Insulin is a powerful pan-metabolic hormone. A major physiological effect of insulin is maintenance of whole-body glucose homeostasisReference Saltiel and Pessin1. Insulin regulates glucose homeostasis mainly by increasing the transport of glucose into the skeletal muscle. Insulin binding to the α-subunits of its receptor triggers its intrinsic tyrosine kinase activity of the β-subunits. The auto-phosphorylated insulin receptor phosphorylates the tyrosine residues of insulin receptor substrate-1 (IRS-1). IRS-1 tyrosine phosphorylation promotes the transcriptional and mitogenic activity of insulin through a mitogen-activated protein kinase cascade, while activation of the phosphatidyl inositol-3 kinase pathway is engaged with the hormone's metabolic effectsReference Pessin and Saltiel2. In muscle and fat cells, the glucose transport is mediated through the insulin-stimulated translocation of intracellular GLUT 4 isoforms to the cell surface via phosphatidyl inositol-3 kinase activation.
Insulin resistance is the salient feature of type 2 diabetes mellitus. Insulin resistance occurs when normal circulating concentrations of the hormone fail to regulate body glucose homeostasisReference Shulman3. Although many insulin-sensitizing agents are widely available, interest is evoked in dietary adjuvants that possess hypoglycaemic properties. More than 400 herbal products have been used to lower blood glucose, but only a small number of these have received scientific and medical evaluation to assess their efficiencyReference Sathishseker and Subramanian4. Among them, Momordica charantia, commonly known as bitter gourd (BG), bitter melon and karela, belonging to the Curcurbitaceae family has been a popular herbal recourse. It is a climber plant, cultivated throughout southern Asia and its fruits are available throughout the year. BG has received widespread attention in the scientific community due to its beneficial effects, including anti-diabetic, anti-cancer and anti-inflammatory effects in laboratory studiesReference Grover and Yadav5.
BG has been used to treat diabetes since the 16th century. Although various parts (roots, stems, leaves and fruits) of BG are used traditionally, studies have shown that the fruit extract of BG possesses potent hypoglycaemic propertiesReference Yibchok-Anun, Adisakwattana, Yao, Sangvanich, Roengsumran and Hsu6–Reference Miura, Itoh, Iwamoto, Kato and Ishida7, while the seeds and other parts have little or no effectsReference Ali, Khan, Mamun, Mosihuzzaman, Nahar, Nur-e-Alam and Rokeya8. Hypoglycaemic effects of BG fruit extracts have been demonstrated in various animal models of insulin resistanceReference Ahmed, Lakhani, Gillett, John and Raza9–Reference Miura, Itoh, Iwamoto, Kato, Kawai, Park and Suzuki10. Recent in vitro studies in C2C12 myocytes and 3T3-L1 adipocytes demonstrated the role of BG extract in improving insulin-stimulated glucose uptakeReference Yibchok-Anun, Adisakwattana, Yao, Sangvanich, Roengsumran and Hsu6, Reference Roffey, Atwal, Johns and Kubow11. Even though the insulin-sensitizing property of BG has been noted, the molecular mechanisms by which BG improves insulin sensitivity are not clear. To the best of our knowledge the effect of BG on insulin signalling has not been studied. In view of the above, this study was designed to explore the effect of crude BG fruit extract on insulin sensitivity and insulin signalling in high-fat diet (HFD) induced insulin-resistant rats, which mimic the obesity/dyslipidemia-induced insulin resistance in human subjects.
Materials and methods
Materials
Insulin receptor β-subunit, IRS-1 and phosphotyrosine antibodies were purchased from Cell Signalling Technology, Inc. (Danvers, MA, USA). Protein-A agarose was purchased from Bangalore Genei (Bangalore, India). Human recombinant insulin and all other chemicals were purchased from Sigma Chemicals (St.Louis, MO, USA).
