Over two billion adults worldwide are overweight or obese. Reference Ng, Fleming, Robinson, Thomson, Graetz and Margono1 As prevalent as obesity is in the general population, it disproportionately affects people with bipolar disorder. In North American studies, obesity rates in people with bipolar disorder ranged from 22% to 53% compared with 9–32% in people without bipolar disorder. Reference Fagiolini, Frank, Scott, Turkin and Kupfer2–Reference Fiedorowicz, Palagummi, Forman-Hoffman, Miller del and Haynes4 European and Asian patients had lower rates of 10–41%, but these were still elevated compared with the 5–32% reported in non-psychiatric comparator groups from the same countries. Reference Sicras, Rejas, Navarro, Serrat and Blanca5–Reference Lee, Kim, Cho, Lee, Chang and Kang7 The largest study to date reported that people with bipolar disorder were two-thirds again more likely to be obese than the age-, gender- and ethnically adjusted general population. Reference Goldstein, Liu, Zivkovic, Schaffer, Chien and Blanco8 Adipose tissue is a key endocrine organ that plays a critical role in regulating metabolism and immune functioning. It does so by producing a plethora of systemically acting molecules – over 50 have been identified – including adipokines such as leptin and adiponectin, and cytokines such as tumour necrosis factor-alpha (TNF-α) and interleukins. As weight increases and adipose tissue expands, it becomes infiltrated with activated macrophages, leading to increased production of inflammatory, oxidative and thrombotic factors such as TNF-α, leptin, peroxynitrite and plasminogen activator inhibitor-1, and reduced synthesis of anti-inflammatory molecules such as interleukin-10 and adiponectin. Reference Ouchi, Parker, Lugus and Walsh9 This proinflammatory, oxidative, hypercoagulable state has been causally linked to the development of obesity-related medical conditions such as diabetes. Reference Uysal, Wiesbrock, Marino and Hotamisligil10 Converging evidence suggests that the brain is also susceptible to obesity-related damage. People who are obese have reduced grey matter volume compared with normal weight individuals, a finding that was recently demonstrated in children as young as 5. 11 Obesity-related cognitive impairments are also detectable throughout the lifespan, Reference Gunstad, Paul, Cohen, Tate, Spitznagel and Gordon12 and people who are overweight or obese in mid-life have 35% and 104% increased risks, respectively, of developing Alzheimer's disease. Reference Anstey, Cherbuin, Budge and Young13 Finally, animals assigned to experimental weight gain conditions develop pathological brain changes, Reference Colman, Anderson, Johnson, Kastman, Kosmatka and Beasley14 whereas weight loss interventions in humans lead to greater improvement in cognition than control conditions, Reference Siervo, Arnold, Wells, Tagliabue, Colantuoni and Albanese15 suggesting a causal relationship between weight and brain structure and function.
In keeping with the adverse brain effects of obesity, patients with bipolar disorder who are obese experience a more severe mood illness than those patients who are normal weight, including more frequent mood episodes, lower response rates to mood-stabilising medications, more suicide attempts and greater inter-episode cognitive impairment. Reference Fagiolini, Kupfer, Houck, Novick and Frank16–Reference Depp, Strassnig, Mausbach, Bowie, Wolyniec and Thornquist18 However, the mechanisms by which obesity has an impact on clinical outcomes in bipolar disorder are unclear. This led us, and others, to investigate whether elevated body mass index (BMI) is associated with abnormalities in brain regions relevant to bipolar disorder. Several magnetic resonance imaging (MRI) studies have since reported that patients with first-episode mania, but not healthy comparator controls, had BMI-related grey and white matter volume reductions and reduced white matter integrity in limbic brain areas implicated in bipolar disorder. Reference Bond, Ha, Lang, Su, Torres and Honer19,Reference Kuswanto, Sum, Yang, Nowinski, McIntyre and Sim20 Thus, elevated BMI is associated with unique brain changes early in bipolar disorder, such that the structural brain changes characteristic of the illness are more prominent in overweight/obese patients. The current study extends these findings by examining whether the most consistently reported neurochemical abnormality in bipolar disorder, increased glutamate/glutamine (Glx), was also more pronounced with elevated BMI. We used single-voxel proton magnetic resonance spectroscopy (1H-MRS) to investigate the relationship between BMI and hippocampal Glx in patients with bipolar disorder at recovery from their first manic episode, and a comparison group of healthy controls. We focused on the hippocampus as it is implicated in the pathophysiology of bipolar disorder and our MRI studies suggested that temporal lobe structures may be particularly susceptible to BMI-related changes. We chose to investigate Glx because it is the most extensively studied neurochemical signature in bipolar disorder, and a meta-analysis of 1H-MRS studies demonstrated that Glx activity is elevated in patients. Reference Gigante, Bond, Lafer, Lam, Young and Yatham21 Moreover, elevated glutamate levels are neurotoxic, and BMI-related Glx increases thus suggest a possible explanation for the reduced brain volumes we previously observed. We therefore hypothesised that higher BMI would be associated with greater hippocampal Glx in patients, but not healthy controls.
