Introduction
There have been some important developments in policy on medicines optimisation in England in recent years. In 2013, the Royal Pharmaceutical Society (RPS) (Picton and Wright, Reference Picton and Wright2013) and The King’s Fund (Duerden et al., Reference Duerden, Avery and Payne2013) separately published independent reports describing the rise of polypharmacy and promoting strategies for optimising the growing use of multiple medicines by individual patients. These reports were followed by the production of official guidelines on medicines optimisation by the National Institute for Health and Social Care Excellence (NICE, 2015a). In this paper, we critically examine the ways in which patient perspectives have been incorporated in these influential independent reports and official guidelines. We also highlight gaps in research on people’s experiences of polypharmacy that need to be addressed in order to inform the future development of more inherently person-centred medicines optimisation policy and practice in the National Health Service (NHS).
Methods
We carried out a non-systematic review of the English-language research literature relating to patient experiences of polypharmacy. The review was wide-ranging, covering relevant empirical, theoretical and methodological work, as well as UK policy documents on the topic, without date restrictions. It was carried out between February and July 2015 as a precursor to a research proposal being developed to design, implement and evaluate a complex intervention based in primary care in the NHS for optimising medicines use by patients who take multiple medications (Reeve et al., Reference Reeve, Dickenson, Harris, Ranson, Dohnhammer, Cooper, Krska, Byng and Britten2015).
We did not attempt a systematic review of the literature on patients’ experiences of polypharmacy with or without multimorbidity at this stage in our preparatory work because we were aware that such evidence was hard to locate (and a sub-project in itself). For example, studies on this topic tend to use different terminology; relevant findings are also often reported in the context of wider studies of patients’ experiences of chronic illness using qualitative or mixed methods. For these reasons, relevant evidence is not easily found using mechanical search procedures. Instead, we did three things. We started with the literature that we knew on patient experiences of using medicines and followed up relevant references. Then we began compiling a list of terms that we might use to search the literature more systematically. Finally, we used some of these terms to search Google, PubMed and other targeted sources (such as specific journals) for relevant papers, while continuing to compile our vocabulary of terms for a future more comprehensive review.
This process was very revealing, highlighting major issues with the array of terms used (and variously defined) in research on polypharmacy and medicines usage. A list of the terms we have compiled to date is available (see Supplementary Appendix). For the purposes of this paper, we refer to a few of the 34 studies we identified to illustrate key findings and gaps in this literature. We hope to carry out a more thorough and complete narrative review of this literature using the terms we have identified as part of our ongoing work.
In our initial reading of the policy documents, we noted some variation in how central patients were in them, as well as how limited reference was to relevant research on patient experiences of using medicines. We carried out the present documentary analysis to examine in more depth how patient perspectives had been incorporated and to consider how future research might better inform policy on medicines optimisation.
In the paper, we focus on the current policy advice produced for healthcare professionals in England. This is because, as we describe below, it differs from that of Scotland in promoting person-centred care values and in positing medicines optimisation as a way of managing the rise of polypharmacy and some of the problems associated with it. Our discussion of the implications of our findings is, however, relevant to researchers and policymakers across the UK and in other countries where the rise of polypharmacy is a matter of concern.
Overview of medicines optimisation guidelines in England
‘Medicines optimisation’ is a relatively new model of prescribing that is currently being promoted by the NICE and by independent organisations to succeed the established ‘medicines management’ approach in England. In this section, we examine how the new model is conceptualised and differentiated from the old one in major policy reviews and guidelines as being more centred around patients and less concerned with processes and systems of prescribing.
In 2013, the RPS published Medicines optimisation: helping patients to make the most of medicines (Picton and Wright, Reference Picton and Wright2013). It states that
Medicines optimisation is about ensuring that the right patients get the right choice of medicine, at the right time. By focusing on patients and their experiences, the goal is to help patients to: improve their outcomes; take their medicines correctly; avoid taking unnecessary medicines; reduce wastage of medicines; and improve medicines safety. Ultimately medicines optimisation can help encourage patients to take ownership of their treatment.
