Depression and obesity are leading causes of disease burden and disability, as well as major public health concerns worldwide. Reference Mathers and Loncar1 Both conditions are highly prevalent and major risk factors for chronic physical diseases such as type 2 diabetes, cardiovascular disease and hypertension. Reference Farmer, Korszun, Owen, Craddock, Jones and Jones2 Shared aetiological factors, including genetic risk factors, between depression, obesity and physical disorders have been reported. Reference Farmer, Korszun, Owen, Craddock, Jones and Jones2,Reference Afari, Noonan, Goldberg, Roy-Byrne, Schur and Golnari3
The nature of the association between obesity and depression remains unclear. In a review and meta-analysis of longitudinal studies, obesity was found to increase the risk of depression, whereas depression was predictive of the development of overweight and obesity, suggesting a bidirectional relationship between depression and obesity. Reference Luppino, de Wit, Bouvy, Stijnen, Cuijpers and Penninx4
The FTO (fat mass and obesity associated) gene has consistently been associated with common forms of human obesity. Reference Loos and Bouchard5 Since the 2007 discovery, Reference Frayling, Timpson, Weedon, Zeqqini, Frealthy and Lindgren6,Reference Dina, Meyre, Gallina, Durand, Körner and Jacobson7 the role of FTO in body mass regulation and predisposition to obesity has been confirmed in multiple populations in many independent studies Reference Cotsapas, Speliotes, Hatoum, Greenawalt, Dobrin and Lum8–Reference Fawcett and Barroso10 (see reference Reference Fawcett and Barroso10 for a review), as well as in large genome-wide association studies (GWAS). Reference Scuteri, Sanna, Chen, Uda, Albai and Strait11–Reference Thorleifsson, Walters, Gudbjartsson, Steinthorsdottir, Sulem and Helgadottir13
Animal studies have shown that FTO is widely expressed in the brain, with high expression in hypothalamic nuclei, which are involved in regulating energy balance. Reference Gerken, Girard, Tung, Webby, Saudek and Hewitson14
The single-nucleotide polymorphism (SNP) rs9939609 is one of the most extensively studied FTO polymorphisms. The body mass index (BMI)-increasing ‘A’ allele has been associated with increased energy intake Reference Cecil, Tavendale, Watt, Hetherington and Palmer15 and diminished satiety, Reference Wardle, Carnell, Haworth, Farooqi, O'Rahilly and Plomin16 also implicating FTO in appetite regulation. Furthermore, the FTO gene, BMI and depression have been associated with structural brain differences in humans. Reference Cole, Boyle, Simmons, Cohen-Woods, Rivera and McGuffin17,Reference Ho, Stein, Hua, Lee, Hibar and Leow18
In 2012, we reported the first study identifying an interaction effect between FTO genotype, depression and increased risk of obesity Reference Rivera, Cohen-Woods, Kapur, Breen, Ng and Butler19 in two independent samples of patients with depression and psychiatrically healthy controls, in which the effect of FTO was increased in those individuals who had experienced depression.
In the current study, we first replicate the FTO rs9939609 depression interaction in 3 independent cohorts and then combine results with the 2 original cohorts for a total combined meta-analysis of 6902 patients with depression and 6799 psychiatrically healthy controls.
Method
Samples
This meta-analysis includes data from five different studies: Radiant, PsyCoLaus, GSK, MARS and NESDA/NTR.