Method
The Systematic Treatment Optimization Program for Early Mania (STOP-EM)
STOP-EM is a comprehensive study of clinical outcomes, brain morphology and neurochemistry in patients with bipolar disorder with a first episode of mania. A detailed description of the programme was published previously. Reference Yatham, Kauer-Sant'anna, Bond, Lam and Tam22 Briefly, patients with bipolar disorder aged 14–35 who experienced their first DSM-IV-TR-defined manic episode 23 within the previous 3 months were recruited from the University of British Columbia (UBC) Hospital Mood Disorders Clinical Research Unit and affiliated sites (the bipolar group). Patients may have had a pure or mixed mania, with or without psychosis, and with or without comorbid conditions. They received treatment for bipolar disorder according to Canadian clinical practice guidelines. Reference Yatham, Kennedy, Parikh, Schaffer, Beaulieu and Alda24 Healthy comparison controls aged 14–35 were recruited from the greater Vancouver metropolitan area through print advertisements and online forums such as Craigslist (control group). The UBC Clinical Research Ethics Board approved the procedures described here, and written informed consent was obtained prior to any study activities taking place.
Clinical assessments
At enrolment, the diagnoses of bipolar disorder and first manic episode were based on a comprehensive interview with an academic research psychiatrist and confirmed with the Mini International Neuropsychiatric Interview (MINI). Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller25 The control group were administered the MINI and were enrolled if they had no personal or self-reported family history of psychiatric illness in first- or second-degree relatives. Sociodemographic and clinical data were collected using a standardised protocol. Mood and psychotic symptoms were quantified with the Young Mania Rating Scale (YMRS), Reference Young, Biggs, Ziegler and Meyer26 the Montgomery–Åsberg Depression Rating Scale (MADRS) Reference Montgomery and Åsberg27 and the Brief Psychiatric Rating Scale (BPRS). Reference Overall and Gorham28 Participants were weighed in a non-fasting state in light clothing with footwear removed. BMI was calculated as weight (kg)/height (metres2). Underweight was defined as BMI<18.50, normal weight as BMI 18.50–24.99, overweight as BMI 25.00–29.99 and obese as BMI≥30.00.
MRI/MRS protocols and data extraction
T 1-weighted magnetic resonance images were acquired with a Philips Achieva 3.0 Tesla scanner (Amsterdam, The Netherlands), typically on the same day as clinical assessment, using a three-dimensional axial inversion recovery-weighted spoiled gradient recalled sequence with the following parameters: field of view (FOV) = 25.6 cm, matrix: 256×256, isotropic voxels (1×1×1 mm3), autoshim, repetition time (TR)/echo time (TE) = autoset shortest, transmit/receive head coil, flip angle: 8°, sensitivity encoding (SENSE) = 0, and 1 mm thick contiguous 180 slices of the whole brain. Hippocampal volumes were estimated using Freesurfer v5.1 subcortical segmentation. The grey matter, white matter and cerebrospinal fluid (CSF) composition of the MRS voxel was determined with the FSL v4.1.9 FAST tool (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl/). Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens and Johansen-Berg29,Reference Woolrich, Jbabdi, Patenaude, Chappell, Makni and Behrens30
MRS signals were acquired with the Philips 3.0 T unit. T 2-weighted coronal, sagittal and axial images for anatomical parameters were first obtained. A point resolved spectroscopy (PRESS) sequence (TE = 35 ms, TR = 2000 ms) was then used to acquire data from 30 mm×15 mm×15 mm voxels in the hippocampus on both sides of the brain. Using the sagittal image, the voxel was placed with the long axis angled along the hippocampus. Its position in the medial/lateral and superior/inferior directions was adjusted based on the coronal and axial images to include the maximum amount of hippocampus and avoid CSF. Voxel placement and tissue composition are shown in online Fig. DS1(a–c). Chemical shift selective (CHESS) pulses were used to suppress the water signal during data acquisition, and water-unsuppressed signals were also obtained for eddy current correction and to reference metabolite signals. In total, 128 water-suppressed and 16 non-water-suppressed averages were acquired from each voxel.