(Picton and Wright, Reference Picton and Wright2013: introduction).
Developed with input from healthcare professionals, patients and the pharmaceutical industry, the report provides guidance on good practice for healthcare professionals in England, based around four key principles: (1) aim to understand the patient’s experience; (2) evidence-based choice of medicines; (3) ensure medicines use is as safe as possible; and (4) make medicines optimisation part of routine practice. The outcomes that these principles are intended to influence are shown in Table 1.
Table 1 Royal Pharmaceutical Society’s four guiding principles of medicines optimisation and their intended outcomes
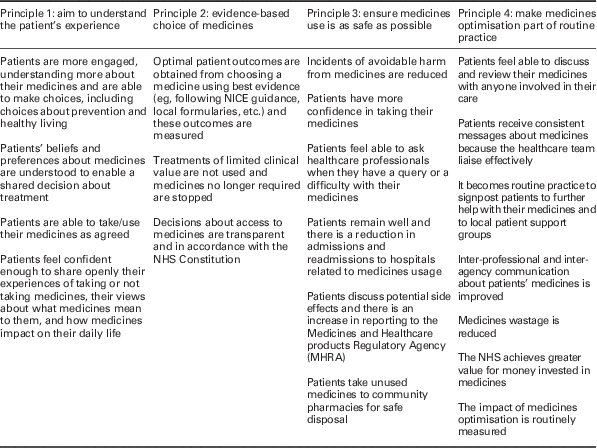
NICE=National Institute for Health and Clinical Excellence; NHS=National Health Service
Source: Picton and Wright (Reference Picton and Wright2013)
The RPS principles have been agreed by NHS England and used to inform the ongoing development of its Medicines Optimisation Prototype Dashboard (www.england.nhs.uk/ourwork/pe/mo-dash) and wider Medicines Optimisation strategy. They have also been agreed by The Association of the British Pharmaceutical Industry, The Royal College of Nursing, The Royal College of General Practitioners and The Academy of Medical Royal Colleges (Keele Centre for Medicines Optimisation, 2015: 6).
In the same year, The King’s Fund, an independent ‘think tank’, issued Polypharmacy and medicine optimisation: making it safe and sound (Duerden et al., Reference Duerden, Avery and Payne2013). The authors examine the nature and extent of the problem of polypharmacy in the UK, and consider the implications for policy and practice. A key distinction is made between ‘appropriate’ and ‘problematic’ polypharmacy. The former is achieved when: ‘prescribing for an individual for complex conditions or for multiple conditions in circumstances where medicines use has been optimised and where the medicines are prescribed according to best evidence’, whereas the latter occurs when ‘multiple medications are prescribed inappropriately, or where the intended benefit of the medication is not realised’ (Duerden et al., Reference Duerden, Avery and Payne2013: 1).
According to the report, the ‘overall intent for the combination of medicines prescribed should be to maintain good quality of life, improve longevity and minimise harm from drugs’ (Duerden et al., Reference Duerden, Avery and Payne2013: 1). So medicines optimisation is intended to support the achievement of appropriate polypharmacy for these various needs and minimise the occurrence of problematic polypharmacy. However, the latter occurs for a number of reasons, such as when the treatments are not evidence based, or the risk of harm is likely to outweigh benefit, or the combination of drugs is hazardous because of interactions between them, or the demands of medicine-taking are unacceptable to patients, or the demands make it difficult to achieve clinically useful medication adherence, or when medicines are prescribed to treat the side effects of other medicines even though other solutions are available to reduce the number of medicines prescribed (Duerden et al., Reference Duerden, Avery and Payne2013: 1).
The authors go on to propose some solutions to problematic forms of polypharmacy based on processes associated with medicines management and the newer notion of ‘medicines optimisation’. They claim that the latter model, with its wider focus on how medicines are or are not used by people, is fundamental to addressing problematic polypharmacy. As they put it: ‘Medicines optimisation, or robust medicines management, helps to ensure more appropriate polypharmacy so that the various trade-offs of harm, benefit and patient acceptability and choice have been considered and an explicit decision on the drug to use has been made with the patient’ (Duerden et al., Reference Duerden, Avery and Payne2013: 2). Throughout the report, they stress the need for clinicians to involve patients in decisions on drug use.
