Introduction
Hand-foot-mouth disease (HFMD) is an acute contagious disease caused by the human enterovirus (EV) family, with EV A71 (EV-A71) and coxsackievirus A16 (CV-A16) being considered to be the major pathogens [Reference Zhao1]. Children aged <5 years are particularly susceptible to HFMD [Reference Hooi-Ling2]. Over the last decades, large amounts of HFMD cases have been reported across the Asia-Pacific region, becoming a growing public health concerns [Reference Nguyen3, Reference Ang4]. The same HFMD epidemics occur in most parts of China every year since its first emergence in Shanghai in 1981 [Reference Zeng5]. The Chinese government designated HFMD as a category-C notifiable communicable disease on 2 May 2008 after two large outbreaks which led to thousands of cases and dozens of deaths in Linyi and Fuyang city of China [Reference Xing6]. According to the data released by the National Health and Family Planning Commission of China, 12 167 721 HFMD cases and 2863 deaths were reported in mainland China during 2010–2015. Averagely more than 2 million HFMD cases were reported annually, ranking the top first among notifiable communicable diseases [7].
Although the neutralizing antibodies elicited after infection can provide certain protection for the same EV subtype of HFMD, they cannot protect against the other EV subtypes [Reference Liu8]. In lack of cross-protection between different EV serotypes, patients could be reinfected with HFMD, which therefore increases the prevalence of HFMD and heavens the associated social health burden. Reinfection of HFMD has been reported in some researches, and the reported reinfection rates ranged from 0.16% to 3.73% [Reference Chen9–Reference Shu11]. However, these studies mainly focused on the basic epidemiological description of reinfection. The hazard and potential risk factors associated with reinfection of HFMD remain poorly understood, which impedes the development of targeted interventions. The present study aims to analyse the reinfection hazard of HFMD in Wuhan, China, using Cox-proportional hazard regression model.
Methods
Study site
Wuhan is a city locating at the central China with the total area of about 8494 km2. By the end of 2016, the total population in Wuhan amounted to more than 10 million. Wuhan is well known as the ‘Jiusheng Tongqu (the nine provinces’ leading thoroughfare)’, which means Wuhan is a major transportation hub, with hundreds of railways, roads and expressways passing through the city and connecting to major cities in Mainland China [12]. Taking into consideration the key role that Wuhan played in transportation, it is reasonable to believe that infectious disease could spread broadly outside once it outbreaks in Wuhan.
Statistics showed that since 2010, HFMD has always been the contagious disease with the largest number of cases in Wuhan city. More than 10 000 cases were reported annually with an incidence rate of over 100 per 100 000 populations [Reference Peng13], posing serious public health threats.
Case definition
The HFMD cases were diagnosed according to the guidelines for HFMD diagnosis and treatment (2010) issued by the National Health and Family Planning Commission of China [14]. A clinical case of HFMD was defined as a patient presenting with vesicular rashes or maculopapular on hands, feet, mouth and/or buttocks, with or without fever. A laboratory-confirmed case was defined as a clinical case with one of the following laboratory evidences including specific EV RNA (+), isolation of EV (EV-A71, CV-A16 or other EV), and at least fourfold increase of the neutralizing antibodies titre between acute and convalescent phase. The reinfected case was defined as a patient who was infected with HFMD twice or more (>15-day intervals between infections).
Data resources
The current data were obtained from the Chinese Information System for Disease Control and Prevention (CISDCP), which is an internet-based surveillance system established in 2004 by the Chinese government [Reference Wang15]. It was enforced that healthcare providers had to report any clinically diagnosed or laboratory-confirmed HFMD cases via the CISDCP within 24 h since 2 May 2008. Basic information of the case including card ID, name, gender, age, group classification, current address, date of disease onset, date of diagnosis, case classification (clinical or laboratory-confirmed) was input to the system. A series of measures have been taken to insure the quality of data reporting, such as regular training of healthcare providers on data entry, routine audits of data by the local Center for Disease Control and Prevention (CDC), field investigations of reporting accuracy and under-reporting of cases [Reference Wang15].
Totally, 95 290 HFMD cases were reported via the CISDCP in Wuhan during the period of 2 May 2008 to 31 December 2015. Data cleaning was done by excluding logically erroneous and incomplete data. Finally, 95 209 cases were used for the current study.