Animals
Male Wistar rats weighing 150–200 g were housed at 22 ± 2°C with a 12:12 h dark–light schedule. All the experimental protocols used in this study were approved by the Institute Animal Experimentation Ethics Committee. Animals were randomly assigned to three groups. The control group was maintained on normal rodent chow for 10 weeks. The experimental groups were treated with a purified HFD for 10 weeks (HFD group) or with a HFD for 10 weeks supplemented with BG extract for the last two weeks of high-fat feeding (HFD+BG group). Each group consisted of eight animals. The semi-purified HFD was prepared as described previouslyReference Storlien, James, Burleigh, Chisholm and Kraegen12, with 59 % of total calories derived from fat, 21 % from protein and 20 % from carbohydrate. The energy content of the HFD was 21·84 kJ/g (5·2 kcal/g), whereas that of the chow diet was 13·86 kJ/g (3·3 kcal/g). The rats were provided with the respective diet and water ad libitum.
Preparation of bitter gourd fruit extract
The crude BG fruit extract was prepared as described previouslyReference Leatherdale, Panesar, Singh, Atkins, Bailey and Bignell13. Fresh BG fruits purchased from the local market were washed thoroughly with tap water, cut open and the seeds were removed. The washed fruits were cut into small pieces and then crushed with pestle and mortar, followed by the collection of juice through muslin to remove the debris. The yield was found to be about 15 ml/100 g BG. The juice was administered to the animals once daily in the morning through oral gavage at a dose of 10 ml/kg per d.
Intraperitoneal glucose tolerance test
An intraperitoneal glucose tolerance test was done at the baseline, after 8 weeks and after 10 weeks of HFD treatment as described previouslyReference Minsheng, Nicky, Jongsoon, Lone, Zhi-Wei, Micheal and Shoelson14. After an overnight fast, rats were injected intraperitonealy with glucose (2·0 g/kg body weight) and blood samples were collected at different time intervals to estimate plasma glucose. Area under the curve of each group was calculated using NCCS software.
Intraperitoneal insulin tolerance test
Intraperitoneal insulin tolerance test was done at the baseline, after 8 weeks and after 10 weeks of HFD treatment as described previouslyReference Minsheng, Nicky, Jongsoon, Lone, Zhi-Wei, Micheal and Shoelson14. After 6 h fasting, rats were injected intraperitonealy with 2·0 U insulin/kg and blood samples were collected at different time intervals to estimate plasma glucose. Area under the curve of each group was calculated using NCCS software.
Analysis of plasma metabolic profile
After 10 weeks, glucose, TAG and total cholesterol were estimated in overnight fasting plasma samples using standard reagent kits in 550 Express plus autoanalyser (Bayers Diagnostics, New York, USA). Overnight fasting plasma insulin was estimated using rat insulin ELISA kit (Boehringer, Mannheim, Germany).
Insulin stimulation
At the end of the 10th week animals were sacrificed with and without insulin stimulation. Insulin stimulation was performed by intraperitoneal injection of 15U human recombinant insulin/kg. After a wait of 30 min for the maximum effect of insulin to occurReference Youngren, John and Barnard15, the animals were sacrificed and the gastrocnemius muscle was removed, frozen in liquid N2 and stored at − 70°C for subsequent immunoblotting analysis.
Preparation of muscle homogenate and Western blotting
Muscle homogenates were prepared in homogenization buffer (50 mm Tris-HCl pH 7·4, 1 % Nonidet P-40, 0·25 % sodium deoxycholate, 150 mm NaCl, 1 mm sodium vandate, 1 mm phenyl methyl sulfonyl fluoride, 1 mm aprotinin, 1 mm leupeptin, 0·5 μg okadaic acid/ml) as described previouslyReference Saad, Araki, Miralpeix, Rothenberg, White and Kohn16. Homogenates were centrifuged at 4°C at 12 000 g for 15 min, supernatant collected and protein content was estimated by the method of BradfordReference Bradford17. Muscle homogenates (100 μg protein) were resolved by 8·0 % sodium dodecyl sulphate PAGE, electrotransferred onto nitrocellulose membrane, and immunoblotted for insulin receptor and IRS-1 with specific antibodies. Protein bands were visualized by an enhanced chemiluminescence method using ECL-kit (Amersham Pharmacia Biotech, New Jersey, USA). Bands were scanned using a densitometer (Biorad, Model GS-710, Hercules, California) and quantified by Quantity 1 software (Biorad).