In March 2015, following a public consultation from 2013 to 2014, NICE published the guideline NG5: Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes (NICE, 2015a). The NICE guideline adopts The King’s Fund’s definitions of appropriate and problematic polypharmacy and describes medicines optimisation as ‘a person-centred approach to the safe and effective use of medicines’ (NICE, 2015a: 5). It notes that this approach replaces the previous focus on systems, processes and behaviours that was characteristic of medicines management, although the latter is still viewed as ‘an important enabler of medicines optimisation’ (NICE, 2015a: 8). The eight topic areas covered by the NICE recommendations for practice, and the four for research, are listed in Table 2.
Table 2 National Institute for Health and Social Care Excellence recommendations for practice and research on medicines optimisation: topic areas

Source: National Institute for Health and Clinical Excellence (NICE) (2015a).
Elsewhere in the UK, NHS Scotland’s Polypharmacy Guidance, originally published in 2012, was recently updated in 2015 (Scottish Government Model of Care Polypharmacy Working Group, 2012; 2015; Wilson et al., Reference Wilson, Mair, Dreischulte and Witham2015). Although published over a similar timescale, these guidelines differ in some important respects from those for NHS England. First, they define the positive and negative forms of polypharmacy slightly differently, preferring the terms ‘appropriate’ and ‘inappropriate’ polypharmacy, and describing the particular conditions when each is present (Scottish Government Model of Care Polypharmacy Working Group, 2015: 5; Wilson et al., Reference Wilson, Mair, Dreischulte and Witham2015: 109). Second, they do not promote medicines optimisation as a model for dealing with inappropriate forms of polypharmacy. Instead, the Scottish report outlines a ‘7-step’ Medication Review Process to inform patients’ and clinicians’ decision making about medicines. Finally, this process is not described as a person-centred (or patient-centred) approach as such.
In the rest of the paper we focus on how, unlike Scotland, policy in England has promoted a shift to the more person-centred medicines optimisation model as a strategic response to the rise of polypharmacy. Although the RPS, The King’s Fund and NICE guideline all support this strategy, as we show below, their reports vary in how integral patient perspectives and person-centred care values are to them, and also in the extent to which they draw on evidence from existing research on people’s experiences of medicines use in general, and polypharmacy in particular.
Centrality of patient perspectives and person-centred care in the guidelines
In the RPS report, medicines optimisation is represented as a ‘step change’ from the medicines management approach, focussing on patients and the outcomes that matter to them, rather than on professionals and the systems and processes that shape how medicines are used (Picton and Wright, Reference Picton and Wright2013: introduction). While the RPS report emphasises the divergence between the two models, both The King’s Fund report and NICE guideline highlight the convergence: they tend to represent medicines optimisation as a broad approach that is facilitated by processes and systems of medicines management.
This difference in perception of the two models is reflected in the extent to which the respective guidelines are formulated around patients. In the RPS guidelines, the need to understand patient experiences is relatively strongly represented, forming one of its four fundamental principles of medicines optimisation. Many of the RPS recommendations are also written from the patient’s viewpoint (see Table 1).
In The King’s Fund report, the importance of determining patient perspectives is a running theme, although here it is represented more as a matter for clinicians to address on an individual basis rather than, say, as a guiding principle of care or a systemic issue, and there are no references to practical information on how to go about this. There is just a short section – less than a page in the 56-page report – summarising what is known about patient experiences of polypharmacy.
Similarly, the 47-page NICE guideline has a one-page section describing ‘person-centred care’ (which it treats as equivalent to ‘patient-centred care’) that is somewhat separate from, and not integrated with, the rest of the report. Moreover, the actual recommendations are mainly concerned with processes and systems for making safe and cost-effective decisions with less space given to ways of facilitating the identification and achievement of person-centred goals. This is also reflected in the companion information NICE produced for the public (NICE, 2015b).