The concentrations of Glx were extracted using LCModel v. 6.3 and normalised to the unsuppressed water spectrum. Sample 1H-MRS spectra from a patient and a healthy control, showing typical data quality, are displayed in online Fig. DS1(d–e). Glx-to-water ratios were converted to institutional absolute concentrations in millimolar units by correcting for the mean water concentration of the voxel, taking into account the fractions of grey matter, white matter and CSF in the voxel and their respective water concentrations, and water and Glx signal T 1 and T 2 relaxation during the acquisition (see online supplement DS1). Values for water and Glx T 1 and T 2 relaxation times were taken from the literature. Reference Posse, Otazo, Caprihan, Bustillo, Chen and Henry31
Data analysis and statistics
Statistical analyses were carried out using IBM SPSS Statistics for Windows 19.0. Comparisons were two-tailed, with a significance level of α = 0.05. We examined sociodemographic and clinical variables with t-tests, factorial ANOVA, χ2-tests or log-linear analysis as appropriate. For our primary analysis, we constructed linear regression models separately for the bipolar and control groups, with mean (left(L)+right(R)/2) hippocampal Glx as the dependent variable. Predictors were entered using the forced-entry method. For patients, they included BMI, age, YMRS and MADRS scores, and treatment with lithium, valproate semisodium and second-generation antipsychotics (in chlorpromazine equivalents). To ensure that BMI-related differences in the composition of the MRS voxel did not have an impact on our results, mean (L+R/2) hippocampal tissue volume in the voxel was also entered as a predictor. For the control group, predictors included BMI, age, and mean voxel hippocampal volume. The primary analysis allowed us to determine whether the continuous variable of BMI significantly predicted mean Glx, and importantly, allowed us to confirm this in patients when potentially confounding clinical and treatment variables were controlled for.
As a secondary analysis, we used factorial ANCOVA to assess the impact of diagnosis (bipolar v. control group), BMI category (normal weight v. overweight/obese) and their interaction on mean Glx. We covaried this analysis for age and mean voxel hippocampal volume. The secondary analysis enabled us to investigate whether the impact of weight on hippocampal neurochemistry differed based on diagnosis. Participants with Cramer-Rao lower bounds >25% for Glx were excluded from the primary and secondary analyses.
Results
Bipolar and control groups
In total, 57 of 71 patients and 31 of 42 healthy controls enrolled in STOP-EM had baseline BMI and neurochemical data. Participants with missing data had either declined to be weighed or to undergo the MRS procedure, or had their MRS data discarded because of artefacts. Of those with complete data, six patients and three controls were excluded for having Cramer-Rao lower bounds >25% for Glx, leaving 51 patients and 28 healthy controls for our analyses. Reflecting their early illness stage and short duration of pharmacotherapy, the bipolar group did not differ from the control group with respect to mean BMI (24.11 (s.d. = 4.12) v. 23.27 (s.d. = 2.92); F = 0.910, d.f. = 1, P = 0.343), the proportions with normal weight (66.7% v. 78.6%), overweight (23.5% v. 17.9%) or obesity (9.8% v. 3.6%) (χ2 = 1.556, d.f. = 2, P = 0.459) or mean hippocampal Glx (2.3% greater in the bipolar group; 13.52 (s.d. = 2.38) v. 13.21 (s.d. = 2.95) mmol/L, F = 0.106, d.f. = 1, P = 0.742).