Statistical analysis
Descriptive statistics such as rate and proportion were used to summarise the sample characteristics and to estimate the infection and reinfection rates of HFMD. The Kaplan–Meier method was used to estimate the cumulative hazard probabilities of HFMD first reinfection stratified with gender, age, group classification, region and pathogenic results, and the differences were assessed by the log-rank test. Hazard ratios (HR) with 95% confidence intervals (CI) were estimated by Cox-proportional hazard regression analysis, to investigate the factors associated with the risk of reinfection. P < 0.05 was considered to be statistically significant. All analyses were conducted with Statistics software SPSS V 16.0 (SPSS Inc., Chicago, Illinois, USA).
Ethical statement
According to the law on the prevention and treatment of infectious diseases in China, personal identifiers of individual cases should be collected in the CISDCP, for the purposes of public health surveillance and response. The National Health and Family Planning Commission of China identified that the collection of individual data for all notifiable diseases including HFMD was part of an ongoing public health response, and was thus exempt from institutional review board assessment [Reference Takahashi16].
Strict regulations were established and supervised by the China CDC to protect patients’ privacy. The local CDCs were given access to the surveillance data for the purpose of research. The individual HFMD data for this study were anonymised by deleting the personal identifiers (such as patient name, address and telephone number). No informed consent was required since there were no ethical issues relevant to the study design and data analysis.
Results
General characteristics of the cases
As shown in Table 1, of 95 209 cases, 62.6% were males, 82.4% aged 3 years and below, 36.2% lived in rural area, 0.1% diagnosed as severe case and 48.6% were laboratory-confirmed with EV-A71 being the primary pathogen.
Table 1. Basic characteristics of HFMD cases, 2008–2015, Wuhan
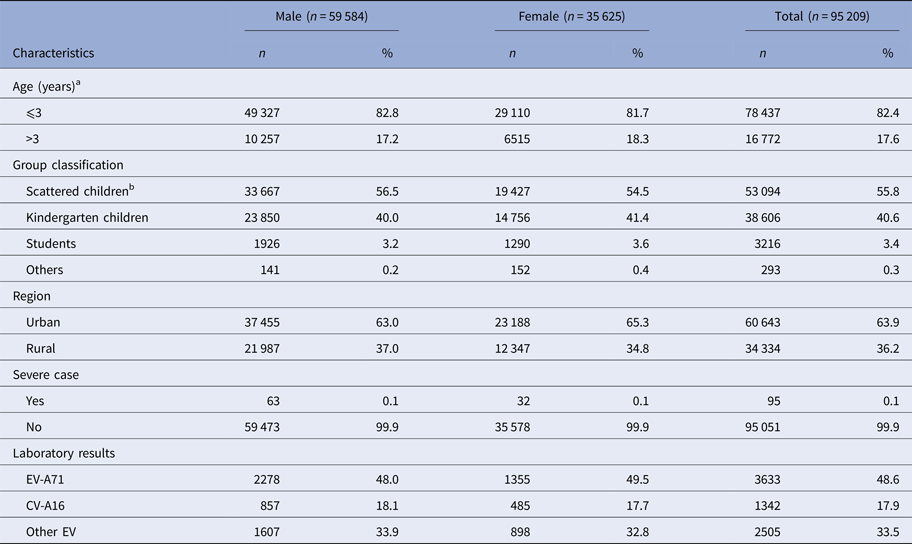
a The age when the children were into the study.
b The children who have not attend kindergarten or school yet.
HFMD reinfection characteristics
Of all cases, 1842 were reinfected with the reinfection rate of 1.93%. Among reinfected cases, 1798 (97.61%) cases were infected twice, 42 (2.28%) cases three times and two (0.11%) cases four times. The median interval time between infections was 13.33 (inter-quartile range: 8.53–22.55) months. Table 2 showed that the reinfection rate was significantly higher in male, children aged 3 years and below, scattered children, urban areas, patients first infected with CV-A16 and CV-A16 predominant years, compared with their counterparts (P < 0.05).
