Table of Contents
Preamble
-
Introduction
-
Methodology and Evidence Review
-
Organization of the Writing Committee
-
Document Review and Approval
-
Health Policy Objectives
-
Top 10 Take-Home Messages
-
-
Permanent Pacemakers
-
Introduction
-
Isolated Sinus Node Dysfunction
-
Isolated Congenital Complete Atrioventricular Block
-
Atrioventricular Block: Other Considerations
-
Postoperative Atrioventricular Block
-
Congenital Heart Disease: Specific Considerations
-
Post Cardiac Transplantation
-
Neuromuscular Diseases and Other Progressive Cardiac Conduction Diseases
-
Neurocardiogenic Syncope
-
Cardiac Channelopathies
-
Inflammation/Infection
-
-
Implantable Cardioverter Defibrillators
-
Introduction
-
General Recommendations for Implantable Cardioverter Defibrillator Therapy
-
ICD Indications for Cardiac Channelopathies
-
Long QT Syndrome
-
Catecholaminergic Polymorphic Ventricular Tachycardia
-
Brugada Syndrome
-
-
ICD Indications for Cardiomyopathies
-
Hypertrophic Cardiomyopathy
-
Arrhythmogenic Cardiomyopathies
-
Nonischemic Dilated Cardiomyopathy
-
-
ICD Indications for Congenital Heart Disease
-
-
Insertable Cardiac Monitors
-
CIED Lead Management
-
CIED Follow-up and Ancillary Testing
-
Special Considerations
-
CIEDs and Magnetic Resonance Imaging
-
CIEDs and Sports Participation
-
Shared Decision-Making
-
-
Knowledge Gaps and Future Research
-
References
-
Appendix 1 Author Relationships With Industry
-
Appendix 2 Reviewer Relationships With Industry
Preamble
Guidelines for the implantation of cardiac implantable electronic devices (CIEDs) have evolved since publication of the initial ACC/AHA pacemaker guidelines in 1984. Reference Frye, Collins and DeSanctis1 CIEDs have evolved to include novel forms of cardiac pacing, the development of implantable cardioverter defibrillators (ICDs) and the introduction of devices for long term monitoring of heart rhythm and other physiologic parameters. In view of the increasing complexity of both devices and patients, practice guidelines, by necessity, have become increasingly specific. In 2018, the ACC/AHA/HRS published Guidelines on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay, Reference Kusumoto, Schoenfeld and Barrett2 which were specific recommendations for patients >18 years of age. This age-specific threshold was established in view of the differing indications for CIEDs in young patients as well as size-specific technology factors. Therefore, the following document was developed to update and further delineate indications for the use and management of CIEDs in pediatric patients, defined as ≤21 years of age, with recognition that there is often overlap in the care of patents between 18 and 21 years of age.
This document is an abbreviated expert consensus statement (ECS) intended to focus primarily on the indications for CIEDs in the setting of specific disease/diagnostic categories. This document will also provide guidance regarding the management of lead systems and follow-up evaluation for pediatric patients with CIEDs. The recommendations are presented in an abbreviated modular format, with each section including the complete table of recommendations along with a brief synopsis of supportive text and select references to provide some context for the recommendations. This document is not intended to provide an exhaustive discussion of the basis for each of the recommendations, which are further addressed in the comprehensive PACES-CIED document, Reference Shah, Silka and Avari Silva3 with further data easily accessible in electronic searches or textbooks.
Introduction
Methodology and evidence review
The principles in the development of this document are 1) new recommendations or changes to previous recommendations are based on data, when possible; 2) these recommendations are consistent with current ACC/AHA/HRS adult guidelines when reasonable; and 3) all recommendations have been critically reviewed, initially by the writing committee and editors, followed by the PACES executive committee, and subsequently by external HRS, ACCF, AHA, and AEPC representatives. Any revisions or additions to existing recommendations require approval of at least 80% by the members of the PACES writing committee.
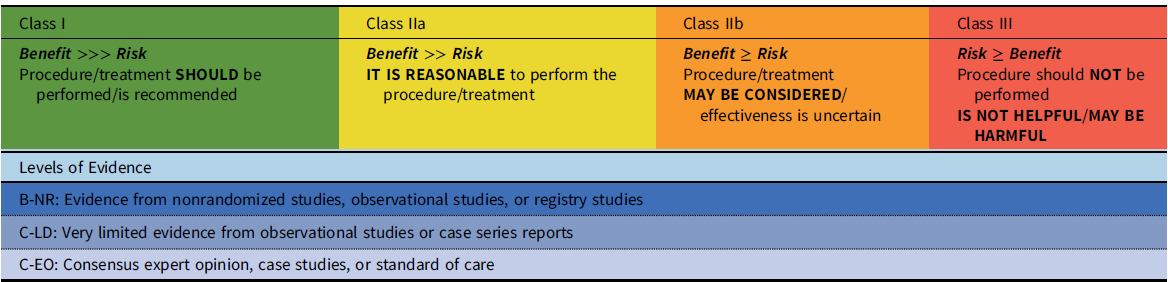
These recommendations have been developed with standard guideline methodology, i.e., with both a class of recommendation (COR) and a level of evidence (LOE) (Table 1). The class of the recommendation indicates the strength of recommendation, based on the estimated magnitude or certainty of benefit in proportion to risk. The level of evidence rates the quality of evidence based on the type, quantity, and consistency of data from clinical trials and other sources. A recommendation with a Level of Evidence C-EO does not imply that the recommendation is weak. Many of the questions addressed in this (and other) documents either do not lend themselves to clinical trials or are rare disease entities. However, there may be unequivocal consensus that a particular intervention is either effective or necessary.
Table 1. Class of recommendation and level of evidence categories*
*Adapted from Halperin, et al.Reference Halperin, Levine and Al-Khatib100
Organization of the writing committee
The writing committee consisted of members of PACES who were selected by the PACES executive committee. The writing committee members included junior and senior pediatric electrophysiologists as well as allied health professionals and represented diverse genders, countries, and cultures. The writing committee also included external representatives from the ACC, AHA, HRS, and AEPC. Prior to final publication, all committee members were required to verify their specific contributions to this document. Appendix 1 lists writing committee members’ relevant relationships with industry.
Document review and approval
Following internal review by the PACES executive committee, this document was then reviewed by the PACES writing committee. Following considerations of these comments and approval by an independent PACES reviewer, the recommendations were opened for public comment to PACES members. An official reviewer each nominated by HRS, ACC, AHA, and AEPC provided independent external review. This document was then approved for publication by the PACES executive committee and endorsed by all collaborators and the Asia Pacific Heart Rhythm Society (APHRS), the Indian Heart Rhythm Society (IHRS), and the Latin American Heart Rhythm Society. Appendix 2 lists reviewers’ relevant relationships with industry.