Despite their common support for medicine optimisation, a model that is conceptually distinguished from medicines management by its greater focus on patient perspectives, only The King’s Fund report engages with real examples of the sorts of issues patients have experienced, albeit briefly. In a short section on ‘Polypharmacy and the patient experience’, the authors describe some of the evidence about people’s strategic use of medicines and difficulties coping with the demands of their drug regimens (Duerden et al., Reference Duerden, Avery and Payne2013: 32). They also discuss a key issue that is glossed over in the other reports – that patients and professionals do not always agree about medicines usage – and suggest ways of dealing with this. As they point out, ‘Patients may not want to take multiple medicines, or prefer one treatment over another. Advice should be given on which interventions may be most likely to minimise side effects, reduce symptoms and improve outcomes. Regimens may need to be tailored to fit with patient preferences and “compromise” may be required’ (Duerden et al., Reference Duerden, Avery and Payne2013: 1–2).
Expanding on these ideas, the authors go on to add that
Compromises may often need to be reached between the view of the prescriber in delivering interventions intended to improve outcome, and the choice made by the patient, based on the demands of the medication regimen. The alternative is the potentially wasteful process of prescribing where the patient does not take the medicines appropriately, or does not take them at all, but the prescriber unwittingly continues to supply prescriptions. Various estimates of long-term drug use indicate that as many as 40 per cent of people on long-term prescriptions do not take them as intended [ref].
(Duerden et al., Reference Duerden, Avery and Payne2013: 32).
Closely related to this theme, which again only The King’s Fund report mentions, is the notion of the ‘demands’ of the drug regimen and the ‘pill burden’ that patients often find unacceptable, which are two of the manifold reasons why patients may sometimes choose not to use medicines (Duerden et al., Reference Duerden, Avery and Payne2013: 1).
Utilisation of research in the guidelines
The three reports were each informed by associated reviews of the literature. However, there was noticeable variation in the extent to which they covered qualitative studies of people’s use of medicines. For example, the RPS report draws on an undated review of evidence (RPS, nd) that it published separately online at www.rpharms.com/medicines-safety/medicines-optimisation.asp. Most of the 16 references in the report itself refer to research on prescribing practices. The consultation document (NICE, 2014) that the NICE guideline was developed from summarises evidence from systematic reviews, randomised controlled trials and observational studies but not qualitative studies of people’s experiences of using medicines. Only The King’s Fund report considers some of the evidence from qualitative studies of people’s experiences of using medicines in its more comprehensive review.
The under-utilisation of qualitative research on people’s experiences of using medicines is disappointing given that there is a large international literature on the topic and on patients’ self-management of chronic conditions, which often includes a focus on medicines usage. This work has examined, among other things, the reasons why some people do not always take their medicines as prescribed (eg, Britten, Reference Britten2007), the disruptive effects of being ill and fitting complex care regimens into everyday life, and the various strategies that people have adopted to minimise these effects (Demain et al., Reference Denford, Frost, Dieppe, Cooper and Britten2015). However, while information on these and other aspects of medicines usage is (and was) already available in the literature and could have been better utilised in the guidelines, this evidence base is not without limitations.
Much of what we know about people’s use of medicines is based on evidence from studies of patients with single conditions, such as diabetes or asthma. Only a small proportion of the existing literature focusses on people who take multiple medications for multiple conditions. This is important because, while people may take two or more medicines for a single condition, those taking multiple medicines for multiple conditions, and those prescribing for them, face some additional issues. Although it is not our intention to fully review the literature on people’s experiences of polypharmacy and multimorbidity here, we highlight below some of the complex issues that have been identified by studies carried out in the UK to date, which are relevant to policy and guidelines on medicines optimisation but not so far addressed by them.
