Glutamine (Gln) is an amino acid with many important metabolic functions. It serves as a fuel for rapidly dividing cells (particularly lymphocytes and enterocytes)( Reference Kew, Wells and Yaqoob 1 , Reference Boelens, Nijveldt and Houdijk 2 ), induces the expression of heat shock proteins (HSP)( Reference Wischmeyer, Musch and Madonna 3 – Reference Zuhl, Lanphere and Kravitz 6 ), prevents apoptosis induced by injury( Reference Evans, Jones and Ziegler 7 ), has immunoregulatory functions( Reference Singleton, Beckey and Wischmeyer 8 , Reference Fan, Meng and Guo 9 ), and is a key precursor for the intestinal synthesis of glutathione, one of the major antioxidants in the body( Reference Wischmeyer, Musch and Madonna 3 , Reference Wischmeyer 10 , Reference Gouvea Junior, Caporossi and Salomao 11 ). Although Gln is the most abundant amino acid in the bloodstream, under certain conditions of stress, such as trauma, sepsis, major surgery, bone marrow transplantation, and intense chemotherapy and radiotherapy, the physiological requirement for Gln may exceed the capacity for endogenous synthesis such that Gln becomes a conditionally essential amino acid( Reference Wischmeyer, Musch and Madonna 3 ). Thus, dietary Gln supplementation may be a useful strategy to improve the body's response to stressful conditions.
Recently, our group has reported that Gln therapy is effective at preserving mucosal integrity and preventing increases in intestinal permeability and bacterial translocation (BT) in mice subjected to an experimental model of intestinal obstruction( Reference dos Santos, Viana and Generoso 12 ). These positive effects of Gln on the intestinal barrier function suggest a potential role for Gln in the prevention of heat stroke, a life-threatening illness characterised by elevated core body temperatures (T core), generally above 40°C.
Heat stroke events are not rare. According to the US Armed Forces Health Surveillance Center( 13 ), the incidences of heat stroke and ‘other heat injury’ events among active-duty military recruits in 2011 were 0·25 and 1·82 per 1000 person-years, respectively. A high incidence of heat stroke was also observed during a heat wave in Europe in the summer of 2003, when tens of thousands of people died from heat-related injuries( Reference Schar and Jendritzky 14 ). The pathogenesis of heat stroke is complex; marked increases in T core are associated with blood flow redistribution, which is characterised by cutaneous vasodilation that occurs at the expense of decreased intestinal blood flow( Reference Hall, Buettner and Oberley 15 – Reference Leon and Helwig 18 ). This splanchnic vasoconstriction may cause ischaemia and limit local vascular heat exchange, thereby promoting bowel tissue hyperthermia. Both intestinal ischaemia and hyperthermia may promote oxidative and nitrosative stresses that stimulate cytoskeletal relaxation, thus contributing to the opening of tight junctions and/or injuries to the epithelium( Reference Bouchama and Knochel 16 , Reference Leon and Helwig 18 – Reference Oliver, Phillips and Novosad 21 ). These morphological and functional changes enhance intestinal permeability( Reference Singleton and Wischmeyer 5 , Reference Lambert, Gisolfi and Berg 19 , Reference Dokladny, Moseley and Ma 20 ), thus facilitating the translocation of bacteria and endotoxins that are normally contained in the intestinal lumen( Reference Singleton and Wischmeyer 5 , Reference Hall, Buettner and Oberley 15 , Reference Lambert, Gisolfi and Berg 19 , Reference Dokladny, Moseley and Ma 20 , Reference Shapiro, Alkan and Epstein 22 , Reference Gathiram, Wells and Raidoo 23 ) and subsequently increasing the risk of a systemic inflammatory response syndrome that may culminate in multi-organ system failure and death( Reference Bouchama and Knochel 16 , Reference Leon and Helwig 18 ).
A previous investigation has already reported Gln-mediated beneficial effects in passively heated animals. Singleton & Wischmeyer( Reference Singleton and Wischmeyer 5 ) observed that Gln administration decreased gut permeability and plasma endotoxin concentrations and also improved survival following lethal hyperthermia. However, they conducted their study in rats under anaesthesia with ketamine and xylazine, which may represent a confounding factor because deep anaesthesia affects thermoregulation( Reference Lenhardt 24 ). In addition to this methodological issue, Singleton & Wischmeyer did not address whether oral Gln supplementation could change the ability to maintain T core during heat exposure. As Gln modulates the release of inflammatory cytokines( Reference Singleton and Wischmeyer 25 , Reference Cruzat, Rogero and Tirapegui 26 ) and as systemic inflammation response syndrome is closely associated with thermoregulatory manifestations( Reference Romanovsky, Almeida and Aronoff 27 ), Gln may also exert beneficial effects in passively heated animals by reducing their thermal strain under extreme environmental conditions.