Health policy objectives
The purpose of this document is to provide guidance to clinicians for the management of pediatric patients who may require a CIED, with a primary focus on the indications for device implantation. The document will be useful to pediatric cardiologists, cardiac surgeons, cardiac intensivists, anesthesiologists, and arrhythmia specialists. This document supersedes the pediatric CIED recommendations made in “ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities” Reference Epstein, Dimarco and Ellenbogen4 and “2012 ACCF/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities.” Reference Tracy, Epstein and Darbar5
Top 10 take-home messages
1. In patients with isolated sinus node dysfunction (SND), there is no minimum heart rate or maximum pause duration where permanent pacing is absolutely recommended. Establishing a temporal correlation between symptoms and bradycardia is critical in the decision as to whether permanent pacing is indicated.
2. Young patients with impaired ventricular function or abnormal cardiovascular physiology may be symptomatic due to sinus bradycardia or the loss of atrioventricular (AV) synchrony at heart rates that do not produce symptoms in individuals with normal cardiovascular physiology.
3. Although the average ventricular rate in newborns and infants with congenital complete atrioventricular block (CCAVB) provides an objective measure regarding the decision for pacemaker implantation, additional factors may equally influence the decision/timing of pacemaker implant. These include birth weight (size), congenital heart defects, ventricular function, and other comorbidities.
4. In patients with postoperative AV block, a period of observation for at least 7–10 days before pacemaker implantation remains advised; in select cases, earlier pacemaker implantation may be considered if AV block is not expected to resolve due to extensive injury to the cardiac conduction system.
5. Atrial pacing with antitachycardia pacing capabilities is reasonable for congenital heart disease (CHD) patients with recurrent intra-atrial reentrant tachycardia when medication and catheter ablation are not effective.
6. There is increased recognition of the need for pacemaker implantation in conditions such as Kearns-Sayre syndrome or certain neuromuscular disorders due to the unpredictable progression of conduction disease.
7. The cause of sudden cardiac arrest (SCA) remains undefined in nearly 50% of pediatric survivors. ICD implantation is recommended provided completely reversible causes have been excluded, other treatments that may be beneficial are considered, and meaningful survival is anticipated.
8. The decisions for implantation of an ICD for primary prevention in cardiac channelopathies or cardiomyopathies remain guided by limited and, at times, conflicting data. Consideration of patient-specific factors and shared decision-making are critically important.
9. In pediatric patients with nonischemic dilated cardiomyopathy (NIDCM), primary prevention ICD implantation for left ventricular ejection fraction (LVEF) ≤ 35%, in the absence of other risk factors, is not clearly supported by published data.
10. In patients with indications for implantation of a CIED, shared decision-making and patient/family-centered care are endorsed and emphasized. Treatment decisions are based on the best available evidence and patient’s preferences.
Permanent pacemakers
Introduction
The most common indications for permanent pacemaker implantation in children, adolescents, and patients with CHD are 1) symptomatic sinus bradycardia, 2) advanced second- or third-degree AV block, and 3) pacing for the prevention or termination of tachyarrhythmias. Reference Epstein, Dimarco and Ellenbogen4 Many indications for pacemaker implantation in adolescents are similar to those in adults. Reference Kusumoto, Schoenfeld and Barrett2 However, in infants and young children, there are important differences. For example, criteria for normal heart rates are an age-dependent variable; whereas a heart rate of 45 bpm is normal in an adolescent, the same rate in a newborn or infant indicates profound bradycardia. In addition, young patients with impaired ventricular function or abnormal physiology may be symptomatic due to sinus bradycardia or loss of AV synchrony at heart rates that do not produce symptoms in individuals with normal cardiovascular physiology. Reference Hernández-Madrid, Paul and Abrams6,Reference Khairy, Van Hare and Balaji7 Hence, the indications for pacemaker implantation in young patients need to be based on the correlation of symptoms with relative bradycardia rather than absolute heart rate criteria.
Significant technical challenges may complicate device and lead implantation in small patients or those with abnormalities of venous or intracardiac anatomy. Epicardial lead placement and innovative use of device technology may be needed to provide pacing or defibrillation in young patients. Furthermore, as device leads may need to be utilized for multiple decades, consideration of the potential consequences from lead failure plays a major role in implantation of pediatric devices.
Isolated sinus node dysfunction
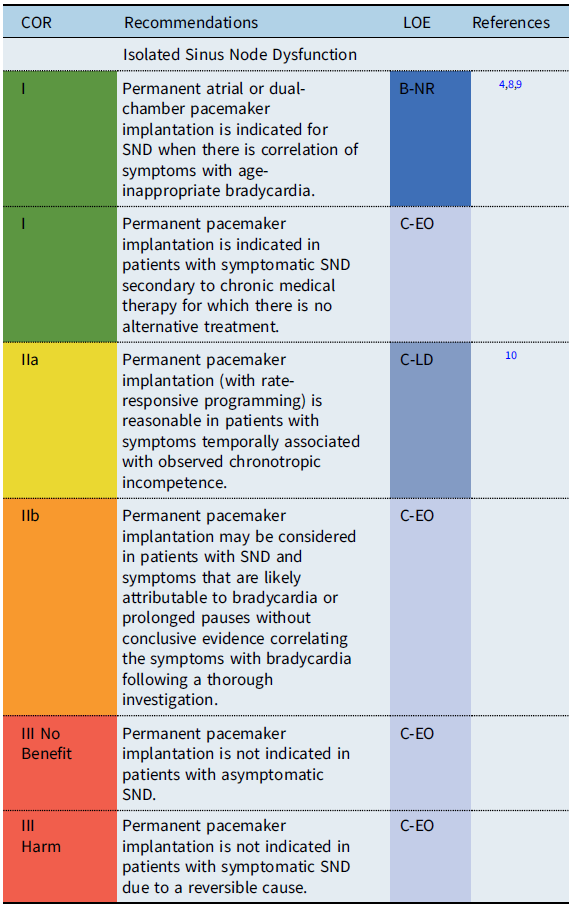
Recommendation-specific supportive text
Sinus node dysfunction (SND) refers to physiologically inappropriate atrial rates, either due to sustained bradycardia or abrupt pauses in the intrinsic cardiac rhythm. In patients with isolated sinus bradycardia without symptoms due to cerebral or systemic hypoperfusion, there is no minimum heart rate or maximum pause duration where permanent pacing is recommended. Reference Kusumoto, Schoenfeld and Barrett2 Establishing a temporal correlation between symptoms and age-related bradycardia is of paramount importance when determining whether permanent pacing is needed. In symptomatic patients with SND, atrial-based pacing is generally recommended over single chamber ventricular pacing. Reference Gillette, Wampler and Shannon11
Isolated congenital complete atrioventricular block