In an early study of the views of people with multimorbidity on their complex drug regimens, Townsend et al. (Reference Townsend, Hunt and Wyke2003; Reference Townsend, Wyke and Hunt2006) found tensions in their experiences of ‘regular’ versus ‘flexible’ regimens for different drugs. They also found that people preferred to minimise their use of prescribed medicines and maximise their use of alternative ways of managing their conditions. Another study of people with type 2 diabetes and cardiovascular diseases found that they believed healthcare professionals were more likely to overprescribe for those with comorbid conditions, and that more people prioritised their medicines prescribed for their diabetes than for their cardiovascular disease (Stack et al., Reference Stack, Elliot, Noyce and Bundy2008). Lindsay (Reference Lindsay2009) found that people with multiple chronic conditions often prioritised one main condition because it was unpredictable, or it was not controlled through tablets, or it tended to set off other problems.
Some studies have also begun to document the strategies that people have developed to deal with the issues of using multiple medications. For example, a qualitative longitudinal study of people’s experiences of multimorbidity, which examined people’s shifting priorities over time, found that the prescription of medicines from different sources and with various instructions led to some confusion, but also to the development of pragmatic routines enabling people to take control (Morris et al., Reference Morris, Sanders, Kennedy and Rogers2011). The authors further observed that: ‘Medication management emerged as an anchor or point around which multiple condition management could be changed (or abandoned) and represented a point whereby a person could either take or abandon control’ (Morris et al., Reference Morris, Sanders, Kennedy and Rogers2011: 158). Another study of patients who were regularly prescribed four or more medicines for chronic conditions found that while some did adapt to their long-term medicines use others did so at a cost to their quality of life (Krska et al., Reference Krska, Morecroft, Poole and Rowe2013).
We believe that the lack of research on these and other issues experienced by people taking multiple medicines for single or multiple conditions in the UK has limited the size and quality of the evidence-base available to potential users. As a result, policymakers have lacked relevant evidence to draw upon in preparing guidelines on polypharmacy, medicines optimisation and multimorbidity; clinicians have lacked meaningful and practical information on how to practice optimal prescribing for people with complex or multiple conditions; and patients have lacked insights from other patients with first-hand experience about how they themselves have developed strategies for self-optimising medications, and whether these have been successful or not from their point of view. In the last part of the paper, we consider some of the ways in which researchers might work to improve the evidence base to inform the future production of person-centred guidelines on medicines optimisation.
Implications for research
Below we suggest five ways in which researchers could help policymakers to both make better use of available knowledge on the topic and provide them with more evidence about patient experiences of polypharmacy, to inform the future development of guidelines on medicines optimisation and ensure that they are truly person-centred.
Facilitate reviews of research
One possible reason why the current policy reviews and guidelines make partial and limited use of the available research on medicines use is because of the amorphous nature of the literature. The literature on patient experiences of using multiple medicines is difficult to identify and review because of a lack of consistency and clarity in the terminology that is used. There is, for example, no commonly accepted definition of the number of medicines involved in polypharmacy. In a review of polypharmacy terminology, Bushardt et al. (Reference Bushardt, Massey, Simpson, Ariail and Simpson2008: 386) found that six or more drugs was the most commonly used number. In a study of polypharmacy among people with stroke and other morbidities, Gallacher et al. (Reference Gallacher, Batty, McLean, Mercer, Guthrie, May, Langhorne and Mair2014) observed that five or ten tend to be the most commonly used thresholds. Other numbers have also been used to indicate progressive levels of polypharmacy. Definitions may be limited to prescription drugs or include over the counter drugs as well.
Similarly, while there is general acceptance that the individualisation of drug treatments is a good thing, there is no common understanding of what this means. As Denford et al. (Reference Demain, Gonçalves, Areia, Oliveira, Marcos, Marques, Parmar and Hunt2014) have shown, the terms ‘personalised’ and ‘individualised’ care have been variously and sometimes vaguely defined in published studies. As previously noted, the terms ‘patient-centred’ and ‘person-centred’ care are sometimes used interchangeably (as in the NICE guideline) or the latter can be used to refer to a philosophy of care that is distinct from that of the former (eg, Ekman et al., Reference Ekman, Swedberg, Taft, Lindseth, Norberg, Brink, Carlsson, Dahlin-Ivanoff, Johansson, Kjellgren, Lidén, Öhlén, Olssen, Rosén, Rydmark and Sunnerhagen2011; Rasmussen et al., Reference Rasmussen, Jørgensen and Leyshon2014). Given the proliferation of terms in this field of research, it is important that researchers carefully select and clearly define the terms used in studies, and differentiate them from alternatives where necessary, to help facilitate the identification and review of relevant work by users of research knowledge.