Immunoprecipitation and immunoblotting
Muscle homogenates (200 μg protein) were incubated overnight at 4°C with antibodies specific to insulin receptor and IRS-1 and the immune complexes were captured by adding 50 μl protein-A agarose beads. Immune complexes were pelleted at 12 000 g for 15 min at 4°C and washed three times with homogenization buffer. The immune complexes were suspended in Laemmli sample bufferReference Laemmli18 and boiled for 5 min. Protein-A agarose was removed from the denatured proteins by centrifugation at 12 000 g for 15 min at 4°C. The supernatant was resolved by 8·0 % sodium dodecyl sulphate PAGE and electrotransferred onto nitrocellulose membrane. Proteins were probed with antibodies specific to phosphotyrosine residues and immunoblot was stripped of bound antibodies and then reprobed with antibody specific to insulin receptor and IRS-1 to normalize the tyrosine phosphorylation with corresponding proteins. Protein band detection and quantification were performed as mentioned earlier.
Statistical analysis
Results were expressed as means with their standard errors. Statistical comparison of means among individual groups was done using one-way ANOVA followed by Tukey post hoc test. A value of P < 0·05 was considered significant.
Results
Effect of bitter gourd on glucose tolerance and insulin sensitivity
Figs. 1 and 2 show the effect of HFD and BG treatment on glucose tolerance and insulin sensitivity in male Wistar rats. High-fat feeding shows impaired glucose tolerance and decreased insulin sensitivity. BG treatment for 2 weeks significantly improved the glucose tolerance and insulin sensitivity in rats fed with HFD.