Recommendation-specific supportive text
The average ventricular rate in neonates and infants with isolated CCAVB provides one objective parameter regarding the decision for pacemaker implantation. However, additional factors including birth weight (size), ventricular dysfunction, and other co-morbidities may equally influence the decision. Therefore, an average heart rate of ≤50 bpm is recommended for infant pacemaker implantation when overt symptoms related to low cardiac output are not present. Beyond the first year of life, permanent pacemaker implantation is generally indicated in symptomatic patients. Natural history studies have demonstrated progressive LV dysfunction and mitral insufficiency with cardiovascular mortality in the 4th or 5th decade in CCAVB patients who did not undergo pacemaker implantation. Reference Michaëlsson and Engle17
Atrioventricular block: other considerations

Recommendation-specific supportive text
Advanced AV block diagnosed during childhood or adolescence may be congenital, related to infiltrative diseases or remain idiopathic. At times, late-onset AV block may be paroxysmal and difficult to document. Exercise testing may be useful regarding the significance of AV block. When progressive AV block occurs during exercise, conduction disturbance within the His-Purkinje system is suspected and is associated with a poor prognosis. Reference Yandrapalli, Harikrishnan, Ojo, Vuddanda and Jain19 With the exception of infiltrative or inflammatory causes, the criteria for pacemaker implantation are similar to those for CCAVB.
Postoperative atrioventricular block

Recommendation-specific supportive text
Postoperative AV block complicates 3–8% of congenital heart surgeries, with 1–3% of patients requiring permanent pacemaker implantation for persistent postoperative AV block. Reference Romer, Tabbutt and Etheridge21 A very poor prognosis has been established for CHD patients with permanent postoperative AV block who do not receive permanent pacemakers. Among patients who regain AV conduction following transient AV block, ≥85% have recovery of AV conduction by post-operative day 7 and ≥95% AV conduction by postoperative day 10. Reference Weindling, Saul and Gamble20,Reference Romer, Tabbutt and Etheridge21 Although patients who regain AV conduction have a favorable prognosis, there is a small risk of late-onset complete AV block in transient postoperative AV block patients. Reference Villain, Ouarda and Beyler22 Permanent pacemaker implantation may be considered for transient postoperative third-degree AV block that reverts to normal AV node conduction in patients with forms of CHD which may develop progressive AV block such as discordant AV connections, AV septal defects and heterotaxy syndromes.
Congenital heart disease: specific considerations

Recommendation-specific supportive text
Patients with CHD often have important structural and functional lesions which influence both the indications for pacing as well as the type of pacing lead(s) utilized. Therefore, pacemaker implantation in these patients is not an isolated procedure. Bradycardia and scar related tachycardias are common following surgery, and in the absence of high-grade AV block, atrial pacing is preferred to avoid pacing-induced ventricular dysfunction. Permanent pacemaker and/or lead implantation may be considered at the time of surgery in patients with restricted vascular access or evidence of conduction disease in heart defects with a known natural progression to advanced heart block. Decisions regarding pacemaker implantation must also consider the complexity of the patient’s anatomy, surgical repair and hemodynamic status.
Post cardiac transplantation