Table 2. Reinfection rates of HFMD by social demographic characteristics
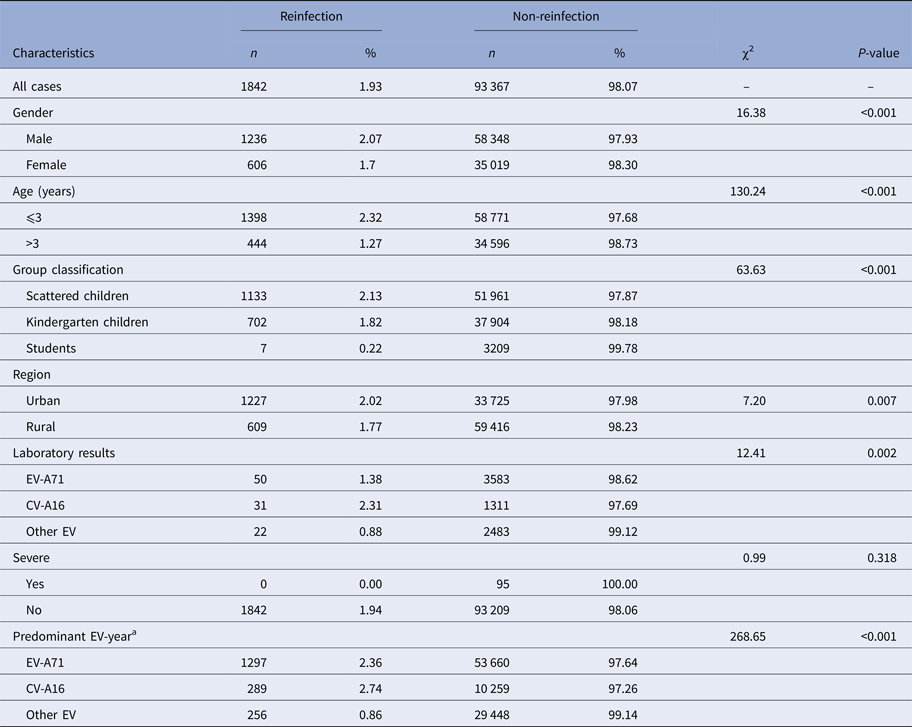
a Routine pathogenic surveillance of HFMD in Wuhan has been carried out since 2008. According to the predominant circulating EV in each year shown by the surveillance data, we categorised 2008–2015 into EV-A71 predominant year, CV-A16 predominant year, and other EV predominant year.
The hazard of HFMD reinfection
Generally the reinfection hazard sharply increases before 40 months, with 0.64% of cases reinfected within 10 months, 1.55% within 20 months, 2.03% within 30 months and 2.36% within 40 months after first infection. Figure 1 showed the cumulative hazard probability curves of HFMD reinfection stratified by gender, age, group, region and serotypes with the proportional hazard model. Higher reinfection hazards were detected among those who were males, aged 3 years and below, scattered children, belonging to urban areas and first infected with CV-A16.