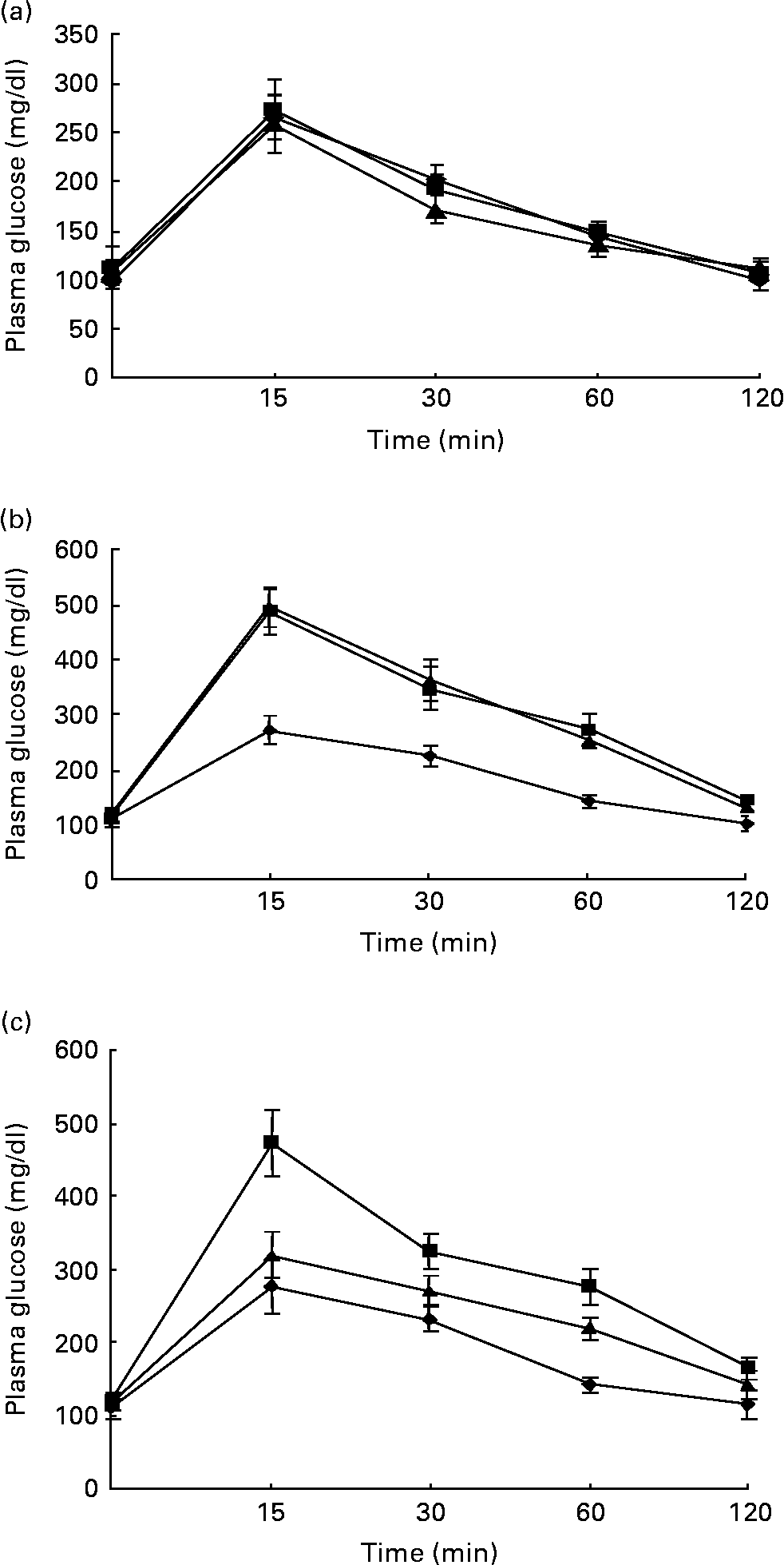
Fig. 1 Intraperitoneal glucose tolerance test on rats from experimental dietary groups (♦, control, n 8; ■, high-fat diet (HFD), n 8 and ▲, HFD + bitter gourd (BG) extract, n 8) at (a) baseline, (b) 8 weeks and (c) 10 weeks. After an overnight fast, rats were injected with glucose intraperitonealy (2·0 g/kg body weight) and blood samples were taken at different time intervals to estimate plasma glucose. Area under the curve (AUC) of different groups was calculated using NCCS software. Data are expressed as means with their standard errors indicated by vertical bars. Statistical comparisons among the individual groups were evaluated by using one-way ANOVA followed by Tukey's post hoc test. The AUC for the control, HFD and HFD+BG groups at baseline were 10655 (se 663), 10864 (se 427) and 10144 (se 714) mg/dl × min, respectively. The AUC for the control, HFD and HFD+BG groups at 8 weeks were 11297 (se 761), 18664 (se 1405) and 18586 (se 1165) mg/dl × min, respectively. AUC for the HFD and HFD+BG groups at 8 weeks were significantly different from that of the control group (P < 0·05). The AUC for the control, HFD and HFD+BG groups at 10 weeks were 11315 (se 746), 18106 (se 1413) and 13922 (se 689) mg/dl × min, respectively. AUC for the HFD and HFD+BG groups at 10 weeks were significantly different from that of the control group (P < 0·05). AUC for the HFD+BG group at 10 weeks was significantly different from that of the HFD group (P < 0·05).