Heat stroke is a deadly event that affects not only immunocompromised, aged people, but also healthy, young people. Therefore, the present study aimed to assess T core, intestinal permeability and BT to the blood and extra-intestinal organs in unanaesthetised and unrestrained mice exposed to a high ambient temperature. Moreover, because Gln preserves gastrointestinal function under several stressful conditions, another relevant question that was addressed was whether dietary Gln supplementation can alleviate the functional changes in the intestine and the increase in T core that are induced by a passive heating protocol.
Experimental methods
Animals and diets
In total, seventy-eight male Swiss mice (4 weeks old) weighing 20·3 (sem 1·9) g were obtained from the animal care centre at the Faculty of Pharmacy (Federal University of Minas Gerais, Brazil) and were used in all the experiments. Mice were housed in individual cages under controlled light (05.00–19.00 hours) and temperature (24·0 ± 2·0°C) conditions with water and chow provided ad libitum. The experiments were approved by the local Ethics Committee for Animal Experimentation (protocol number: 007/2011) and carried out in compliance with the Guide for the Care and Use of Laboratory Animals published by the Institute of Laboratory Animal Resources.
Mice were surgically implanted with abdominal temperature sensors, allowed to recover for 5 d, and then randomly allocated to three groups: (1) group fed the standard AIN-93G diet and maintained at room temperature during the experimental trials (control and non-supplemented group; C-NS); (2) group fed the standard AIN-93G diet and subjected to a passive heating protocol (hyperthermic and non-supplemented group; H-NS); (3) group fed the AIN-93G diet supplemented with Gln and subjected to a passive heating protocol (hyperthermic and Gln-supplemented group; H-Gln). The standard AIN-93G diet was originally formulated to support the growth, pregnancy and lactation of rodents by the American Institute of Nutrition( Reference Reeves, Nielsen and Fahey 28 ) and has been used extensively. In the supplemented diet, a portion of casein (equivalent to 4·375 mg/g) was replaced with l-Gln (Sigma-Aldrich). Thus, the standard and Gln-supplemented diets were isoenergetic and isoproteic.
Mice were fed the assigned diets for 7 d( Reference dos Santos, Viana and Generoso 12 , Reference de Oliveira, Lemos and Diniz 29 , Reference Santos, Quirino and Viana 30 ). During this period, water was provided ad libitum and body weight and food intake were measured once and twice a day, respectively. During the light phase of the day, all mice were given access to the AIN-93G diet ad libitum. In contrast, during the dark phase, mice were given access to only 4 g of food, an amount that they fully consumed on most days. Mice from the H-Gln group had access to 4 g of the supplemented diet, which corresponded to a daily intake of 17·5 mg of Gln or 500 mg Gln/kg body weight (i.e. mice had an average body weight of 35 g on the day of the experimental trials). This dose of Gln was selected based on previous findings that showed decreased BT to the liver, lungs and spleen of mice subjected to intestinal obstruction after the enteral administration of 500 mg/kg of Gln once a day for 7 d( Reference de Oliveira, Lemos and Diniz 29 ).
After Gln supplementation (or free access to the AIN-93G diet) for 7 d, mice were subjected to the experimental trials: a passive heating protocol or resting under temperate conditions. All experimental trials were performed during the light phase of the day. To achieve the goals of the study, three different sets of animals were used, with mice being always allocated to the three groups described earlier (i.e. C-NS, H-NS and H-Gln). The first set of mice (n 12 per group) was used to measure intestinal permeability, whereas the second (n 8 per group) and third (n 6 per group) sets were used to measure BT and secretory IgA (sIgA) concentrations in the intestinal fluid, respectively. The abdominal temperature of all mice subjected to different protocols was recorded during the experimental trials.
Implantation of the abdominal temperature sensor
A telemetry transmitter was surgically implanted in each mouse for recording T core (G2 E-Mitter series; Mini Mitter). Mice were weighed and anaesthetised with ketamine (60 mg/kg body weight, intraperitoneally) and xylazine (8 mg/kg body weight, intraperitoneally). During an aseptic procedure, the device was implanted in the abdominal cavity via a midline laparotomy and fixed to the lateral abdominal wall with sutures. Then, the abdominal muscles and skin were sutured in layers( Reference Costa, Soares and Wanner 31 , Reference Wanner, Costa and Soares 32 ). To prevent surgical hypothermia, the surgery was performed by placing the mice on a pad that was heated to 33°C.
Mice were allowed to recover from this surgery for 5 d. This period was sufficiently long for the mice to recover and overcome their presurgical body weight (26·2 (sem 0·7) g postsurgical v. 20·4 (sem 0·4) g presurgical, P< 0·001; the telemetric probes had an average weight of 1·1 g). During this recovery period, each mouse was individually housed and maintained under standard environmental conditions and given access to the AIN-93G diet (control) and tap water ad libitum.