Original cohorts
Extended Radiant
The depressive disorder sample included 2442 individuals sourced from several studies described in detail elsewhere: the Depression Case Control (DeCC) study, Reference Cohen-Woods, Gaysina, Craddock, Farmer, Gray and Gunasinghe20 Depression Network (DeNT) study Reference Farmer, Breen, Brewster, Craddock, Gill and Korszun21,Reference McGuffin, Knight, Breen, Brewster, Boyd and Craddock22 and the Genome-Based Therapeutic Drugs for Depression (GENDEP) study. Reference Uher, Huezo-Diaz, Perroud, Smith, Rietschel and Mors23 The DeCC is a case–control study that recruited individuals from three UK sites (London, Cardiff and Birmingham). Reference Cohen-Woods, Gaysina, Craddock, Farmer, Gray and Gunasinghe20 The DeNT sibling pair linkage study includes cases of recurrent unipolar depression collected at seven European sites and one US site. Reference Farmer, Breen, Brewster, Craddock, Gill and Korszun21,Reference McGuffin, Knight, Breen, Brewster, Boyd and Craddock22 All participants in the DeCC and DeNT studies had experienced at least two episodes of major depression of at least moderate severity. The GENDEP study includes individuals with one or more episodes of depression of at least moderate severity recruited from nine European centres. Reference Uher, Huezo-Diaz, Perroud, Smith, Rietschel and Mors23
Diagnosis of major depressive disorder (MDD) was ascertained using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) interview in all three studies. Reference Wing, Babor, Brugha, Burke, Cooper and Giel24 The control sample comprised 809 controls from the UK who were screened for lifetime absence of any psychiatric disorder using a modified version of the Past History Schedule. Reference McGuffin, Katz and Aldrich25 All cases and controls were of White European ancestry.
PsyCoLaus study
The PsyCoLaus study focused on psychiatric symptoms in a population-based cohort randomly selected from the list of residents of the city of Lausanne (Switzerland) and originally assessed for cardiovascular risk factors (CoLaus study). All 35- to 66-year-old individuals of the CoLaus sample were invited to participate in the psychiatric evaluation for the PsyCoLaus substudy (see Firmann et al Reference Firmann, Mayor, Vidal, Bochud, Pécoud and Hayoz26 and Preisig et al Reference Preisig, Waeber, Vollenweider, Bovet, Rothen and Vandeleur27 for a detailed description). The PsyCoLaus sample included 1296 individuals who fulfilled lifetime criteria for MDD according to DSM-IV based on assessment using the Diagnostic Interview for Genetic Studies (DIGS). Reference Nurnberger, Blehar, Kaufmann, York-Cooler, Simpson and Harkavy-Friedman28 The control sample included 1698 PsyCoLaus participants who had never fulfilled criteria for MDD. The PsyCoLaus study has been described in more detail elsewhere. Reference Preisig, Waeber, Vollenweider, Bovet, Rothen and Vandeleur27
Replication cohorts
GSK study
Participants from this study were recruited at the Max Planck Institute of Psychiatry in Munich, Germany, and at two satellite recruiting hospitals in the Munich area (BKH Augsburg and Klinikum Ingolstadt). A total of 821 White European individuals diagnosed with recurrent MDD and 856 White European age- and gender-matched unaffected controls were included in the meta-analysis. All patients with depression were evaluated using the SCAN interview and were included in the study if they had experienced at least two moderate to severe depression episodes according to DSM-IV. Controls were excluded if anxiety and mood disorders were present using the Composite International Diagnostic Screener (CIDS). The study has been described in detail elsewhere. Reference Lucae, Salyakina, Barden, Harvey, Gagné and Labbé29
MARS study
The Munich Antidepressant Response Signature (MARS) project at the Max Planck Institute of Psychiatry in Munich, Germany (www.mars-depression.de) is a naturalistic clinical study of in-patients with a major depressive episode. Individuals with a DSM-IV diagnosis of major depressive episode or recurrent depression (n = 575) were included in this meta-analysis. Five hundred and forty-one controls randomly selected from Munich community registries and screened for the absence of a lifetime history of DSM-IV Axis I disorders were included in the analyses. All patients and controls were of White European origin. Study details have been described previously. Reference Hennings, Owashi, Binder, Horstmann, Menke and Kloiber30
NESDA/NTR studies
This sample is part of the Netherlands Study of Depression and Anxiety (NESDA) Reference Penninx, Beekman, Smit, Zitman, Nolen and Spinhoven31 and the Netherlands Twin Register (NTR). Reference Willemsen, de Geus, Bartels, van Beijsterveldt, Brooks and Estourgie-van Burk32 NESDA is a naturalistic multicentre and longitudinal cohort study designed to examine the course and consequences of individuals with depressive and anxiety disorders. Recruitment of participants was from the general population, general practices and mental health organisations.