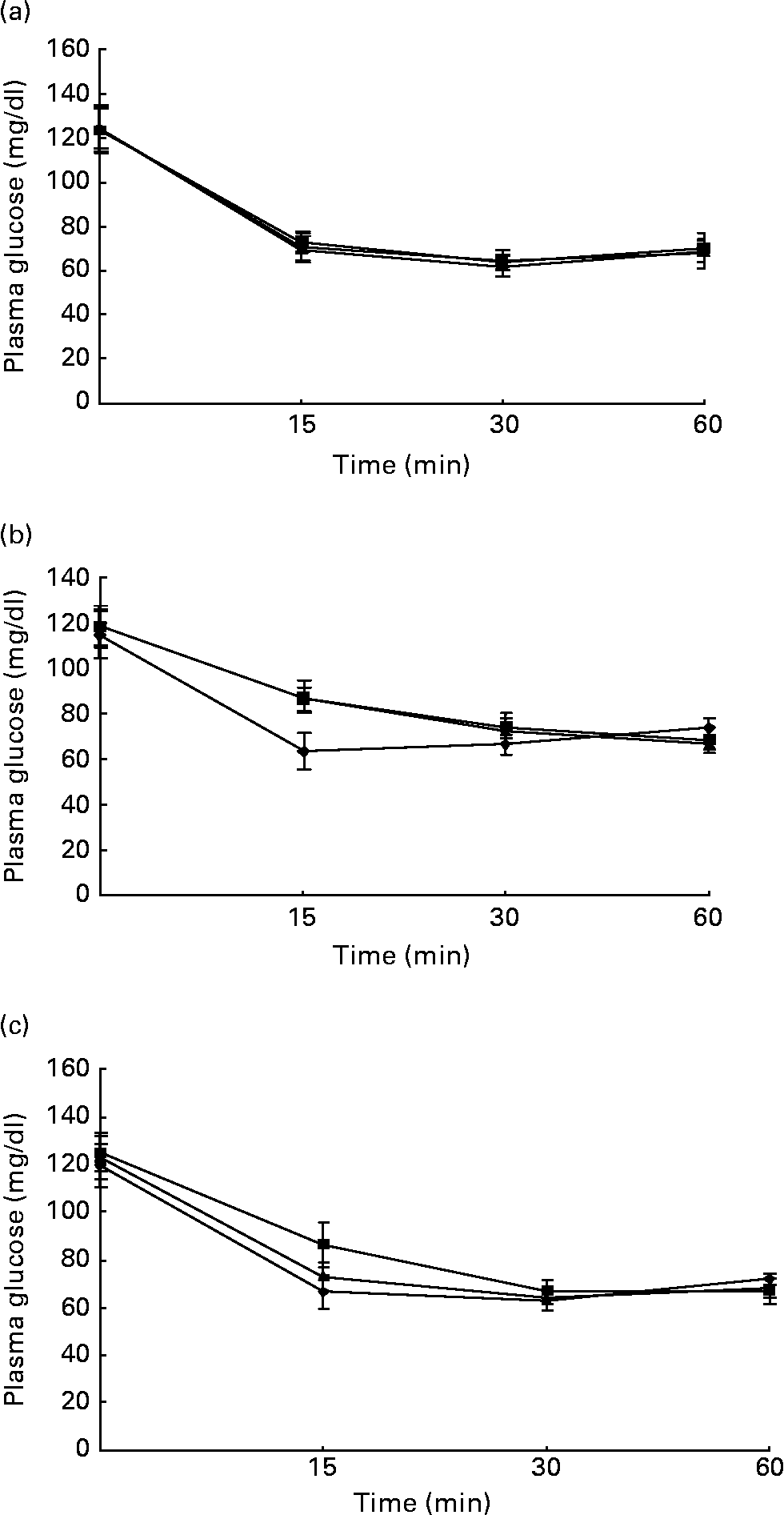
Fig. 2 Intraperitoneal insulin tolerance test on rats from experimental dietary groups (♦, control, n 8; ■, high-fat diet (HFD), n 8 and ▲, HFD + bitter gourd (BG) extract, n 8) at (a) baseline, (b) 8 weeks and (c) 10 weeks. After 6 h fast, rats were injected with insulin intraperitonealy (2·0 g/kg body weight) and blood samples were taken at different time intervals to estimate plasma glucose. Area under the curve (AUC) of different groups was calculated using NCCS software. Data are expressed as means with their standard errors indicated by vertical bars. Statistical comparisons among the individual groups were evaluated by using one-way ANOVA followed by Tukey's post hoc test. The AUC for the control, HFD and HFD+BG groups at baseline were 4232 (se 106), 4458 (se 76) and 4286 (se 95) mg/dl × min, respectively. The AUC for the control, HFD and HFD+BG groups at 8 weeks were 4315 (se 76), 5297 (se 107) and 5342 (se 121) mg/dl × min, respectively. AUC for the HFD and HFD+BG groups at 8 weeks were significantly different from that of the control group (P < 0·05). The AUC for the control, HFD and HFD+BG groups at 10 weeks were 4329 (se 92), 5676 (se 113) and 4973 (se 79) mg/dl × min, respectively. AUC for the HFD and HFD+BG groups at 10 weeks were significantly different from that of the control group (P < 0·05). AUC for the HFD+BG group at 10 weeks was significantly different from that of the HFD group (P < 0·05).