Experimental trials
Mice were weighed and orally administered diethylenetriaminepentaacetic acid (DTPA) radiolabelled with technetium (99mTc) for the assessment of intestinal permeability or 99mTc-Escherichia coli for the assessment of BT. Mice were then allowed to rest for 30 min in a room with the ambient temperature maintained at 24°C. Mice from the H-NS and H-Gln groups were then transferred from their home cages to an acrylic chamber (25·5 cm long × 14 cm wide × 13·5 cm high) that was preheated to 39°C. An electric fan positioned at one end of the chamber generated an air flow rate of 2·0–2·5 m/s. The environment inside the chamber was heated by placing an electric heater (model AB 1100; Britânia) at the same level 20–30 cm away from the fan and turned on at 1200 W( Reference Lima, Pires and Fonseca 33 ).
The ambient temperature was set at 39°C based on previous findings showing that this environment was sufficiently hot to raise the abdominal temperature of mice above 42°C within 4 h( Reference Leon, Gordon and Helwig 34 ). The ambient temperature was measured using a thermocouple (400A; Yellow Springs Instruments). The heat exposure protocol lasted 2 h or the time required for an animal to exhibit the T core limit of 42°C. This T core value was chosen as a criterion for interrupting the passive heating protocol because it induces autonomic and behavioural responses related to heat stroke( Reference Leon, Gordon and Helwig 34 ) without causing mortality in mice( Reference Leon, DuBose and Mason 35 ).
Rather than being exposed to heat, mice from the C-NS group were allowed to move freely in their home cages at an ambient temperature of 24°C for 2 h. Food and water were not provided to the mice while they were resting in the cage or during the heat exposure protocol. However, from the end of the heat exposure or control period to the time of killing, they were given free access to water and food again.
Measurements
Abdominal temperature
Abdominal temperature was measured by telemetry at 30 s intervals and was considered to represent T core. The radiowave pulses emitted by the temperature sensors were captured by a receiving plate, which was positioned next to the chamber (during the experiments in the hyperthermic groups) or below the home cage of mice (in the C-NS group). The information received by the plate was sent to a data acquisition system (Vital View; Mini Mitter), which converted the frequency values into temperature values.
Telemetry is a technique that allows the measurement of temperature in conscious and freely moving animals without affecting their ability to engage behavioural and autonomic thermoeffectors to deal with environmental challenges. This method eliminates the influence of confounders such as restraint of the animals, stress associated with the insertion of rectal temperature probes, and anaesthesia on the study( Reference Leon, DuBose and Mason 35 ).
Intestinal permeability
Intestinal permeability was assessed based on the diffusion of a DTPA solution labelled with 99mTc that was administered orally. The DTPA probe is large (molecular weight: 500–700 Da), allowing for the evaluation of intestinal permeability through the paracellular pathway( Reference Jorgensen, Nielsen and Espersen 36 ).
The experimental trials (i.e. heat exposure protocol or resting under temperate conditions for 2 h) were carried out 30 min after the administration of 13 MBq of99mTc-DTPA in a volume of 0·1 ml. At 3, 8 and 18 h after the experimental trial, groups of six mice were anaesthetised and killed by decapitation. Trunk blood samples (300 μl) were collected and placed in appropriate tubes for radioactivity measurement in an automatic gamma counter (Wallac Wizard model 1480; Perkin Elmer).
The percentage of the administered dose present in the blood was calculated using the following equation( Reference Santos, Quirino and Viana 30 , Reference Viana, Santos and Generoso 37 – Reference Quirino, Cardoso and Santos 40 ):
where cpm represents the counts of radioactivity/min.
Bacterial translocation
BT translocation was measured in three more groups of mice (n 8 each). Mice were orally administered 0·1 ml of 99mTc-E. coli ATCC-10 536 (1·8 MBq) containing 108 colony-forming units. The radiolabelling of E. coli was performed as described by Diniz et al. ( Reference Diniz, Resende and Nunan 41 ). The percentage of 99mTc incorporated into the bacterial cells was determined using the following equation:
Mice were subjected to the heat exposure protocol or allowed to rest under temperate conditions 30 min after the administration of 99mTc-E. coli. BT was evaluated 3 h after the heat exposure protocol because within the time points studied this time point corresponded to the period when increased intestinal permeability was observed. Blood, mesenteric lymph nodes, liver, spleen, brain and lungs were collected, weighed and placed into tubes for radioactivity measurement in an automatic gamma counter (Wallac Wizard model 1480; Perkin Elmer). The results are expressed as cpm relative to the mass of tissue analysed( Reference Santos, Quirino and Viana 30 , Reference Viana, Santos and Generoso 37 – Reference Quirino, Cardoso and Santos 40 ).