Recommendation-specific supportive text
Transient sinus bradycardia is common immediately after transplantation and typically resolves. In rare cases, symptomatic sinus bradycardia may persist, with at least one week allowed for recovery of sinus node function. Analysis of the United Network Organ Sharing database reported that 1% of heart transplant patients <18 years of age required a pacemaker in the acute post-transplant interval. Factors associated with need for pacing were bi-atrial anastomosis, older donor age and antiarrhythmic use. Reference El-Assaad, Al-Kindi and Oliveira28 Late onset conduction disorders (sinus node or AV node dysfunction) may be related to cardiac allograft vasculopathy or allograft rejection. Patients should be evaluated for the presence of transplant coronary artery disease, as late onset bradycardia may be the first manifestation. The role of prophylactic ICD implantation is not well established but may be considered in patients who require pacemakers.
Neuromuscular diseases and other progressive cardiac conduction diseases

Conditions include Duchenne muscular dystrophy, Becker muscular dystrophy, myotonic dystrophy type 1, Friedreich ataxia, Emery-Dreifuss muscular dystrophy, facioscapulohumeral muscular dystrophy, Barth syndrome, Kearns-Sayre syndrome, lamin A/C mutations, and desmin-related myopathies.
Recommendation-specific supportive text
Progressive cardiac conduction diseases are genetic disorders with deterioration of the conduction system either in isolation or in conjunction with other diseases such as neuromuscular and mitochondrial diseases. Reference Feingold, Mahle and Auerbach30 Variable degrees of conduction abnormalities may occur, from first-degree AV block to complete AV block with an unpredictable progression. Laminopathies caused by mutations in the LMNA genes is a wide spectrum disorder with cardiac conduction abnormalities often observed before the onset of heart failure symptoms. Reference Wahbi, Meune and Porcher32 Among the mitochondrial diseases, Kearns-Sayre syndrome, with progressive ophthalmoplegia and myopathy, has a high risk for AV block and sudden cardiac death (SCD). Reference Di Mambro, Tamborrino and Silvetti31 Currently, an HRS expert consensus statement on the evaluation and management of arrhythmic risk in neuromuscular disorders is under development. Therefore, the above recommendations may be subject to modification as newer data become available.
Neurocardiogenic syncope
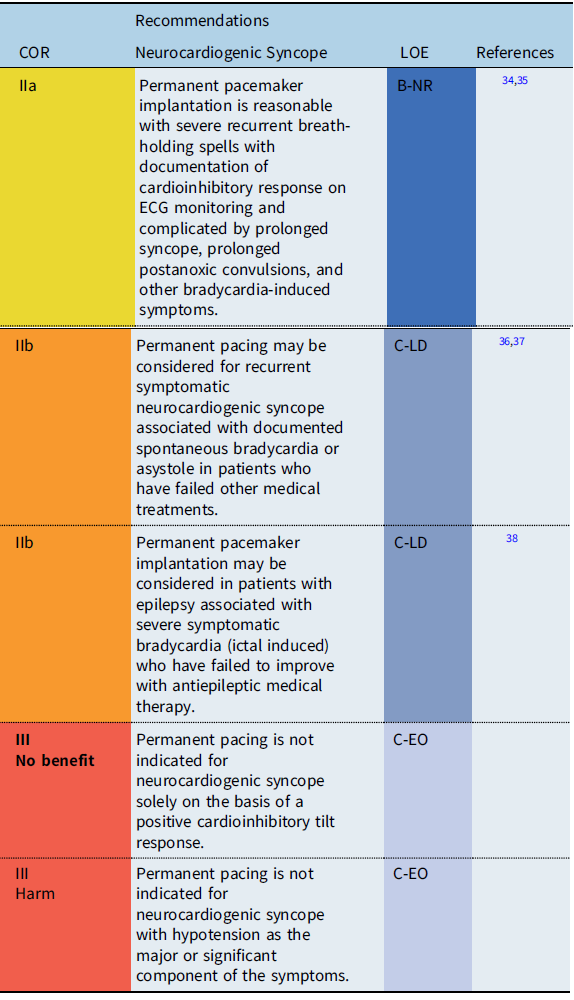
Recommendation-Specific Supportive text
In the vast majority of cases, neurocardiogenic syncope is a limited disease and pacemaker implantation is not required. However, in some patients, recurrent syncopal events may significantly impair quality of life and may result in traumatic injury, particularly when the dominant feature of reflex syncope is cardioinhibitory. Therefore, in a highly select group of patients who fail more conservative treatment options, pacemaker therapy may be useful by preventing profound bradycardia or prolonged asystole. Reference Shen, Sheldon and Benditt36 Because the efficacy of pacing depends on the clinical setting, a clear relationship between symptoms and bradycardia or asystole should be established prior to pacemaker implantation. Reference Shen, Sheldon and Benditt36,Reference Brignole, Menozzi and Moya37
Cardiac channelopathies
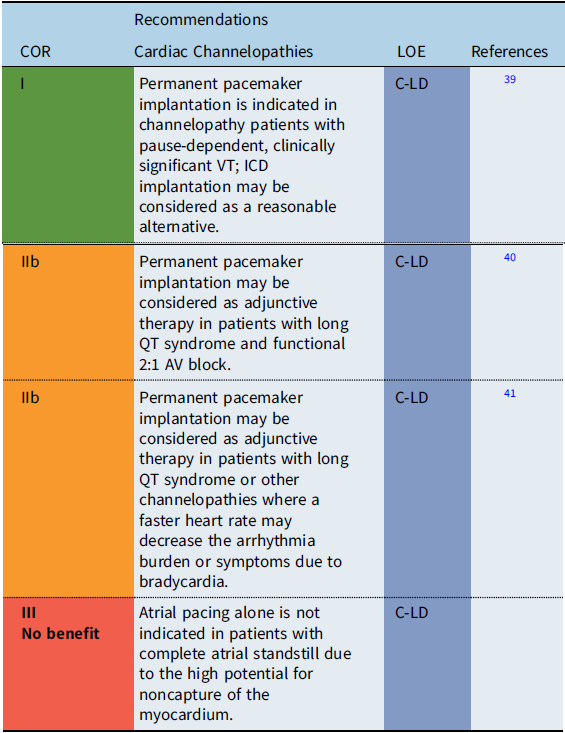
Recommendation-specific supportive text
The utility of pacing as adjunctive therapy in the various channelopathies is not well defined. In patients with bradycardia-related or pause-related initiation of ventricular tachyarrhythmias, permanent pacemaker implantation may provide benefit. Also, pacing has been reported to improve outcomes in infants with prolonged QT-related functional 2:1 AV block. Reference Aziz, Tanel and Zelster40 Limited data also suggest that atrial pacing faster than the intrinsic rate may shorten the QT interval and reduce the rate of recurrent syncopal events in select high-risk long QT syndrome (LQTS) patients. Reference Kowlgi, Giudicessi and Brake41
Inflammation/infection

