Giraudoux and Rieffel, pers. obs.), which may also feed the sustainable transmission of E. multilocularis (however, see Liccioli et al., Reference Liccioli, Giraudoux, Deplazes and Massolo2015). Third, the only specimen of E. tancrei found to be infected by E. multilocularis was also the only specimen caught by hand by children. This might indicate that the animal found infected in the present study might have been caught not by chance but as the result of an increased vulnerability to capture induced by the parasite. This possibly altered host-behavioural aspect of the transmission ecology of E. multilocularis appears not to have been mentioned previously in the literature, and should be investigated carefully, using appropriate methods.
Acknowledgements
We thank Alexander Mastin, Mike Rogan and Bermet Mytynova for their help in obtaining the domestic dog sample.
Financial support
Financial support was received from the Wellcome Trust (#094325/Z/10/Z programme). This research has been conducted within the context of the GDRI (International research network) ‘Ecosystem health and environmental disease ecology’.
Conflict of interest
None.