Immunoglobulin analysis
The small intestines of mice from all groups (C-NS, H-NS and H-Gln; n 6 for each group) were removed after killing the mice. The intestinal contents (500 mg) were withdrawn, weighed and resuspended in 2 ml of PBS supplemented with an anti-protease cocktail (1 μm-aprotinin, 25 μm-leupeptin, 1 μm-pepstatin and 1 mm-phenylmethanesulphonyl fluoride); this anti-protease cocktail was used by Santos et al. ( Reference Santos, Quirino and Viana 30 ). The concentrations of sIgA in the intestinal fluid were measured by ELISA using a goat anti-mouse IgA (Sigma Chemical Company) and a horseradish peroxidase-conjugated goat anti-mouse IgA (Sigma), as described previously by Martins et al. ( Reference Martins, Silva and Vieira 42 ). The measurements were performed in duplicate. sIgA is an important component of the intestinal protective immunity and acts by reducing the number of epithelium-adherent bacteria, thus limiting BT through the epithelium( Reference Wiest and Rath 43 ).
Statistical analyses
The variables studied were tested for normality using the Shapiro–Wilk test. All the variables, except BT data, were normally distributed. The BT data are expressed as medians and analysed using the non-parametric Kruskal–Wallis test. When significance was detected, Mann–Whitney post hoc tests were conducted to identify differences among the experimental groups.
Normally distributed data are expressed as means with their standard errors. The abdominal temperature curves were compared between the experimental groups and time points using a two-way ANOVA, with repeated measures only for the time factor. Body weight gain, intestinal permeability, sIgA concentrations and food, energy, protein and nitrogen intakes were compared among the three groups using one-way ANOVA. Tukey's test was used as the post hoc test for variables that had a variation coefficient < 15 % and Duncan's test for variables that had a variation coefficient >15 %( Reference Sampaio 44 ). Differences in the preoperative and postoperative body weight were assessed using paired Student's t test (data from the three experimental groups were pooled for this analysis). The role of Gln supplementation in the prevention of the increase of T core to 42°C during the passive heating protocol was assessed using the log-rank test( Reference Bland and Altman 45 ).
The AUC of T core across time points was calculated using trapezoidal integration. The correlation between the thermoregulatory parameters (final abdominal temperature, maximal temperature achieved and area under the temperature curve) and intestinal permeability or sIgA concentrations was assessed using Pearson's coefficient analysis. The correlation between the thermoregulatory parameters and BT was assessed using Spearman's coefficient (non-parametric) analysis.
All analyses were performed using Sigma Plot version 11.0 (Systat Software, Inc.), and P< 0·05 was defined as statistically significant.
Results
Body weight gain and food intake
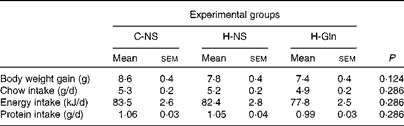
There were no significant differences in the nutritional parameters evaluated among the three experimental groups (P>0·05 for all nutritional parameters; Table 1). The only exception was the intake of Gln, which was augmented by approximately 17·5 mg/d in mice from the H-Gln group when compared with those from the non-supplemented groups. Mice exhibited an average body weight gain of 7·9 (sem 0·2) g (pooled data for the three experimental groups) across the 7 d of treatment with different diets. Their average daily intakes of food, energy and protein were 5·2 (sem 0·1) g, 81·3 (sem 1·5) kJ and 1·03 (sem 0·02) g, respectively.
Table 1 Body weight gain and daily chow, energy and protein intakes in the C-NS (non-supplemented mice maintained under temperate conditions), H-NS (non-supplemented mice subjected to heat stress) and H-Gln (glutamine-supplemented mice subjected to heat stress) groups during the 7 d before the experimental trials (Mean values with their standard errors)
Core temperature
Before the experimental trials (at time 0), mice exhibited an average T core that ranged from 37·56 to 37·75°C, and no differences were observed among the three experimental groups (Fig. 1). At an environmental temperature of 24°C, the T core of mice from the C-NS group remained stable at approximately 37·5°C for 20 min and then gradually decreased to 37·0°C, a value that was sustained until the end of the recording period. The fact that T core decreased in mice from the C-NS group as the experiments progressed indicates that the trials were initiated when the mice were still under the effects of stress hyperthermia induced by the handling and oral administration procedures.

Fig. 1 Abdominal temperature of mice during the experimental trials. C-NS group, non-supplemented mice maintained under temperate conditions (–○–); H-NS group, non-supplemented mice subjected to heat stress (–●–); and H-Gln group, glutamine-supplemented mice subjected to heat stress (![]() ). Values are means (n 26 per group), with their standard errors represented by vertical bars. * Mean values for the H-NS and the H-Gln group were significantly different that of the C-NS group (P< 0·001). † Mean value was significantly different from that of the H-NS group (P< 0·05).
). Values are means (n 26 per group), with their standard errors represented by vertical bars. * Mean values for the H-NS and the H-Gln group were significantly different that of the C-NS group (P< 0·001). † Mean value was significantly different from that of the H-NS group (P< 0·05).