Recommendation-specific supportive text
Systemic infections may cause myocardial inflammation or infiltration presenting with bradycardia or complete AV block. In most cases, there is recovery of AV conduction. However, in chronic Chagas disease, advanced heart block in Chagas is permanent and pacemaker implantation is indicated. Reference Nunes, Beaton and Acquatella43 Limited data suggest that children who develop AV block due to coronavirus 2019 (COVID-19)–related multisystem inflammatory syndrome will have recovery of normal AV conduction. Reference Dionne, Mah and Son44
Implantable cardioverter defibrillators
Introduction
The following recommendations for ICD implantation are primarily based on contemporary adult guidelines, and with some modifications, applied to younger patients. Adult ICD guidelines have been established based on a specific diagnosis or presumed risk factor for a sudden cardiac event, such as ischemia, cardiomyopathy, or genetic cardiovascular disease. Reference Epstein, Dimarco and Ellenbogen4,Reference Al-Khatib, Stevenson and Ackerman45,Reference Priori, Blomström-Lundqvist and Mazzanti46 In contrast, studies of pediatric sudden cardiac arrest (SCA) survivors demonstrate that in approximately 50% of cases, the cause of the event remains undefined despite an extensive systematic evaluation. Reference Cunningham, Roston and Franciosi47,Reference Rucinski, Winbo and Marcondes48 Furthermore, in young patients with diagnoses such as catecholaminergic polymorphic ventricular tachycardia (CPVT) or Brugada syndrome (BrS), SCA is often the initial presentation of the disease. Reference van der Werf, Lieve and Bos49,Reference Minier, Probst and Berthome50 Therefore, while development of pediatric ICD recommendations based on specific cardiovascular diagnoses would be preferable, the following recommendations for ICD implantation will begin with general considerations for young patients, followed by more nuanced recommendations for ICD implantation when a specific cause or a defined risk factor for SCA has been identified. There remain extensive “gaps” in current ICD recommendations, irrespective of age, for many of the diseases associated with SCD in pediatrics. Reference Kusumoto, Calkins and Boehmer51 The recommendations that follow are largely based on limited clinical data or expert opinion and consensus and require the application of case-specific clinical judgment and a shared decision approach.
General recommendations for implantable cardioverter defibrillator therapy

Recommendation-specific supportive text
The decisions regarding ICD implantation pediatric patients are made in the context of both the unique aspects of device implantation as well as pathogenesis of the disease, which may evolve over time. Therefore, a pediatric cardiologist should be involved in the ICD implant decision and the procedure should be performed by a cardiologist or surgeon with special training/experience in CIED implantation in the pediatric age group. ICD implantation should be a shared decision between the patient, family, and physician. This includes the physical and psychological impact of an ICD on the patient’s well-being. Furthermore, the indications for the ICD should be reconsidered at each reintervention with respect to current guidelines, especially after a period of nonuse, as discontinuation of device therapy may be considered in select cases. Reference Kini, Soufi and Deo54
ICD indications for cardiac channelopathies
Long QT syndrome

Recommendation-specific supportive text
Both phenotypic and genotypic characteristics are used to guide risk stratification when patients with LQTS may require ICD therapy. Reference Liu, Jons and Moss61 Phenotypic risk factors include the onset of symptoms at age <10 years, patients with prior SCA or those with recurrent syncope. Additional high-risk factors include a QTc ≥ 550 ms regardless of genotype, QTc ≥ 500 ms with LQT1, females with LQT2 and males with LQT3 genotype. Non-selective beta blockers are considered first line therapy and can significantly decrease subsequent cardiac events in patients, especially in those with KCNQ1 mutations. Reference Al-Khatib, Stevenson and Ackerman45 In addition, beta-blockers and cardiac sympathetic denervation without ICD may be appropriate in carefully selected patients. Reference Moss, Zareba and Hall58 Conversely, ICD implantation in an asymptomatic low-risk patient with LQTS for a positive family history of LQTS related SCD is not clearly supported by published data and requires case-specific decision making.
Catecholaminergic polymorphic ventricular tachycardia