Effect of bitter gourd on plasma metabolic profile and adiposity
High-fat feeding for 10 weeks significantly increased the plasma TAG, total cholesterol, insulin, body weight and epidydimal fat weight in male Wistar rats (Table 1). BG supplementation for 2 weeks to the high-fat-fed rats significantly reduced the plasma TAG, total cholesterol, insulin and epidydimal fat weight, but there was no significant effect on plasma fasting glucose and body weight.
Table 1 Plasma metabolic profile of experimental dietary groups after 10 weeks. Body weight and epidydimal fat weight were measured in male Wistar rats. Plasma parameters were measured in overnight-fasted rats. Statistical comparison among the individual groups was evaluated by using one-way ANOVA followed by Tukey's post hoc test
(Mean values with their standard errors)
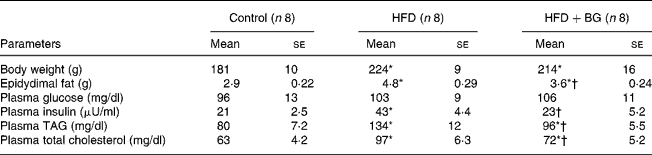
HFD, high-fat diet; BG, bitter gourd.
Mean values were significantly different from those for the control group: *P < 0·05.
Mean values were significantly different from those for the HFD group: †P < 0·05.
Effect of bitter gourd on insulin signalling
High-fat feeding and BG supplementation did not have any significant effect on the amount of insulin receptor (Fig. 3(a)), IRS-1 (Fig. 3(c)) and insulin-stimulated tyrosine phosphorylation of insulin receptor compared to control rats (Fig. 3(b)). High-fat feeding significantly reduced the insulin-stimulated IRS-1 tyrosine phosphorylation compared to control rats. BG supplementation for 2 weeks together with HFD showed a significant increase in insulin- stimulated tyrosine phosphorylation of IRS-1 compared to rats fed with HFD alone (Fig. 3(d)).
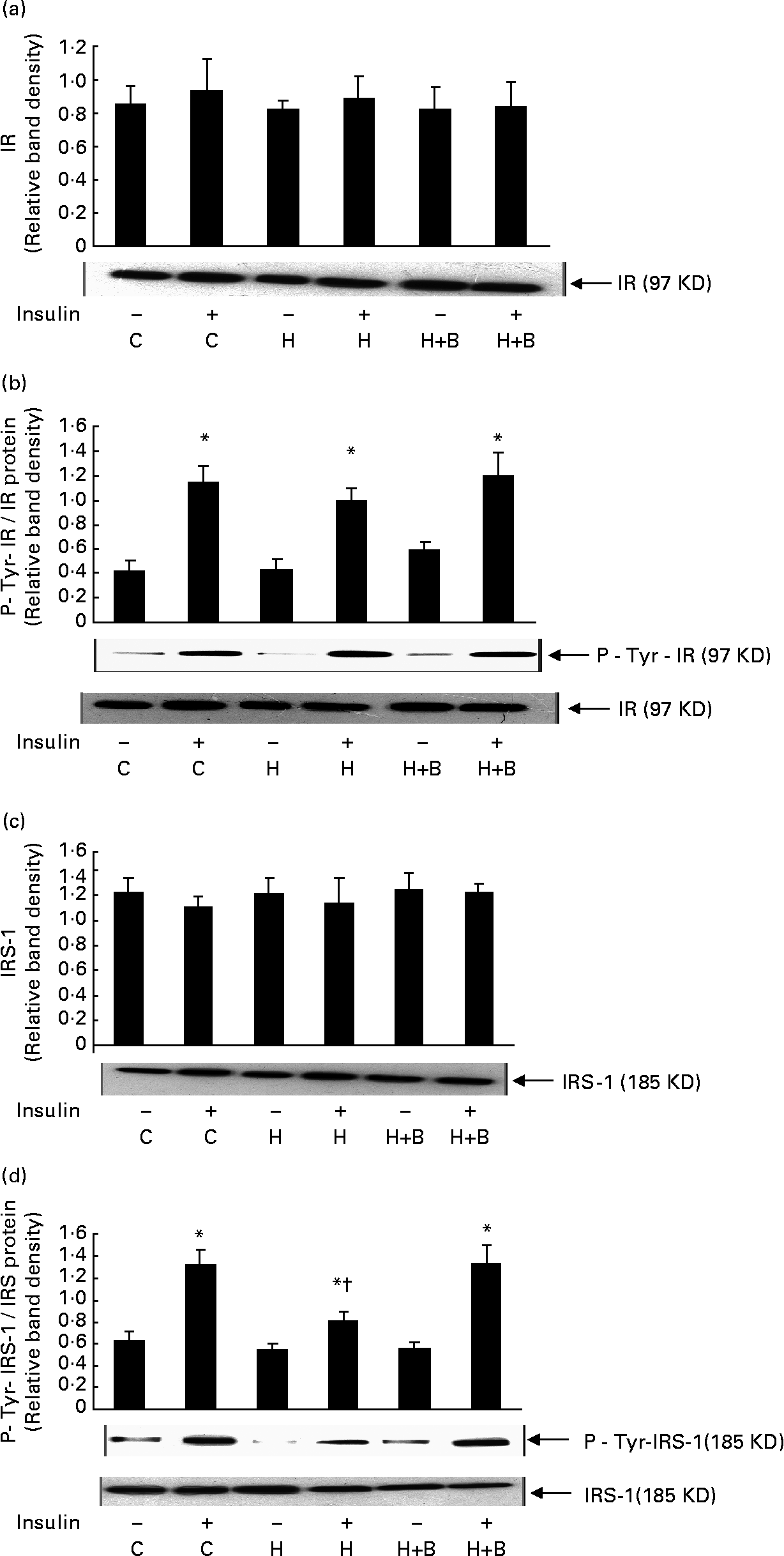