The heat-exposed mice (i.e. the H-NS and H-Gln groups) exhibited a steep increase in T core because of the exposure to an uncompensated environmental heat load (Fig. 1). Exposure to a high ambient temperature (39°C) significantly increased the T core of mice from the H-NS group relative to those from the C-NS group from the 3rd min to the end of the heat exposure protocol (40·90 (sem 0·17) v. 37·02 (sem 0·07)°C at the end; P< 0·001). Heat exposure also increased the T core of mice from the H-Gln group relative to those from the C-NS group; however, Gln-supplemented mice exhibited attenuation of hyperthermia compared with non-supplemented mice from the 49th to the 70th min of heating (39·86 (sem 0·11) v. 40·33 (sem 0·15)°C at the 70th min; P< 0·05; Fig. 1).
This Gln-induced protective thermoregulatory effect was also evident from the analysis of the percentage of mice in which the T core limit of 42°C was reached during the passive heating protocol (P< 0·05; log-rank test). Of the twenty-six mice from the H-NS group, six (23 %) exhibited a T core of 42°C before the end of the 2 h heat exposure protocol (Fig. 2). In contrast, this temperature limit was reached in only one mouse from the H-Gln group (4 %). It is worth noting that during the passive heating protocol, one mouse from the H-NS group died with a T core value of 41·5°C after being exposed to heat for 100 min (this mouse was excluded from all analyses). No mice from the H-Gln group died during the experimental trials.

Fig. 2 Percentage of mice that did not attain the abdominal temperature limit of 42°C at different time points during the passive heating protocol (n 26 per group). H-NS group, non-supplemented mice subjected to heat stress (–●–), and H-Gln group, glutamine-supplemented mice subjected to heat stress (![]() ). * Percentage of mice along the whole experimental trial was significantly different from that of the H-NS group (P< 0·05).
). * Percentage of mice along the whole experimental trial was significantly different from that of the H-NS group (P< 0·05).
Intestinal permeability
Mice from the C-NS group exhibited a physiological range of intestinal permeability values at 3, 6 and 18 h after the experimental trial (Fig. 3). At 3 h after the heat exposure protocol, mice from the H-NS group exhibited an increased intestinal permeability that was approximately eleven to twelve times higher than that of mice from the C-NS group (Fig. 3); however, this augmented intestinal permeability was transient, and no differences were observed between these two groups at 6 and 18 h after the experimental trial. Dietary Gln supplementation prevented the increase in intestinal permeability that was observed in mice from the H-NS group at 3 h after the heat exposure protocol. In fact, the uptake of 99mTc-DTPA in the blood samples of mice from the H-Gln group was similar to that in mice from the C-NS group at every time point studied.

Fig. 3 Intestinal permeability of mice at 3, 6 and 18 h after the experimental trials. C-NS group, non-supplemented mice maintained under temperate conditions (–○–); H-NS group, non-supplemented mice subjected to heat stress (–●–); and H-Gln group, glutamine-supplemented mice subjected to heat stress (![]() ). Values are means (n 12 per group; four for each time point), with their standard errors represented by vertical bars. ** Mean value was significantly different from those of the C-NS and H-Gln groups (P< 0·01). DTPA, diethylenetriaminepentaacetic acid; % dose = ((cpm in blood × 100)/cpm of administered dose), where cpm = counts/min.
). Values are means (n 12 per group; four for each time point), with their standard errors represented by vertical bars. ** Mean value was significantly different from those of the C-NS and H-Gln groups (P< 0·01). DTPA, diethylenetriaminepentaacetic acid; % dose = ((cpm in blood × 100)/cpm of administered dose), where cpm = counts/min.
Bacterial translocation
Mice from the C-NS group exhibited a physiological level of BT to the blood and all organs at 3 h after the experimental trial (which corresponded to the time point when increased intestinal permeability caused by passive heating was observed; Table 2). Heat exposure increased BT, as demonstrated by a significantly increased uptake of 99mTc-E. coli by the blood and liver (P< 0·05) and a tendency for higher uptake by the lungs (P= 0·064) in mice from the H-NS group compared with those from the C-NS group (Table 2). Dietary Gln supplementation prevented the hyperthermia-induced BT to the blood, liver and lungs; the uptake of 99mTc-E. coli in mice from the H-Gln group was lower than that in mice from the H-NS group (P< 0·05) and did not differ from that in mice from the C-NS group. No differences were observed in the uptake of 99mTc-E. coli by the mesenteric lymph nodes, spleen and brain among the three experimental groups.
Table 2 Bacterial translocation (counts of radioactivity per minute (cpm)/g) to the blood and extra-intestinal organs in the C-NS (non-supplemented mice maintained under temperate conditions), H-NS (non-supplemented mice subjected to heat stress) and H-Gln (glutamine-supplemented mice subjected to heat stress) groups* (Median and 25th and 75th interquartile ranges (IQR))

MLN, mesenteric lymph nodes.
a,bMedian values with unlike superscript letters were significantly different between the experimental groups (P< 0·05).
* Simultaneous comparison was performed among the different experimental groups (n 8 per group).