The NTR project, in 1994, started collecting longitudinal data from twins and their families to create a resource for genetic studies on health, lifestyle and personality.
In both cohorts, similar inclusion and exclusion criteria were used to select MDD cases. The Composite Interview Diagnostic Interview (CIDI) Reference Ter Smitten, Smeets and Van der Brink33 was used to diagnose depressive disorders according to the DSM-IV criteria. The control group had no lifetime diagnosis of depression or anxiety disorders. Controls were partly confirmed by the absence of lifetime diagnoses of psychiatric disorders, and partly by repeated measures of low genetic liability for MDD (determined by factor score derived from longitudinal measures of neuroticism, anxiety and depressive symptoms). 34
Participants included in these studies were required to report western European ancestry. These studies have been previously described in more detail. Reference Penninx, Beekman, Smit, Zitman, Nolen and Spinhoven31,Reference Willemsen, de Geus, Bartels, van Beijsterveldt, Brooks and Estourgie-van Burk32
The case sample comprised 1636 individuals from NESDA and 132 from NTR. The controls were mainly from NTR (n = 2470) with 424 additional controls from NESDA.
Phenotypic data
In all studies, BMI was defined as weight in kilograms divided by height in metres squared (kg/m2). In Radiant, self-reported height and weight were obtained during the SCAN interview for cases and telephone interview for controls. The reliability of self-report of height and weight was assessed in the GENDEP data-set (n = 811) where we had also measured height and weight. The correlations for measured v. self-reported height, weight and BMI were 0.97, 0.95 and 0.95, respectively.
In the PsyCoLaus sample, weight and height were measured at the out-patient clinic at the Centre Hospitalier Universitaire Vaudois (CHUV). Reference Firmann, Mayor, Vidal, Bochud, Pécoud and Hayoz26 In the GSK and MARS studies, anthropometric measures for patients and controls were taken at the Max Planck Institute and associated study sites by trained technicians and study nurses. Reference Lucae, Salyakina, Barden, Harvey, Gagné and Labbé29,Reference Hennings, Owashi, Binder, Horstmann, Menke and Kloiber30
Weight and height were measured by medical examination at the study clinic during the visit for NESDA, Reference Penninx, Beekman, Smit, Zitman, Nolen and Spinhoven31 and during the home visit after blood sampling for NTR. Reference Willemsen, de Geus, Bartels, van Beijsterveldt, Brooks and Estourgie-van Burk32
In all studies the distribution of BMI was positively skewed. We therefore transformed the data to log10(BMI) to achieve a closer approximation to normal distribution.