Fig. 3 Effect of bitter gourd on insulin signalling. (a), (c), Muscle homogenate (100 μg protein) was resolved by 8 % sodium dodecyl sulphate PAGE, electrotransferred onto nitrocellulose membrane, and immunoblotted with antibody specific to insulin receptor (IR) or insulin receptor subtrate-1 (IRS-1). (b), (d), Muscle homogenate (200 μg protein) was incubated overnight at 4°C with antibody specific to IR or IRS-1 and the immune complex was captured by adding 50 μl protein-A agarose beads, suspended in Laemmli sample buffer, resolved by 8·0 % sodium dodecyl sulphate PAGE and transferred to nitrocellulose membrane. Proteins were immunoblotted with antibody specific to phosphotyrosine (P-Tyr) and immunoblot was stripped of bound antibodies and then reprobed with antibody specific to IR or IRS-1. A representative immunoblot of three independent experiments is shown. Results shown are means with standard errors of three experiments. C, Control; H, high-fat diet (HFD); H+B, HFD + bitter gourd; –, baseline and +, insulin stimulation. Mean values were significantly different compared to baseline: *P < 0·001. Mean values were significantly different compared to insulin-stimulated control: †P < 0·001.
Discussion
In the present study, we have investigated the effect of BG fruit extract on high-fat-fed rats. High-fat feeding to male Wistar rats caused insulin resistance, increased the plasma TAG, cholesterol, body weight and adiposity. BG extract treatment for 2 weeks (10 ml/kg per d) along with HFD improved insulin sensitivity, decreased the plasma lipids and adiposity. BG supplementation also improved the skeletal muscle proximal insulin signalling which was impaired by HFD.