Secretory IgA concentrations in the intestinal fluid
At 3 h after the experimental trial, no differences were observed in sIgA concentrations among the three experimental groups (P= 0·20; Fig. 4).

Fig. 4 Secretory IgA (sIgA) concentrations in the intestinal fluid of mice at 3 h after the experimental trials. C-NS group, non-supplemented mice maintained under temperate conditions (□); H-NS group, non-supplemented mice subjected to heat stress (■); and H-Gln group, glutamine-supplemented mice subjected to heat stress (![]() ). Values are means (n 6 per group), with their standard errors represented by vertical bars.
). Values are means (n 6 per group), with their standard errors represented by vertical bars.
Correlation analyses
Correlation analyses were performed to determine whether the beneficial effects of Gln supplementation on intestinal function were associated with Gln-mediated changes in thermoregulation. Intestinal permeability, BT and sIgA concentrations were found to be not significantly associated with any of the evaluated thermoregulatory parameters (i.e. the T core after 2 h of heat exposure, the highest T core value recorded or the area under the T core curve during the passive heating protocol; Table 3). The only exception was the positive and significant correlation between sIgA concentrations and the highest T core attained during heat exposure (r 0·63; P< 0·05).
Table 3 Correlations between thermoregulatory parameters and intestinal permeability, bacterial translocation and secretory IgA (sIgA) concentrations in the H-NS group (non-supplemented mice subjected to heat stress) and the H-Gln group (glutamine-supplemented mice subjected to heat stress)
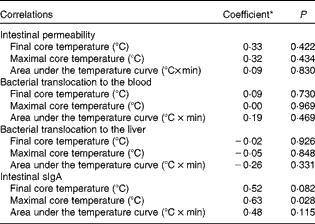
* Pearson's coefficient (r) was used for correlations between thermoregulatory parameters and intestinal permeability or sIgA concentrations, whereas Spearman's coefficient (r s) was used for correlations between thermoregulatory parameters and bacterial translocation.
Discussion
The results of the present study demonstrate that the non-supplemented vigil mice subjected to acute heat stress attained high T core values that were sustained above 40°C for approximately 100 min (Fig. 1). This marked hyperthermia was associated with increased intestinal permeability (Fig. 3) and BT to the blood and liver (Table 2). The results also demonstrate that dietary Gln supplementation improved the intestinal barrier function, thereby preventing the increase in intestinal permeability and limiting the BT induced by the passive heating protocol. Another interesting finding was that dietary Gln supplementation reduced the magnitude of passive hyperthermia (Fig. 1) and reduced the risk of reaching the T core limit of 42°C (Fig. 2).
All the experimental groups were fed isoenergetic and isonitrogenous diets and exhibited similar food intake and body weight gain (Table 1). These data indicate that mice tolerated the supplemented diet well, despite the possibility that the addition of Gln, a virtually tasteless powder( Reference Choi, Lee and Oh 46 ), may have changed the taste of the diet. This possibility is an important concern because malnutrition induces BT( Reference Welsh, Farmery and MacLennan 47 ) and decreases T core ( Reference Yoda, Crawshaw and Yoshida 48 ) . These data also indicate that the beneficial effects observed in the supplemented groups can be exclusively attributed to the actions of Gln.
Increased intestinal permeability was transiently observed in the passively heated animals; when compared with control animals (C-NS), these hyperthermic animals (H-NS) exhibited higher intestinal permeability at 3 h after the experimental trial, but not after 6 and 18 h (Fig. 3). This increased intestinal permeability is in agreement with the results of previous studies in which anaesthetised animals were subjected to different passive heating protocols( Reference Singleton and Wischmeyer 5 , Reference Hall, Buettner and Oberley 15 , Reference Lambert, Gisolfi and Berg 19 , Reference Oliver, Phillips and Novosad 21 ) and with the demonstration of rapid repair of the intestinal epithelium after hyperthermia-induced injury( Reference Blikslager, Moeser and Gookin 49 ). Moreover, as we measured the radioactivity levels of 99mTc-DTPA in the blood to determine intestinal permeability, we conclude that the increased permeation observed at 3 h most probably occurred through the paracellular pathway.
The results of the present study do not exclude the possibility that intestinal permeability may have been even more pronounced in the period between the end of exposure and before the 3 h recovery period. This hypothesis is supported by other studies showing significant increases in intestinal permeability shortly after passive hyperthermia( Reference Hall, Buettner and Oberley 15 , Reference Lambert, Gisolfi and Berg 19 , Reference Oliver, Phillips and Novosad 21 ).