Recommendation-specific supportive text
SCA/SCD is reported in 3 to 13% of CPVT patients. Reference van der Werf, Lieve and Bos49,Reference Roston, Jones and Hawkins62 High-risk factors include male gender, previous history of cardiac arrest, multiple genetic variants, and younger age at diagnosis. Complex ventricular ectopy on exercise testing despite optimal medical therapy is also associated with worse outcome. Treatment with nonselective beta blockers is associated with a significant reduction in adverse cardiac events, while the addition of flecainide to refractory patients may provide further benefit. In general, ICD implantation should be reserved for CPVT patients with prior SCA or with refractory ventricular arrhythmias on combination medical therapy. Reference van der Werf, Lieve and Bos49,Reference Roston, Jones and Hawkins62 Inappropriate shocks are reported in 20–30% of CPVT patients with ICDs with cardiac sympathetic denervation recommended in patients who experience recurrent ICD shocks. Reference Roston, Jones and Hawkins62 In selected patients with aborted SCA as the initial presentation of CPVT, pharmacologic therapy and/or cardiac sympathetic denervation without ICD may be considered as a possible alternative.
Brugada syndrome

Recommendation-specific supportive text
Although Brugada syndrome presents typically in the 4th to 5th decade, it may have onset during childhood, with rapid progression leading to life-threatening arrhythmias. The ICD remains the only therapy with proven efficacy for the management of ventricular arrhythmias or SCA in patients with Brugada syndrome. Reference Gonzalez Corcia and Sieira65 Adult recommendations for risk stratification including ventricular stimulation have been established, but have not been validated in pediatrics. Findings associated with high risk of ventricular arrhythmias and SCD in children include in order of relevance: the presence of symptoms (SCD or arrhythmogenic syncope), spontaneous coved type ST elevation (type I electrocardiogram [ECG] pattern), atrial arrhythmias and/or sinus node dysfunction and conduction abnormalities (AV block or intra-ventricular conduction delay). Reference Gonzalez Corcia, Sieira and Pappaert67 Conversely, implantation of an ICD is not indicated in asymptomatic patients in the absence of risk factors. Further studies are necessary to further characterize risk factors and primary prevention ICD indications for pediatric patients with Brugada syndrome.
ICD indications for cardiomyopathies
Hypertrophic cardiomyopathy
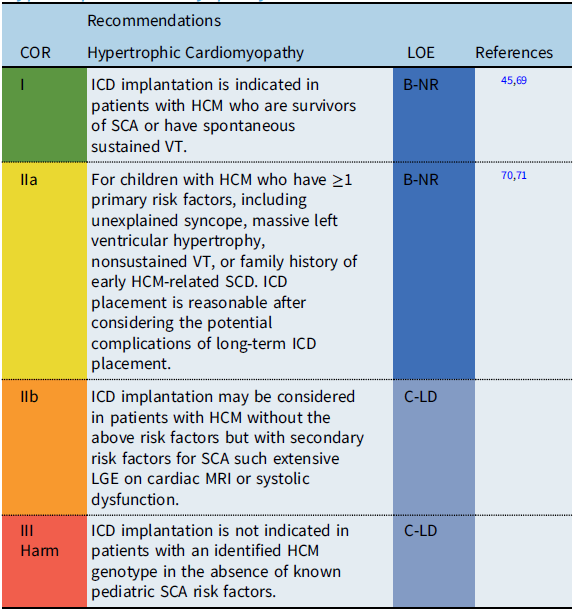
Recommendation-specific supportive text
Estimates for SCD rates in childhood hypertrophic cardiomyopathy (HCM) vary widely, with epidemiologic studies reporting rates between 1 and 7% per year. While ICDs have improved the outcomes for HCM patients resuscitated from SCD, accurate identification of risk factors to guide primary prevention ICD implantation remains a challenge, particularly given the potential progression of the disease process over time. Reference Balaji, DiLorenzo and Fish71 Adult clinical practice guidelines define high risk for SCD in HCM by the presence of ≥1 defined clinical risk factors. Reference Al-Khatib, Stevenson and Ackerman45,Reference Ommen, Mital and Burke69 However, recent pediatric studies suggest that the significance of adult risk factors may differ in children. A multi-center pediatric study reported that an LV posterior wall thickness z score ≥5 was associated with SCA, while a meta-analysis of pediatric studies reported a maximum LV wall thickness ≥30 mm or a z-score ≥6 associated with an increased risk of SCD. Reference Balaji, DiLorenzo and Fish71 The significance of secondary risk factors for SCD, such as late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (MRI) and the role of genetic testing for specific “malignant” sarcomere mutations, remains debated and requires further investigation before inclusion as specific risk factors for SCD in pediatric patients with HCM.
Arrhythmogenic cardiomyopathies

Recommendation-specific supportive text
Arrhythmogenic cardiomyopathy (ACM) encompasses a spectrum of primary myocardial disorders with the key feature of presentation with sustained arrhythmias. Reference Towbin, McKenna and Abrams72 This includes genetic disorders such as arrhythmogenic right/left ventricular cardiomyopathy, lamin A/C mutations, filamin-C, phospholamban, and cardiac amyloidosis. These entities are infrequent before puberty, and often overlap with other cardiomyopathies. Overall, SCD occurs in 2–15% of young patients with ACM. Patients presenting with SCD and/or sustained VT have a class I ICD indication. Although risk stratification data are minimal, ICD implantation is reasonable in ACM patients with hemodynamically tolerated sustained VT, syncope presumed due to ventricular arrhythmia, or an LVEF ≤ 35%. Heart transplantation and whether a wearable external defibrillator is reasonable should be considered on an individual basis for those patients with advanced heart failure.
Nonischemic dilated cardiomyopathy