Numerous studies have shown the development of insulin resistance as a result of increased intake of dietary fat Reference Storlien, James, Burleigh, Chisholm and Kraegen12, Reference Youngren, John and Barnard15, Reference Hansen, Han, Marshall, Nolte, Chen, Mueckler and Holloszy19. An impaired ability of insulin to stimulate glucose uptake in skeletal muscle with high-fat feeding both in vivo and in vitro has been documentedReference Barnard, Berger, Ong and Kern20, Reference Barnard, Roberts, Varon and Berger21. Previous studies in high-fat-fed rats demonstrated decreased insulin-stimulated phosphatidyl inositol-3 kinase activity and GLUT4 translocationReference Oakes, Cooney, Camilleri, Chisholm and Kraegen22. These reports suggest a defect in the proximal insulin signalling pathway as a result of fat intake. In the present study, high-fat feeding and BG supplementation did not affect skeletal muscle insulin receptor content or its insulin-stimulated tyrosine phosphorylation. However, our results show no effect of HFD on IRS-1 content, but HFD decreases insulin-stimulated IRS-1 tyrosine phosphorylation. BG supplementation to high-fat-fed rats improves the insulin-stimulated IRS-1 tyrosine phosphorylation and insulin sensitivity. Studies have shown the importance of IRS-1 in insulin signallingReference White23, Reference Tamemoto, Kadowaki and Tobe24. IRS-1 knock out mice show impaired insulin signalling and severe insulin resistanceReference Yamauchi, Tobe and Tamemoto25. Recent studies demonstrate decreased insulin-stimulated IRS-1 tyrosine phosphorylation as a potential molecular mechanism for insulin resistanceReference Tanti, Gremeaux and Van Obberghen26, Reference De Fea and Roth27.
Over the years, several mechanisms have been put forward to explain the hypoglycaemic effect of BG. Similarly to glucosidase inhibitors, a fraction that competitively inhibits intestinal glucose absorption has been reportedReference Grover and Yadav28. An 11 kDa protein isolated from BG shows hypoglycaemic activityReference Yibchok-Anun, Adisakwattana, Yao, Sangvanich, Roengsumran and Hsu6. Studies have shown BG induced insulin release from isolated pancreatic islet cellsReference Xiang, Huang, Chen, Rao and Ke29. In the present study the insulin sensitizing action of BG, whether it is via regulation of insulin release or altered glucose metabolism or by its insulin-like effect is not clear. Recent studies have shown activation of PPAR by BG extractReference Chuang, Hsu, Chao, Wein, Kuo and Huang30, Reference Chao and Huang31. PPAR are ligand-dependent transcription factors that belong to the steroid hormone nuclear receptor family and control lipid and glucose metabolism in the bodyReference Beatrice and Walter32. PPARγ is mainly expressed in adipocytes and mediates their differentiationReference Kersten, Desvergne and Wahli33. The thiazolidinediones are PPARγ agonists, improve insulin signalling and insulin sensitivityReference Spiegelmen34. PPARα is widely expressed in liver, muscle and kidneyReference Ji Ming, Patric and Miguel35 and regulates expression of genes promoting fatty acid oxidationReference Forman, Chen and Evans36, Reference Guerre-Millo, Gervois and Raspe37. The fibrate class PPARα agonists are effective drugs in clinical use to reduce circulating lipidsReference Chao and Huang31, Reference Ji Ming, Patric and Miguel35–Reference Guerre-Millo, Gervois and Raspe37. In the present study the insulin sensitizing and hypolipidaemic property of BG might be due to the activation of PPARγ and PPARα. Recent reports show high-fat induced activation of serine kinases, which can down regulate IRS-1 tyrosine phosphorylation through increased IRS-1 serine phosphorylationReference Werner, Lee, Hansen, Yuan and Shoelson38, Reference Kim, Kim and Fillmore39. Increased IRS-1 serine phosphorylation impairs its interaction with the juxtamembrane domain of insulin receptor and may thus render it a poorer substrate for insulin receptor tyrosine kinaseReference Paz, Hemi, LeRoith, Karasik, Elhanany, Kanety and Zick40. Several studies show that agents which inhibit serine kinases, improve IRS-1 tyrosine phosphorylation and insulin sensitivityReference Yuan, Konstantopoulos, Lee, Hansen, Li, Karin and Shoelson41, Reference Sykiotis and Papavassiliou42. Another possible explanation, by which BG improves IRS-1 tyrosine phosphorylation may be by preventing or inhibiting the activation of serine kinases. Further studies are clearly warranted to firmly establish these links.
Conclusion
We found high-fat feeding to rats causes insulin resistance and impaired insulin signalling. Treatment with BG for two weeks significantly improved insulin signalling and insulin sensitivity in high-fat-fed rats. Identification of potential molecule(s) present in BG and its molecular basis of improving insulin signalling in insulin resistance might lead to the discovery of novel therapeutic targets and agents to prevent, reverse or delay the onset of insulin resistance.