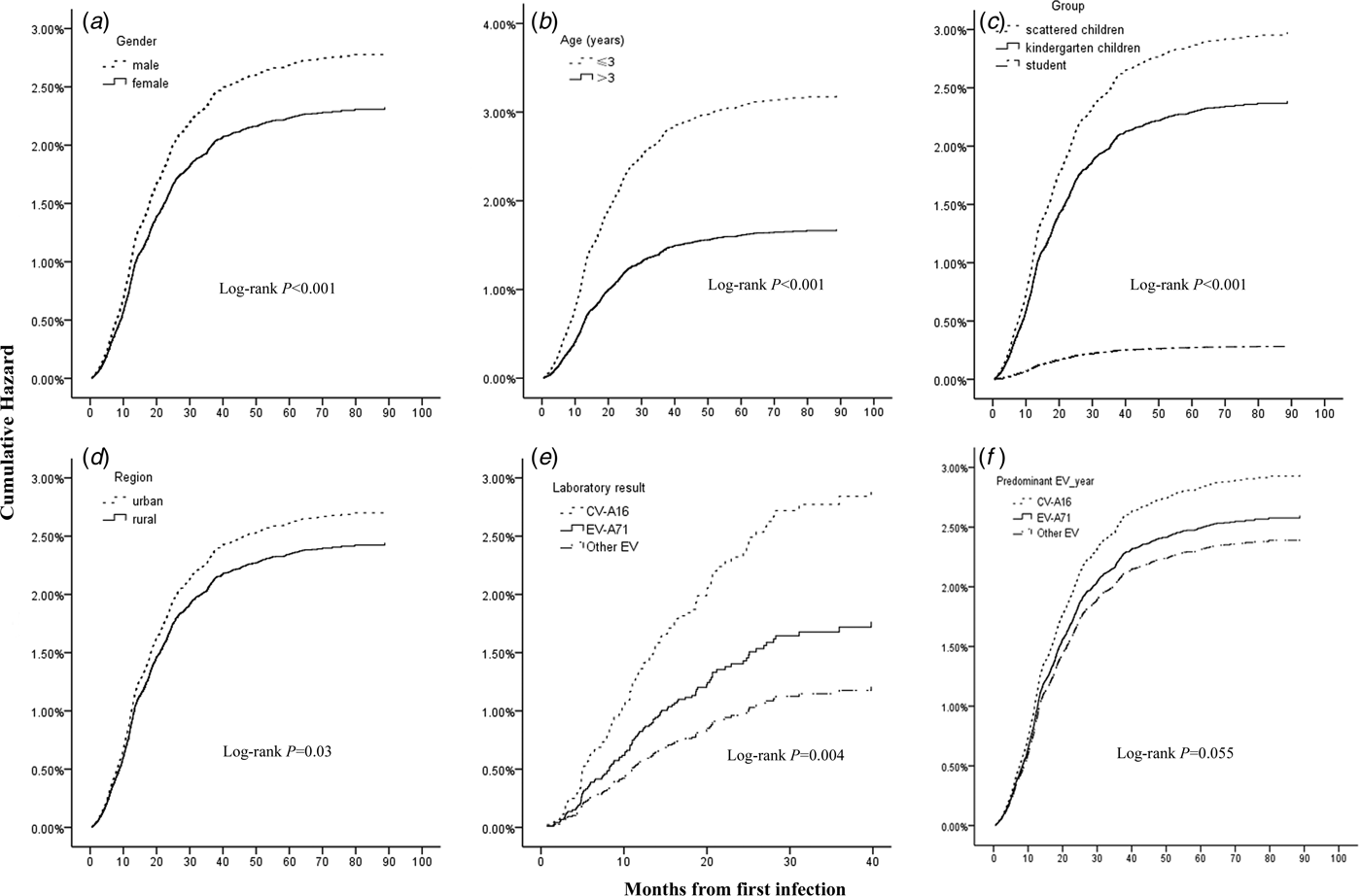
Fig. 1. The Kaplan–Meier curves showing the cumulative hazard of HFMD reinfection.
The results of Cox-proportional hazard regression analysis adjusted for the covariates were shown in Table 3. The data suggested that patient's gender, age, group, living area and serotypes of first infection had significant effect on reinfection even after adjusting for potential confounding effects of other selected factors considered in the study. For instance, the reinfection risk was higher among males compared with females (HR 1.187, CI 1.077–1.308); aged 3 years and below compared with more than 3 years old (HR 1.886, CI 1.658–2.146).
Table 3. Results from Cox-proportional hazard regression model depicting the risk of HFMD reinfection
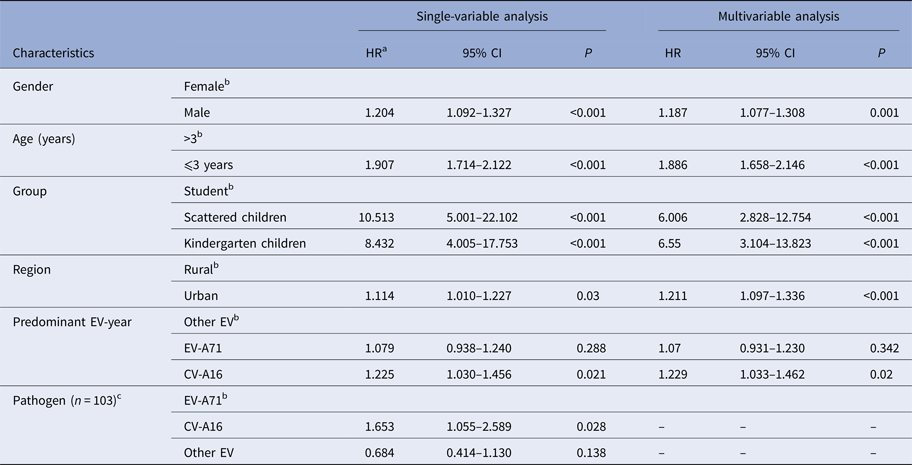
a Hazard ratio.
b Reference category.
c Of 1842 reinfected cases, only 103 were laboratory-confirmed. Thus, the ‘pathogen’ variable has much missing values. It was not included in multivariable analysis.