Recommendation-specific supportive text
The annual incidence of SCD in pediatric patients with NIDCM is 1–5%, which is significantly less than in adult NIDCM patients. Reference Pahl, Sleeper and Canter75 Although studies have shown ICD survival benefit for secondary prevention in pediatric NIDCM, the low incidence of events has made it difficult to establish risk factors to guide recommendations for primary prevention ICD implantation. Reference Dubin, Berul and Bevilacqua73 However, in contrast to studies of adult patients with NIDCM and LVEF ≤ 35%, there is no clear evidence that ICDs implanted for primary prevention improve survival for pediatric patients with NIDCM. The phenotype of NIDCM may overlap with other cardiomyopathies resulting in variable risks of SCD. In the Sudden Death in Childhood Cardiomyopathy study, the cumulative incidence of SCD at 15 years was 5% for NIDCM compared to 23% for left ventricular noncompaction (LVNC). Reference Bharucha, Lee and Daubeney74 Myocardial dysfunction and/or a history of clinically significant arrhythmias were strongly associated with mortality in LVNC. Therefore, factors which influence implantation of a primary prevention ICD include the NIDCM etiology, the cardiomyopathy phenotype, the degree of ventricular dysfunction and the presence of cardiac arrhythmias.
ICD indications for congenital heart disease

Recommendation-specific supportive text
The association between CHD and ventricular arrhythmias is well established. First demonstrated in repaired tetralogy of Fallot, studies have identified risk factors for VT and SCD including residual cardiac defects, abnormal hemodynamics, and scar from prior interventions/surgeries. While correction of residual abnormalities or ablation of arrhythmogenic substrate may improve ventricular function or reduce symptoms, these may be inadequate to prevent subsequent VT or SCA. ICD placement may therefore be appropriate in patients with, or at high risk of, potentially life-threatening arrhythmias. The role of programmed stimulation and presence and degree of ventricular dysfunction as risk factors for SCD in CHD continues to be debated. ICD implantation in patients with CHD must consider anatomy, intracardiac shunts and vascular access. This may require non-standard approaches such as epicardial leads or subcutaneous ICDs.
Insertable cardiac monitors

Insertable cardiac monitors (ICMs) are subcutaneous devices which provide long term rhythm surveillance and provide documentation of rhythm during symptomatic events. Long-term monitoring using an ICM is recommended in highly symptomatic cases when non-invasive investigations are inconclusive, due to either infrequent events or the inability to complete a diagnostic protocol. For adults with syncope, ICM provides the most cost-effective method for establishing a diagnosis and are considered the method of choice when arrhythmogenic syncope is suspected but not proven. For bradyarrhythmias, ICM may be useful in both documentation of the bradycardia and correlation with clinical symptoms. ICM may also be useful for patients at risk for intermittent AV block in conditions such as Kearns-Sayre syndrome. Finally, ICM may be useful for occult arrhythmia detection in asymptomatic patients with potentially lethal cardiac diseases (primary arrhythmia syndromes, cardiomyopathies) and identify events that warrant need for changes in management.
CIED lead management
Lead management involves the decisions of whether or not to perform CIED lead extraction and assessment of the potential risks and benefits. Consensus statements regarding lead management and extraction were published in 2009 Reference Wilkoff, Love and Byrd85 and updated in 2017. Reference Kusumoto, Schoenfeld and Wilkoff86 The following recommendations are complementary to the above guidelines with a perspective focused on pediatrics and patients with CHD. Although major complications during lead extraction are relatively rare (3–4%), significant potential for life-threatening events exists. Reference Atallah, Erickson and Cecchin87 Therefore, lead extraction should only be performed in centers with an institutional commitment to a comprehensive program. This includes facilities, equipment, personnel, and the ability to manage all complications. Reference Bongiorni, Burri and Deharo88 A multi-disciplinary team familiar with CHD is vital to maximizing procedural safety and efficacy. There are extensive gaps in knowledge regarding lead management in children and patients with CHD. This includes limited data in the very young and the impact of repeated extractions on vascular integrity and valvular function. There is also absence of data regarding prophylactic lead extractions, as long-term prospective studies on lead abandonment versus extraction in the young do not exist.

*Recommendations based on adult lead management guidelines.Reference Wilkoff, Love and Byrd85,Reference Kusumoto, Schoenfeld and Wilkoff86
CIED follow-up and ancillary testing
CIED follow-up includes both in-person evaluation (IPE) and remote interrogation and monitoring (RIM) of pacemakers, ICDs and ICMs. The benefits of routine monitoring are well established and include both prolongation of battery life as well as early detection of CIED malfunctions, arrhythmic issues, and adverse events. At present, there are no consensus guidelines for CIED follow up or ancillary testing in the pediatric population. Therefore, the following recommendations are based on Expert Consensus Statements on CIED monitoring Reference Slotwiner, Varma and Akar90,Reference Wilkoff, Auricchio and Brugada91 with select pediatric-relevant modifications. Additional recommendations regarding ancillary testing in conjunction with IPE are also included.