Genotyping
The samples from the different studies were all genotyped with SNP arrays. If rs9939609 was not genotyped directly on the array, genotypes were imputed and best guess genotypes were used to perform the statistical analyses. For the Radiant study, we report results from a larger sample than previously reported for the UK subsample (n = 2174). Reference Rivera, Cohen-Woods, Kapur, Breen, Ng and Butler19 A thorough description of all genotyping and imputation is described for each study in more detail elsewhere. Reference Lucae, Salyakina, Barden, Harvey, Gagné and Labbé29,Reference Hennings, Owashi, Binder, Horstmann, Menke and Kloiber30,Reference Willemsen, de Geus, Bartels, van Beijsterveldt, Brooks and Estourgie-van Burk32,Reference Lewis, Ng, Butler, Cohen-Woods, Uher and Pirlo35–Reference Nivard, Mbarek, Hottenga, Smit, Jansen and Penninx37
Inclusion criteria
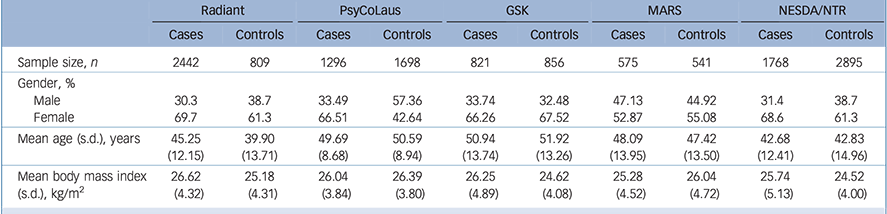
As common inclusion criteria, we looked for studies with information available on a lifetime DSM-IV diagnosis of MDD, BMI and genotype data for the rs9939609 FTO polymorphism. Homogeneous ethnicity (White European) was also required for each study to be included in the meta-analysis to reduce the risk of population stratification. Demographic and clinical characteristics of the participants from the five studies included in the meta-analysis are summarised in Table 1.
Table 1 Demographic and clinical characteristics of the participants from the studies included in the meta-analysis
| Radiant | PsyCoLaus | GSK | MARS | NESDA/NTR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| Sample size, n | 2442 | 809 | 1296 | 1698 | 821 | 856 | 575 | 541 | 1768 | 2895 |
| Gender, % | ||||||||||
| Male | 30.3 | 38.7 | 33.49 | 57.36 | 33.74 | 32.48 | 47.13 | 44.92 | 31.4 | 38.7 |
| Female | 69.7 | 61.3 | 66.51 | 42.64 | 66.26 | 67.52 | 52.87 | 55.08 | 68.6 | 61.3 |
| Mean age (s.d.), years | 45.25 (12.15) |
39.90 (13.71) |
49.69 (8.68) |
50.59 (8.94) |
50.94 (13.74) |
51.92 (13.26) |
48.09 (13.95) |
47.42 (13.50) |
42.68 (12.41) |
42.83 (14.96) |
| Mean body mass index (s.d.), kg/m2 |
26.62 (4.32) |
25.18 (4.31) |
26.04 (3.84) |
26.39 (3.80) |
26.25 (4.89) |
24.62 (4.08) |
25.28 (4.52) |
26.04 (4.72) |
25.74 (5.13) |
24.52 (4.00) |
GSK, GiaxoSmithKline study; MARS, Munich Antidepressant Response Signature project; NESDA/NTR, Netherlands Study of Depression and Anxiety/Netherlands Twin Register.
Statistical analyses
In each study, linear regression models were performed to test for association between rs9939609 polymorphism and log10(BMI) assuming an additive genetic model. Models were tested separately in the cases and controls and in the combined sample. We then tested the interaction effect between rs9939609 variant and depression status on log10(BMI). Gender and age were included as covariates in the regression analyses. Genotype-based principal components were used to control for possible population stratification within each study. Standardised beta coefficients were obtained in each study to allow direct comparison between studies. Statistical analyses were performed using PLINK v1.07. Reference Purcell, Neale, Todd-Brown, Thomas, Ferreira and Bender38
Meta-analyses of main association effects
Fixed effects meta-analyses of the association between rs9939609 variant and log10(BMI) were performed in the whole sample and in cases and controls separately, using PLINK v1.07. Reference Purcell, Neale, Todd-Brown, Thomas, Ferreira and Bender38 Heterogeneity across studies was assessed using Cochran's Q statistic and I 2 heterogeneity index.
Meta-analysis of the Interaction effect
Fixed effects and random effects meta-analyses based on inverse-variance-weighted effect size of the interaction effects were performed using METASOFT, Reference Han and Eskin39 (http://genetics.cs.ucla.edu/meta/index.html). Heterogeneity across studies was assessed using Cochran's Q statistic and I 2 heterogeneity index.