There was no significant difference in sIgA concentrations in the intestinal fluid among the experimental groups (Fig. 4), suggesting that the passive heating protocol did not change the number of immunocompetent cells in the lamina propria and the local production of cytokines involved in IgA synthesis( Reference Lebman, Coffman, Ogra, Mestecky, Lamm, Strober, McGhee and Bienenstock 50 , Reference Kiyono, McGhee, Ogra, Mestecky, Lamm, Strober, McGhee and Bienenstock 51 ). The concentrations of sIgA were positively correlated with the highest T core attained during the heat exposure protocol (Table 3) and tended to be higher in mice from the H-NS group than in those from the C-NS group (Fig. 4). These observations suggest a physiological association between the intestinal concentrations of sIgA and the magnitude of hyperthermia, which should be investigated further. Nevertheless, the positive correlation between the concentrations of sIgA and the magnitude of hyperthermia suggests that the intestinal immune system is acting properly to restrain BT at physiological levels. Previous evidence suggests that the increased intestinal permeability caused by hyperthermia could be attributed to an injury to the epithelial cell lining and/or opening of epithelial tight junctions( Reference Bouchama and Knochel 16 , Reference Leon and Helwig 18 – Reference Dokladny, Moseley and Ma 20 ).
Mice from the C-NS group exhibited a low level of BT to the blood and all evaluated extra-intestinal organs, confirming that BT is a physiological process essential for the maturation and maintenance of a competent gastrointestinal immune system( Reference Wiest and Rath 43 ). In contrast, 3 h after exposure to acute heat stress (exactly at the time when augmented intestinal permeability was observed), mice from the H-NS group exhibited an increased uptake of 99mTc-E. coli in the blood and liver relative to those from the C-NS group. This finding is in agreement with previous reports showing that impaired bowel function increases intestinal permeability, which may facilitate BT( Reference Wiest and Rath 43 , Reference Pearce, Mani and Boddicker 52 , Reference Pearce, Mani and Weber 53 ). It is likely that the bacteria and their products translocated via the portal circulation to the blood and liver in the heat-stressed mice, corroborating recent observations in mice that performed prolonged physical exercise in the heat and also demonstrated increased bacterial levels in the blood and liver( Reference Costa, Soares and Wanner 31 ). According to Wiest & Rath( Reference Wiest and Rath 43 ), bacteria may translocate from the gastrointestinal tract through different routes: (1) via blood vessels to reach the portal system; (2) by direct transmural migration across the intestinal wall; (3) by retrograde migration to the lungs; (4) by lymphatic migration via Peyer's patches to reach mesenteric lymph nodes, followed by movement from the thoracic duct into the left subclavian vein to reach the right side of the heart and then enter the pulmonary circulation. The preferential route for BT probably varies according to the magnitude of the inflammatory insult( Reference Mainous, Tso and Berg 54 ). Our recent findings suggest that BT associated with hyperthermic states (induced by passive heating or physical exercise) occurs preferentially through the portal circulation. In addition, the contribution of a translocation pathway that involves the lungs cannot be excluded because the bacterial content of the lungs of mice from the H-NS group tended to be higher than that of mice from the C-NS and H-Gln groups. We also measured the bacterial content in the brain because hyperthermia may augment the permeation of the blood–brain barrier( Reference Leon and Helwig 18 ). However, the BT to the brain was not affected by acute heat stress or dietary Gln supplementation (Table 2).
Notably, liver failure is frequently associated with heat stroke( Reference Leon and Helwig 18 ). In the present study, mice tolerated the heat stress well, and no deaths were observed in mice in which intestinal permeability was measured 18 h after the experimental trial. In fact, the impairment of gastrointestinal function was short-lived compared with that observed in more severe heat stress protocols( Reference Singleton and Wischmeyer 5 ), in which anaesthetised rats were heated until reaching a rectal temperature of 42°C (a level of hyperthermia that was maintained for 30 min) and consequently exhibited increased permeability 6 and 24 h after the heat exposure protocol had ceased. Therefore, it is likely that the heat exposure protocol used in the present study did not cause major dysfunctions in Küpffer cells. This assumption is supported by the finding that BT to the other extra-intestinal organs was not increased above the physiological levels, suggesting that the liver efficiently acted as a scavenger of bacteria and their products( Reference Wiest and Rath 43 ). Similarly, Hall et al. ( Reference Hall, Buettner and Oberley 15 ) demonstrated higher concentrations of bacterial endotoxin in the portal venous blood, but not in the arterial blood, of anaesthetised rats that were subjected to an increase in colonic temperature from 37·0 to 41·5°C via exposure to an ambient temperature of 40°C, relative to non-stressed animals.
The attenuated increase in T core in Gln-supplemented mice that were passively exposed to heat is a novel finding of the present study (Fig. 1). Our findings are not corroborated by previous observations in humans experiencing endotoxaemia, in whom the magnitude of the febrile response was not influenced by intravascular infusion of Gln( Reference Andreasen, Pedersen-Skovsgaard and Mortensen 55 ). Similarly, a recent investigation subjecting human subjects to treadmill running under a high ambient temperature did not reveal any Gln-induced change in T core at the end of the exercise( Reference Zuhl, Lanphere and Kravitz 6 ). We speculate that this thermoregulatory effect mediated by Gln may be influenced by the dose, frequency and route of Gln administration or may be specific to certain stressful conditions or animal species.