BMI and hippocampal Glx
Overweight/obese and normal weight patients and controls were well-matched on sociodemographic and clinical characteristics (Tables 1 and 2), and had similar mean hippocampal volumes and amounts of hippocampal and non-hippocampal grey and white matter in the MRS voxel (online Table DS1). Measures of MRS data quality, including signal-to-noise ratio (SNR) and full-width at half maximum (FWHM), also did not differ between the groups (Table DS1).
Table 1 Sociodemographic characteristics of the bipolar disorder and control groups a

| Bipolar group (n = 51) | Control group (n = 28) | ||||
|---|---|---|---|---|---|
| Overweight/obese (n = 17) |
Normal
weight (n = 34) |
Overweight/obese (n = 6) |
Normal
weight (n = 22) |
P | |
| Age, years: mean (s.d.) | 23.35 (4.41) | 22.55 (4.43) | 22.67 (4.89) | 22.50 (3.79) | 0.794 |
| Years of education, mean (s.d.) | 14.59 (1.97) | 13.82 (2.38) | 14.83 (3.97) | 15.00 (2.39) | 0.492 |
| Gender, n (%) | 0.235 | ||||
| Male | 7 (13.7) | 18 (35.3) | 4 (14.3) | 10 (35.7) | |
| Female | 10 (19.6) | 16 (31.4) | 2 (7.1) | 12 (42.9) | |
| Ethnicity, self-reported: n (%) | 0.489 | ||||
| White | 15 (29.4) | 26 (51.1) | 4 (14.3) | 15 (53.6) | |
| Asian | 1 (1.9) | 7 (13.7) | 2 (7.1) | 7 (25.0) | |
| Other | 1 (1.9) | 1 (1.9) | 0 | 0 | |
a. The variables in the table are related to both diagnosis and body mass index (BMI). The P-values thus take both into account by showing the probability of diagnosis×BMI interactions – i.e. the probability that age, gender, etc. are distributed different y based on weight categories in the bipolar and control groups. For categorical variables (age and gender), percentages add to 100% (plus or minus rounding error) in the combined patient columns (participants who are overweight/obese patients and normal weight) and the combined healthy control group columns.
Table 2 Clinical and treatment characteristics of the bipolar group
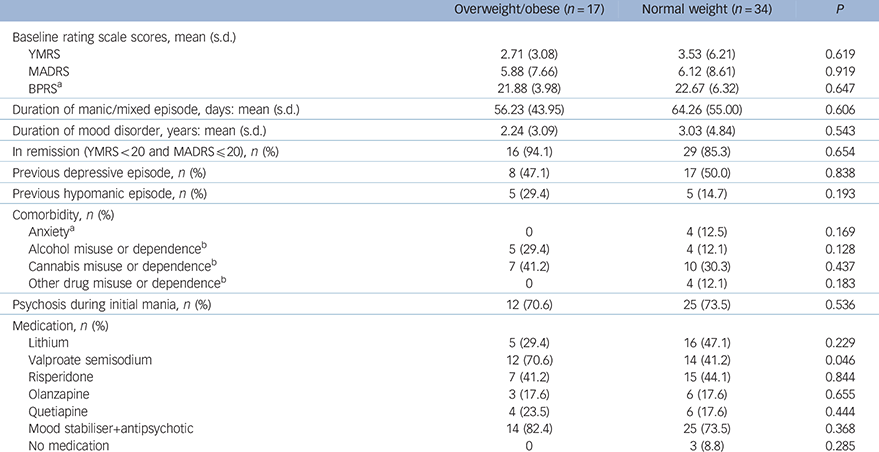
| Overweight/obese (n = 17) | Normal weight (n = 34) | P | |
|---|---|---|---|
| Baseline rating scale scores, mean (s.d.) | |||
| YMRS | 2.71 (3.08) | 3.53 (6.21) | 0.619 |
| MADRS | 5.88 (7.66) | 6.12 (8.61) | 0.919 |
| BPRS a | 21.88 (3.98) | 22.67 (6.32) | 0.647 |
| Duration of manic/mixed episode, days: mean (s.d.) | 56.23 (43.95) | 64.26 (55.00) | 0.606 |
| Duration of mood disorder, years: mean (s.d.) | 2.24 (3.09) | 3.03 (4.84) | 0.543 |
| In remission (YMRS<20 and MADRS≤20), n (%) | 16 (94.1) | 29 (85.3) | 0.654 |