Discussion
The main purpose of current study was to reveal the hazards of HFMD reinfection. To the best of our knowledge, our study is the first one to address the reinfection hazards. Our data suggested that the reinfection rate of HFMD in Wuhan city during 2008–2015 was 1.93%, which was higher than 0.35% in Qinhuangdao city [Reference Ren17], lower than 3.36% in Guangzhou city [Reference Chen, Xiao and Ding18] and similar to 2.02% in Anhui province [Reference Chen9]. Furthermore, our further analysis indicated that the hazards of reinfection increased by months and reached peak of 2.36% at 40 months after first infection. Thus, reinfection hazards may bring continuing threats to each susceptibilities even those ever infected with HFMD.
Both single-variable and multivariable analysis showed association between reinfection risk and demographic variables such as gender, age, place of residence. Luo et al. have conducted a systematic review and demonstrated that the antibody level against EV-A71 and CV-A16 in neonates dropped to the lowest level at 1-year-old, and gradually increased thereafter and peaked at 4-year-old [Reference Luo19]. The Kaplan–Meier curves showed the reinfection hazard increased sharply before 30 months from first infection of HFMD in present study. Therefore, children aged <3 years were more prone to HFMD reinfection. We observed that the reinfection risk was higher in boys than in girls, which was in accordance with HFMD incidence outcomes [Reference Xie20]. The study also reported relatively higher reinfection risk in urban areas compared with rural areas. A similar finding has been noticed in other studies also [Reference Pan21]. This finding is somewhat contrary to the general belief that urban has better sanitary and should have lower risk. Possible reason is that urban has better access and utilisation of health care services, and parents have higher sense of seeking medical treatment. Hence, children belonging to urban areas have higher opportunity to be diagnosed and rediagnosed when they get ill with HFMD.
EV-A71 is a major causative agent of HFMD outbreaks worldwide and accounted for more than 90% of laboratory-confirmed cases [Reference Wu22]. Prior to this report, lower EV-A71 prevalence rate was encouraged as it predicted lower case-severity rate and lower HFMD burden [Reference Wang23]. However, findings of our study suggested that the likelihood of reinfection hazard increases with the non-EV-A71 (e.g. CV-A16) first infection, and it corroborates with the previous study [Reference Chen9]. Public health authorities in areas with a lower EV-A71 proportion of laboratory-confirmed cases need to promote hygiene education so that children can reduce the likelihood of reinfection.
Despite, the study observed various important findings. It has several limitations. First, only 7.85% of cases were confirmed with laboratory tests, therefore very few reinfected cases had laboratory results for both infections. Second, we did not strictly define the end-point and follow-up for a certain time to observe the occurrence of reinfection for those who were first infected with HFMD during the last 1 or 2 years (e.g. 2014–2015). Hence, the reinfection risk could be underestimated in the present study.
Conclusion
The study revealed several useful results that have potential bearing on reducing the risk of reinfection among HFMD population. The most useful finding emerged from our analysis was adjusted hazard risks for HFMD reinfection. In summary, boys aged <3 years living in urban areas and primarily infected with non-EV-A71 EV were more susceptible to HFMD reinfection. Therefore, their parents or guardians need to be alert to signs of HFMD, even if their children were previously infected. In addition, administrations need to carry out specific health education programmes to inform susceptible populations and develop polyvalent vaccine, which can provide protection against different EV genogroups to reduce the reinfection hazards.
Acknowledgements
The authors thank the hospitals for data report and the district CDC for the data audit.
Financial support
This study is financially supported by the Special National Project on Research and Development of Key Biosafety Technologies (B.Y., grant number: 2016YFC1201900); the National Key Laboratory of China CDC (B.Y., grant number: 2017SKLID307); and the Health Bureau of Wuhan (D.K., grant number: WG16B03).
Conflict of interest
None.