Facilitated heat loss is a physiological response that could explain the attenuated hyperthermia observed in supplemented animals. However, an increase in cutaneous vasodilation mediated by Gln is unlikely to account for the lower T core because the temperature gradient between the skin and environment is narrow (if not reversed) at an ambient temperature of 39°C; this low gradient hampers dry heat loss from the body. Whether dietary Gln supplementation facilitates evaporative heat loss or behavioural thermoregulation remains to be determined. Another potential explanation for the attenuated hyperthermia is the diminished release of inflammatory cytokines and eicosanoids that provoke fever, consistent with previous findings showing that Gln decreases the plasma and tissue concentrations of TNF-α, IL-6 and PGE2 ( Reference Singleton and Wischmeyer 25 , Reference Cruzat, Rogero and Tirapegui 26 ). Finally, the reduced T core may be the result of a Gln-induced reduction in intestinal blood flow, limiting the delivery of warmed blood from the hot skin to the gastrointestinal system and thereby attenuating the increase in abdominal temperature. The latter hypothesis is supported by Matheson et al. ( Reference Matheson, Harris and Hurt 56 ), who showed that enteral Gln supplementation impairs nutrient-driven absorptive hyperaemia in the colon, pancreas, spleen and throughout the small intestine.
The protective effect exerted by Gln on the intestinal barrier function was independent of the attenuation of hyperthermia (Table 3). The beneficial effects of dietary Gln enrichment on gastrointestinal function have already been reported in rodents subjected to different stressful conditions that are not necessarily associated with hyperthermia, such as intestinal obstruction( Reference dos Santos, Viana and Generoso 12 , Reference Santos, Quirino and Viana 30 ) and burns( Reference Fan, Meng and Guo 9 ). Under these experimental conditions, Gln exerts beneficial effects through several mechanisms, including intestinal tropism( Reference Rhoads, Argenzio and Chen 57 ), inhibition of apoptosis( Reference Evans, Jones and Ziegler 7 ), stimulation of the Th1 inflammatory response( Reference Singleton, Beckey and Wischmeyer 8 ), reinforcement of the immune system against bacteria and endotoxins( Reference Fan, Meng and Guo 9 ), preservation of glutathione( Reference Gouvea Junior, Caporossi and Salomao 11 ) and increased expression of HSP( Reference Wischmeyer, Musch and Madonna 3 , Reference Singleton and Wischmeyer 5 ). In particular, HSP are involved in the most basic mechanisms of cellular protection against stressful conditions( Reference Weitzel and Wischmeyer 58 ). Treatment with Gln has been shown to up-regulate the expression of HSP genes in hyperthermic animals( Reference Wischmeyer, Musch and Madonna 3 , Reference Singleton and Wischmeyer 5 ), similarly to the HSP expression promoted by a heat acclimation protocol( Reference Magalhaes, Amorim and Passos 59 ). Moreover, animals that have undergone genetic knockout of key HSP pathway mediators are not protected against sepsis and lung injury by Gln administration( Reference Singleton and Wischmeyer 25 ). Recently, Zuhl et al. ( Reference Zuhl, Lanphere and Kravitz 6 ) have shown that in vitro Gln supplementation increases the concentrations of HSP70 and heat shock factor-1 (the transcription factor that regulates HSP70) in response to heat stress; moreover, the combined effect of Gln and heat increased the expression of occludin. Similarly, Beutheu et al. ( Reference Beutheu, Ouelaa and Guerin 60 ) demonstrated that Gln prevented changes in the concentrations of occludin, claudin-1 and zonula occludens-1 during chemotherapy-induced mucositis in rats. Together, these findings suggest that the Gln-mediated intestinal protection may also be due to the modulation of the tight-junction proteins.
In conclusion, acute exposure to heat induced marked hyperthermia, increased intestinal permeability and increased BT that most probably occurred via the portal circulation. Dietary Gln supplementation decreased the magnitude of hyperthermia and prevented the increases in BT and intestinal permeability caused by the passive heating protocol. Taking these findings into account, we suggest that Gln supplementation may be an important nutritional strategy for preventing severe hyperthermia and heat-related disorders.
Acknowledgements
The authors are grateful to the following sources for providing financial support: Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq/UFMG); CNPq (the National Council of Technological and Scientific Development); CAPES (the Coordination for the Improvement of Higher Education Personnel); FAPEMIG (the Minas Gerais State Foundation for Research Support). The funding agencies had no role in the design and analysis of the study or in the writing of this article.
The authors’ contributions are as follows: A. D. N. S., K. A. C., S. P. W., C. C. C. and V. N. C. designed the study; A. D. N. S., K. A. C., S. P. W., R. G. C. S. and F. S. M. conducted the study; A. D. N. S., S. P. W., S. O. A. F., J. R. N., C. C. C. and V. N. C. analysed the data; A. D. N. S., S. P. W. and V. N. C. wrote the article; V. N. C. had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.