Special considerations
CIEDs and magnetic resonance imaging

The 2017 MRI and Radiation Exposure in Patients with CIEDs Consensus Statement provides comprehensive recommendations for individuals with both conditional (Food and Drug Administration approved) and non-conditional transvenous devices. Reference Indik, Gimbel and Abe94 However, this document does not make specific recommendations for patients with either abandoned or epicardial CIED leads. For patients with epicardial CIED leads, as there are no MRI conditional epicardial leads, the system is considered non-conditional, even when used with a conditional device. Reference Bireley, Kovach and Morton95 Regarding abandoned leads, in vitro data suggest that epicardial leads generate more heat than transvenous leads; however, small studies of MRIs in patients with both epicardial and transvenous abandoned leads suggest that it can be done safely in the majority of cases. Reference Gakenheimer-Smith, Etheridge and Niu96 In summary, the data on MRI use in epicardial or abandoned leads are inadequate to provide specific recommendations or absolute contraindications. Acknowledging the sparsity of data, but also the importance of MRI, consideration of the risk/benefit ratio of MRI must be made on a “case by case basis.”
CIEDs and sports participation
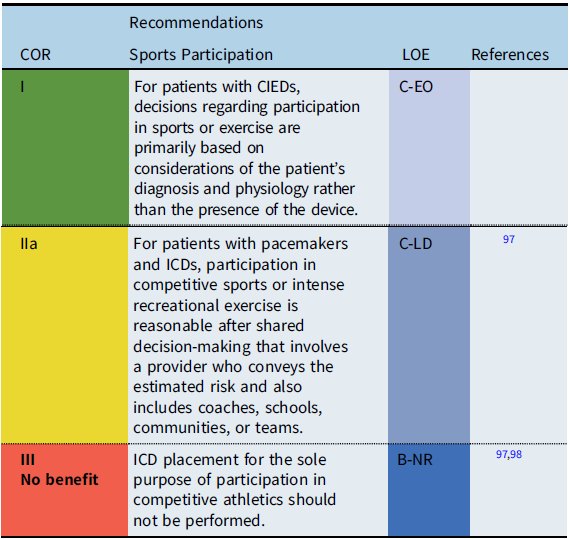
The safety of sports participation for patients with CIEDs continues to evolve. Initial guidelines recommended against competitive sports participation for patients with pacemakers or ICDs. However, subsequent surveys reported that many patients with pacemakers and ICDs had participated in sports without adverse events. Thus, the International ICD Sports Registry was initiated (2013) and reported in 2017. Reference Lampert, Olshansky and Heidbuchel97 The registry consisted of 129 patients <21 years old including high school and collegiate athletes. While shocks occurred during sports, there were no deaths, no resuscitated arrests, and no arrhythmia-related injury during sports. In addition, the rate of lead malfunction was similar to previously reported rates in unselected populations.
When counseling patients with CIEDs and families about sports participation, the decision process is ultimately patient specific, including the underlying cardiac disease and heart rhythm, the type and indication for device implant, patient age, and type of athletic activity. Shared decision making, including the patient, family, coach, school, team and other community members, should be utilized to determine the best course of pursuit for individuals with CIEDs and sporting endeavors.
Shared decision-making

Recommendation specific supportive text
The use of shared decision-making should occur prior to all CIED implantation procedures. Clinicians must estimate and clearly describe the potential benefits and risks for the patient and their family. Some decisions will be relatively straightforward; for example, the decision to implant a permanent pacemaker to treat postoperative surgical complete heart block in a patient who is pacemaker dependent will be largely uncontestable. However, other treatment decisions, such as implantation of an ICD for primary prevention of SCD, are more complex and nuanced and include choice of ICD system, device location, and personalized estimation of risk of life-threatening arrhythmia for the particular patient over time.
Knowledge gaps and future research
Critical knowledge gaps exist is several areas. Reference Goette, Auricchio and Boriani99 One example is the use of ICDs for the primary prevention of SCD. With reduction in device size and the development of novel lead configurations for implantation in smaller patients, the accurate identification of patients at increased risk remains perplexing. Several other important knowledge gaps include but are not limited to the optimal timing of pacemaker implantation after postoperative AV block, contemporary outcomes of patients with isolated CCAVB who do not undergo pacing, risk factors for pacemaker-induced cardiomyopathy, optimal age and body size for transvenous lead implantation, and safety of MRI with abandoned or epicardial leads.
With continuing technological innovations, future research is needed to develop pediatric-specific criteria for application of these new technologies. These include subcutaneous ICDs, leadless pacemakers, and conduction system pacing. Multicenter prospective registries as well as high-quality retrospective data are necessary to provide real-world evidence for new and existing CIED technologies. Future research should be conducted in collaboration with PACES, other relevant scientific societies, the U.S. Food and Drug Administration, and industry partners for development of pediatric “appropriate” CIEDs and device algorithms to specifically benefit young patients and improve their long-term outcomes.
Appendix 1. Author relationships with industry

Number value: 0 = $0; 1 = ≤ $10,000; 2 = > $10,000 to ≤ $25,000; 3 = > $25,000 to ≤ $50,000; 4 = > $50,000 to ≤ $100,000; 5 = >$100,000.
*Research and fellowship support are classed as programmatic support. Sources of programmatic support are disclosed but are not regarded as a relevant relationship with industry for writing group members or reviewers.
Appendix 2. Reviewer relationships with industry

Number value: 0 = $0; 1 = ≤$10,000; 2 = >$10,000 to ≤$25,000; 3 = >$25,000 to ≤$50,000; 4 = >$50,000 to ≤$100,000; 5 = >$100,000.
*Research and fellowship support are classed as programmatic support. Sources of programmatic support are disclosed but are not regarded as a relevant relationship with industry for writing group members or reviewers.