| Previous depressive episode, n (%) | 8 (47.1) | 17 (50.0) | 0.838 |
| Previous hypomanic episode, n (%) | 5 (29.4) | 5 (14.7) | 0.193 |
| Comorbidity, n (%) | |||
| Anxiety a | 0 | 4 (12.5) | 0.169 |
| Alcohol misuse or dependence b | 5 (29.4) | 4 (12.1) | 0.128 |
| Cannabis misuse or dependence b | 7 (41.2) | 10 (30.3) | 0.437 |
| Other drug misuse or dependence b | 0 | 4 (12.1) | 0.183 |
| Psychosis during initial mania, n (%) | 12 (70.6) | 25 (73.5) | 0.536 |
| Medication, n (%) | |||
| Lithium | 5 (29.4) | 16 (47.1) | 0.229 |
| Valproate semisodium | 12 (70.6) | 14 (41.2) | 0.046 |
| Risperidone | 7 (41.2) | 15 (44.1) | 0.844 |
| Olanzapine | 3 (17.6) | 6 (17.6) | 0.655 |
| Quetiapine | 4 (23.5) | 6 (17.6) | 0.444 |
| Mood stabiliser+antipsychotic | 14 (82.4) | 25 (73.5) | 0.368 |
| No medication | 0 | 3 (8.8) | 0.285 |
YMRS, Young Mania Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale, BPRS, Brief Psychiatric Rating Scale.
a. n = 49; two values missing.
b. n = 50, one value missing.
Linear regression models
In the bipolar group, higher BMI significantly predicted greater Glx (α = 0.309, t = 2.036, P = 0.048) (Table 3 and Fig. 1). No other sociodemographic, clinical or treatment variables were associated with Glx. When we repeated our analysis excluding an outlying patient with a BMI of 42.19, BMI remained a significant predictor of Glx (β = 0.382, t = 2.603, P = 0.013). In the control group, there was a non-significant negative relationship between BMI and Glx (β = −0.046, t = −0.223, P = 0.826) and no other significant predictors.
Table 3 Regression analyses showing the relationship between body mass index (BMI) and mean bilateral hippocampal glutamate/glutamine (Glx) in the bipolar disorder and control groups a
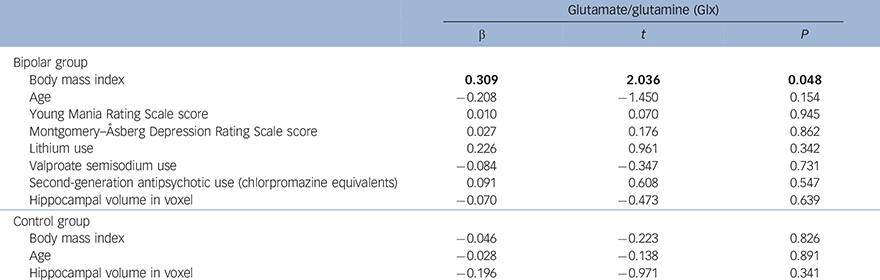
| Glutamate/glutamine (Glx) | |||
|---|---|---|---|
| β | t | P | |
| Bipolar group | |||
| Body mass index | 0.309 | 2.036 | 0.048 |
| Age | −0.208 | −1.450 | 0.154 |
| Young Mania Rating Scale score | 0.010 | 0.070 | 0.945 |
| Montgomery–Åsberg Depression Rating Scale score | 0.027 | 0.176 | 0.862 |
| Lithium use | 0.226 | 0.961 | 0.342 |
| Valproate semisodium use | −0.084 | −0.347 | 0.731 |
| Second-generation antipsychotic use (chlorpromazine equivalents) | 0.091 | 0.608 | 0.547 |
| Hippocampal volume in voxel | −0.070 | −0.473 | 0.639 |
| Control group | |||
| Body mass index | −0.046 | −0.223 | 0.826 |
| Age | −0.028 | −0.138 | 0.891 |
| Hippocampal volume in voxel | −0.196 | −0.971 | 0.341 |
a. Results in bold are significant.