Improve the evidence base on patient experiences of polypharmacy and multimorbidity
As we have noted, relatively little is known about patient experiences of polypharmacy, particularly those with multimorbidity. There is a particular need for more research on the following topics:
-
∙ patient experiences of polypharmacy, especially those taking multiple medicines for multiple chronic conditions, including the disruptiveness and burden of their drug regimens, and the making and balancing of different priorities;
-
∙ patients’ and prescribers’ understanding of and attitudes to medicines optimisation, including their views on the nature and acceptability of compromises;
-
∙ patient and prescriber perspectives on the acceptability, safety and effectiveness of particular interventions designed to optimise medicines use;
-
∙ patient and prescriber perspectives on the barriers and facilitators to the optimisation of medicines prescribed by different practitioners across primary and secondary care settings.
These topics will in some cases require a longitudinal approach, for example, examining how patients’ priorities and use of medicines shift over time, what influences this and how adaptations to drug regimens can be made in response. They also require modes of analysis that bring out the ways in which the design and operation of systems of care are reflected and embodied in individual patient’s experiences. This includes sensitivity to the language used by patients and carers to describe what they regard as good quality care that meets their needs and priorities, which is not always the same as that used by services or in policy. An article on patients’ and carers’ experiences of obtaining repeat prescriptions provides an example of this approach (Wilson et al., Reference Wilson, Kataria and McNeilly2013).
Improve the evidence base on medicine optimisation interventions
Although the policy documents support a move to medicines optimisation in England, they contain little information and practical guidance for healthcare professionals and patients on how to work together to achieve this in practice. Skinner (Reference Skinner2015) has also revealed a lack of clinical protocols for polypharmacy that are specific to primary care, although she acknowledges the difficulties of providing guidelines for professionals treating individual patients with multiple conditions, each of whom have ‘unique’ health priorities (Skinner, Reference Skinner2015: 4–5).
In the proposal that we are developing on the back of this work, we are planning to design and evaluate a generalist approach to prescribing that can be tailored to individuals and their shifting circumstances and priorities (Reeve et al., 2015). However, this is just one possible study of one approach and others are required to investigate the nature and challenges, and benefits and costs, of interventions for optimising medicines in different contexts. A recent study in Ireland of how general practitioners make compromises when prescribing for patients with multimorbidity is a helpful contribution to knowledge in this regard (Sinnott et al., Reference Sinnott, Mc Hugh, Boyce and Bradley2015).
Better use of existing and emerging theories, concepts and tools
There are a number of theoretical frameworks that could be used to inform future conceptual and empirical research on polypharmacy and medicines optimisation. These include debates around the notions of ‘patient-centred’ and ‘person-centred’ care and how these fit with ideas about medicines optimisation and evidence-based medicine. They also include theories such as generalism and how this might provide the basis for reshaping practice around people rather than their diseases (Reeve and Bancroft, Reference Reeve and Bancroft2014). There are also concepts such as ‘burden of treatment’ (May et al., Reference May, Eton, Boehmer, Gallacher, Hunt, MacDonald, Mair, May, Montori, Richardson, Rogers and Shippee2014) and ‘minimally disruptive medicine’ that are salient and informing the development of tools for measuring the impact of interventions in terms that are more relevant to patients (Lepping, Montori and Gionfriddo, Reference Leppin, Montori and Gionfriddo2015).
These theoretical approaches, in their different ways, provide possible alternatives to existing conceptual models of prescribing, which have been largely influenced by ideas about patients’ adherence (and previously compliance) to medication regimens. For a long time, these ideas have failed to fundamentally engage with patients’ perspectives on how they routinely use medicines to better enable them to live their lives and so a new approach is needed.