Fig. 1 Relationship between body mass index (BMI) and mean bilateral hippocampal glutamate/glutamine (Glx) in the bipolar and control groups.
Bipolar group: β = 0.309, P = 0.048; control group: β = −0.046, P = 0.826.
Factorial ANCOVA
Factorial ANCOVA did not detect a main effect of either diagnosis or BMI on hippocampal neurochemistry (diagnosis: F = 1.057, d.f. = 1, P = 0.307; BMI: F = 1.904, d.f. = 1, P = 0.172). However, there was a significant BMI×diagnosis interaction (F = 4.142, d.f. = 1, P = 0.045), indicating the effect of weight on Glx differed in the patient and control groups. This remained significant when the outlying patient was excluded (F = 4.033, d.f. = 1, P = 0.048). Follow-up ANCOVAs demonstrated that in the bipolar group Glx was 17.6% greater in those who were overweight/obese than in those who were normal weight (15.02 (s.d. = 2.30) v. 12.77 (s.d. = 2.06) mmol/L, F = 13.178, d.f. = 1, P = 0.001), with a large effect size (Cohen's d) of 1.03 (Fig. 2). In contrast, in the control group Glx was 3.6% less in those who were overweight/obese than in those who were normal weight (12.82 (s.d. = 1.32) v. 13.31 (s.d. = 3.28) mmol/L, F = 0.091, d.f. = 1, P = 0.765), with a small effect size of −0.20 (Fig. 2).
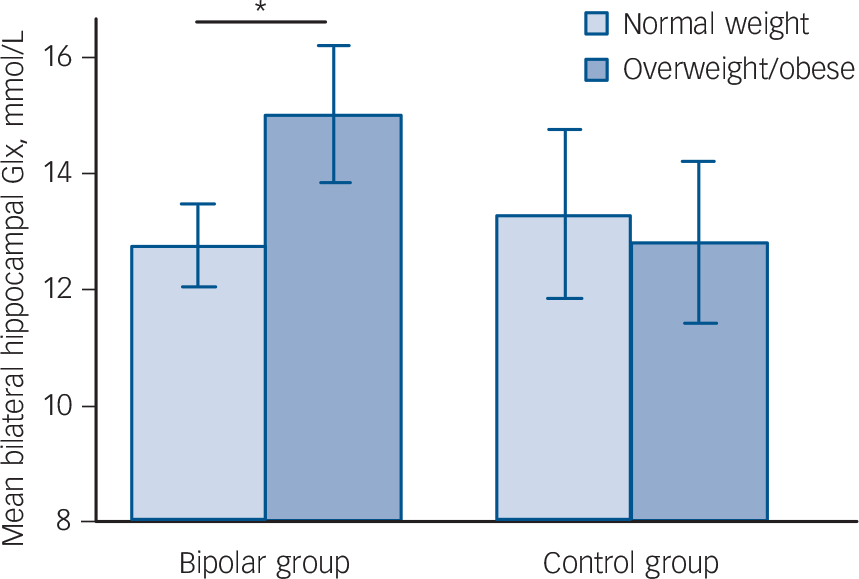
Fig. 2 Mean (95% confidence interval) bilateral hippocampal glutamate/glutamine (Glx) in participants who are overweight/obese and normal weight in the bipolar disorder and control groups.
Vertical bars indicate 95% confidence intervals. *P = 0.001.
Discussion
Main findings
Our results show a relationship between increased weight and altered neurochemistry early in the course of bipolar disorder. Higher BMI was associated with greater hippocampal Glx in patients at recovery from their first manic episode – the first time, in other words, that they could be diagnosed with bipolar disorder. Our findings are particularly noteworthy considering that patients' mean BMI was well within the normal range; that only a third were overweight/obese; and that three-quarters of overweight/obese participants were in fact overweight. This suggests that weight-related neurochemical changes affect patients with bipolar disorder across a broad range of BMIs, not just BMIs in the obese range. Together with our previous volumetric MRI studies, these results suggest that the structural and chemical brain changes typical of bipolar disorder are more pronounced with higher BMI.
The association between BMI and Glx was surprisingly robust – the difference in Glx between those in the bipolar group who were overweight/obese compared with normal weight (17.6% higher) was over seven times greater than the difference between the entire bipolar group and the entire control group (2.3% higher). In contrast, there was not a significant relationship between BMI and Glx in the control group. Whether this was expected is unknown, since no previous studies have examined the relationship between BMI and hippocampal Glx in healthy individuals, or between BMI and Glx in any brain region in healthy individuals in the same age range as ours. Although our sample included a smaller number of healthy controls than patients, resulting in reduced statistical power to detect such a relationship, we are confident that the findings in our patients are unique to bipolar disorder since our factorial ANCOVA detected a BMI×diagnosis interaction for Glx. Moreover, although non-significant, the direction of the relationship was reversed in the control group – i.e. increased BMI was associated with lower Glx.
Importance of Glx
Glutamate is the primary excitatory neurotransmitter in the central nervous system (CNS), and also plays important roles in synaptic plasticity and memory formation. After being released into the synapse, it is taken up by astrocytes and converted to glutamine, which is shuttled back to the presynaptic neuron, where glutamate is resynthesised from it. When glutamate is present in excessive amounts, it activates inotropic receptors in extra-synaptic sites and causes neurotoxicity via calcium influx, nitric oxide synthesis and free radical generation. Meta-analytic data show that increased Glx is the most consistently reported neurochemical alteration in bipolar disorder. Reference Gigante, Bond, Lafer, Lam, Young and Yatham21
Findings from other studies
Our finding of a BMI-related increase in Glx in a bipolar group with a low rate of obesity is in keeping with a large body of evidence demonstrating that the adverse health consequences of elevated BMI begin in the overweight range. One pooled analysis of 89 studies found a ‘dose–response’ relationship between BMI and hypertension, diabetes, ischaemic heart disease, stroke and various cancers, such that the prevalence of these conditions was increased in overweight people (hazard ratios (HRs) = 1.15–3.92) and further increased in obese people (HRs = 1.49–12.42). Reference Guh, Zhang, Bansback, Amarsi, Birmingham and Anis32 Two large meta-analyses, including a combined total of 2.4 million participants, reported similar dose–response relationships between BMI and all-cause mortality over 8–10 years. 33,Reference Berrington de Gonzalez, Hartge, Cerhan, Flint, Hannan and MacInnis34 The HRs for early mortality were 1.03 for mild overweight (BMI 25–27.4), 1.11 for moderate overweight (BMI 27.9–29.9), 1.25 for class I obesity (BMI 30–34.9), 1.59 for class II obesity (BMI 35–39.9), and 1.99 for class III obesity (BMI 40–49.9). Reference Berrington de Gonzalez, Hartge, Cerhan, Flint, Hannan and MacInnis34 A recent study of n = 12 664 adolescents and young adults with a similar age range and BMI distribution to our sample found that a higher BMI within the non-obese range (BMI<30) was associated with significant increases in multiple health risk biomarkers, including lipoproteins, monounsaturated and saturated fatty acids, branched-chain aromatic amino acids, inflammatory markers and adipokines. Reference Wurtz, Wang, Kangas, Richmond, Skarp and Tiainen35 These results largely held true even when the analysis was confined to normal weight individuals (BMI<25).
Possible explanations for our findings
Our MRI and MRS findings suggesting greater limbic brain changes in those in the bipolar group who were overweight/obese have potentially profound relevance for understanding the neurobiology of the illness. Traditional theories of mood dysregulation emphasise structural and functional changes in emotion-generating and -regulating brain areas, and aberrations in the monoamine neurotransmitters that modulate their activity. More recent theories also propose central roles for inflammation, mitochondrial dysfunction and alterations in adipokine levels. Bipolar disorder, like obesity, is an inflammatory condition, with the ratio of serum inflammatory to anti-inflammatory cytokines increased during manic and depressive episodes, but relatively normalised during euthymia. Reference Goldstein, Kemp, Soczynska and McIntyre36 Serum inflammation has an impact on the brain via transport mechanisms for cytokines across the blood–brain barrier and the presence of cytokine receptors on the vagus nerve and the epithelial cells of the blood–brain barrier. Reference Capuron and Miller37 Moreover, inflammation in limbic brain areas in patients with bipolar disorder has been demonstrated in post-mortem brain samples and in vivo in a PET imaging study. Reference Rao, Harry, Rapoport and Kim38,Reference Haarman, Riemersma-Van der Lek, de Groot, Ruhe, Klein and Zandstra39 Persisting obesity-related inflammation during euthymia is thus one possible mechanism to explain the more prominent neurobiological changes in patients with bipolar disorder who are obese.