Improved patient and public involvement in research and in the development of guidelines
One way of improving the centrality of patients in guidelines purporting to promote patient- or person-centred care is to increase their meaningful involvement in research and in the development of guidelines and clinical protocols. It is encouraging to see that NHS England has so far carried out two patient engagement workshops in the course of developing their Medicines Optimisation strategy (www.england.nhs.uk/ourwork/pe/mo-dash). Krahn and Naglie (Reference Krahn and Naglie2008) have argued that patients’ perspectives, experiences and choices should be considered at every stage of the development and implementation of clinical practice guidelines. They suggest five ways of doing this: finding preference-related evidence; integrating preference-related evidence into recommendations; using guidelines in individual decision making; including patients in the guideline development process; and evaluating guidelines with preferences in mind (Krahn and Naglie, Reference Krahn and Naglie2008: 437).
More recently, Montori et al. (Reference Montori, Brito and Murad2013), working out of the Knowledge and Research Unit at the Mayo Clinic in the United States, have similarly called for the incorporation of patient preferences into practice guidelines. They suggest that guideline panels should include frontline patients and clinicians; consult with clinical and methodological experts and seek testimony from individuals who are experts in patient preferences, patients and caregivers’, as well as commission relevant reports; and rely on patient input to ‘drive consideration of the full range of outcomes patients experience and consider critical in deciding what to do’ (Montori et al., Reference Montori, Brito and Murad2013: 2504). They also suggest that panels should refrain from making ‘strong’ recommendations when ‘the best course of action heavily depends on the patient’s context, goals, values, and preferences’; instead they advise panels to make ‘conditional recommendations’ that reflect this scenario and presents options in a way that facilitates shared decision making (Montori et al., Reference Montori, Brito and Murad2013: 2504).
In what we regard as a refreshing dose of realism, they conclude by arguing that
Panels should become much more comfortable with ambiguity, both in the tradeoffs involved and in the recommendations given, and explicitly report how patient preferences and context were considered in formulating the panels’ recommendations. Clinicians need guidance and clear guidance helps and supports efficient practices. Yet, panels must be wise in recognizing when this expediency is appropriate for patient care and when it hinders patient-centred care. Clinicians should remember that taking care of patients is supposed to be difficult. Although guidelines may simplify this task, when patient preferences and context matter, guidelines must not replace clinicians’ compassionate and mindful engagement of the patient in making decisions together. This is the optimal practice of evidence-based medicine.
(Montori et al., Reference Montori, Brito and Murad2013: 2504).
We would reiterate that patients, too, need to be involved as members of panels and, more generally, in discussions around ‘ambiguity’ and ‘compromises’ in prescribing and debates about the ethics and values which inform decision making in prescribing.
Conclusion
In this paper, we have drawn attention to the different ways in which patient perspectives and person-centred care values have been represented in official and influential independent policy documents on medicines optimisation in England. Whereas, understanding patient perspectives is a fundamental principle of the RPS guidelines, the more recent NICE guideline focusses mainly on the safe and effective prescribing of medicines, and less on the identification and achievement of personal goals, which are also important to patients. We have argued that this partly reflects a lack of utilisation of existing qualitative research on patient experiences of polypharmacy, especially where linked to multimorbidity, as well as a lack of basic research specifically on this topic. We have suggested some topics that we believe are priorities for further research, and highlighted ways in which patients can be more fully involved in the process of developing guidelines. We hope that researchers, funders of research and policymakers will use our suggestions to help improve the construction of guidelines on medicines optimisation that are meant to enable patients to get the most from their medicines.
Acknowledgements
We would like to acknowledge the support of other members of the sub-team involved in developing the Programme Grant linked to this work: patient and public representatives Jim Harris and Ed Ranson, and Professor Richard Byng.
Financial Support
This work was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula at the Royal Devon and Exeter NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of Interest
None.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1463423616000207