Aberrations in appetite control hormones may also be relevant to the pathophysiology of bipolar disorder. Alterations in serum adipokines, such as leptin and ghrelin, are a well-known complication of obesity, Reference Ouchi, Parker, Lugus and Walsh9 and have also been reported in patients with bipolar disorder. Reference Kurt, Guler, Serteser, Cansel, Ozbulut and Altinbaş40 Receptors for these molecules are widely distributed throughout limbic brain areas such as the frontal and temporal lobes and the midbrain. Reference Harvey41,Reference Andrews42 raising the possibility that they broadly subserve brain reward circuits relevant to bipolar disorder. Furthermore, adipokines have neuroprotective effects, Reference Signore, Zhang, Weng, Gao and Chen43 promote hippocampal plasticity Reference Harvey, Solovyova and Irving44 and modulate dopamine activity, Reference Opland, Leinninger and Myers45 all functions germane to bipolar disorder. Leptin has direct effects on mitochondrial activity, Reference Porter46 which is particularly relevant to our current results since glutamate is produced in the mitochondrial matrix. Obesity-related biochemical alterations may thus lie at the nexus of inflammation, mitochondrial dysfunction and brain reward circuits, which is increasingly believed to be important to the pathophysiology of bipolar disorder.
Limitations
Our results must be interpreted in light of the limitations of our study. The most important is its naturalistic, cross-sectional design, which precludes us from determining whether there is a causal relationship between BMI and neurochemistry. Other possible explanations for our findings include a subtype of bipolar disorder characterised by both a propensity for weight gain and more pronounced neurochemical changes; and a confounding effect of medications on both weight and neurochemistry, creating an artefactual association between them. However, we note that the association between BMI and Glx was detected in our primary analysis when medication use was controlled for, and that no medications were significantly associated with Glx. Nonetheless, the question of whether elevated BMI causes adverse clinical and neurobiological outcomes in bipolar disorder is among the most pressing to arise from this line of research.
Additional limitations include that rates of overweight and obesity were low in our participants. However, our regression analyses demonstrated that increases in the continuous variable of BMI were associated with similar outcomes as dichotomised overweight/obesity, suggesting that our results in fact hold across the range of BMIs in our sample. We did not gather data on central obesity, or components of the metabolic syndrome, and we were therefore unable to examine whether these measures of adiposity were independently associated with Glx. We also did not systematically collect data on factors that might moderate the impact of obesity on neurochemistry, such as diet and exercise. Finally, our results present the measurement of the combined signal from glutamate and glutamine, preventing the interpretation of either compound individually. Nonetheless, their combined measurement can be interpreted as indicative of the overall activity of the glutamate–glutamine cycle.
Directions for further study
This is the first report to demonstrate that higher BMI is associated with greater Glx in bipolar disorder, or any psychiatric illness. Further studies are needed to confirm our findings, and to determine whether they hold true in untreated patients, those with longer illness durations and those in acute mood episodes. It would also be of interest to investigate whether the relationship between BMI and Glx is present in individuals at risk for bipolar disorder, such as those with family members with the illness. Finally, additional investigations should be carried out to characterise the impact of weight on neurochemistry in other psychiatric illnesses with high obesity rates and alterations in Glx, such as major depressive disorder and schizophrenia.
Funding
The data for this manuscript were generated from the Systematic Treatment Optimization Program for Early Mania (STOP-EM), which was supported by unrestricted grant funding from AstraZeneca Canada. The sponsor had no input into the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.








eLetters
No eLetters have been published for this article.