Results
Main effects of the association between FTO and BMI
Original cohorts: Extended Radiant and PsyCoLaus
In Radiant, as previously reported in a subset of the same data, Reference Rivera, Cohen-Woods, Kapur, Breen, Ng and Butler19 there was a significant association, although strengthened in significance here, between the rs9939609 A variant and log10(BMI) ((β = 0.08, P = 0.001) in the whole sample.
Linear regression analysis in PsyCoLaus also showed that the rs9939609 A variant was significantly associated with log10(BMI) ((β = 0.07, P = 0.006).
Moreover, in both studies the association was strengthened when analysing the cases alone (Radiant: (β = 0.12, P = 6.15 × 10−5; PsyCoLaus: (β = 0.12, P = 8.52 × 10−4). The analyses in the control groups alone showed no significant association with log10(BMI) (Table 2).
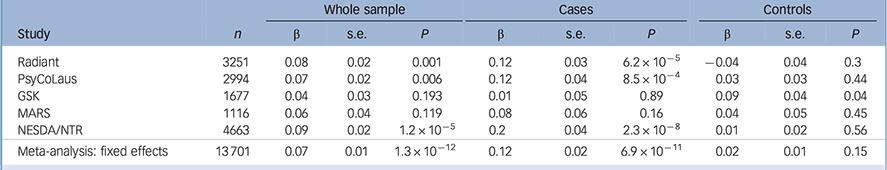
Table 2 Association results between the rs9939609 polymorphism and standardised log10(BMI) and fixed effects meta-analyses in the whole sample and in cases and controls separately
| Whole sample | Cases | Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | n | β | s.e. | P | β | s.e. | P | β | s.e. | P |
| Radiant | 3251 | 0.08 | 0.02 | 0.001 | 0.12 | 0.03 | 6.2 × 10−5 | −0.04 | 0.04 | 0.3 |
| PsyCoLaus | 2994 | 0.07 | 0.02 | 0.006 | 0.12 | 0.04 | 8.5 × 10−4 | 0.03 | 0.03 | 0.44 |
| GSK | 1677 | 0.04 | 0.03 | 0.193 | 0.01 | 0.05 | 0.89 | 0.09 | 0.04 | 0.04 |
| MARS | 1116 | 0.06 | 0.04 | 0.119 | 0.08 | 0.06 | 0.16 | 0.04 | 0.05 | 0.45 |
| NESDA/NTR | 4663 | 0.09 | 0.02 | 1.2 × 10−5 | 0.2 | 0.04 | 2.3 × 10−8 | 0.01 | 0.02 | 0.56 |
| Meta-analysis: fixed effects | 13 701 | 0.07 | 0.01 | 1.3 × 10−12 | 0.12 | 0.02 | 6.9 × 10−11 | 0.02 | 0.01 | 0.15 |
BMI, body mass index; GSK, GlaxoSmithKline study; MARS, Munich Antidepressant Response Signature project; NESDA/NTR, Netherlands Study of Depression and Anxiety/Netherlands Twin Register.
Replication cohorts: GSK, MARS and NESDA/NTR
In the GSK and MARS studies, there were no statistically significant associations between the FTO rs9939609 A variant and log10(BMI) (Table 2). In NESDA/NTR, the rs9939609 A allele was associated with log10(BMI) in the whole sample (β = 0.09, P = 1.24 × 10−5) and showed a stronger effect in the depression group alone than in the combined sample (β = 0.2, P = 2.26 × 10−8), whereas the association in controls was not significant (Table 2).
In all studies, depression status, gender, age and principal components were included as covariates when the analyses were performed in the combined sample; gender, age and principal components were included when cases and controls were analysed separately. The results for the association analyses between the rs9939609 polymorphism and log10(BMI) in the whole sample, and in cases and controls separately, for each individual study are shown in Table 2.
We also explored the association between FTO gene and depression in all the studies and found no association between the rs9939609 A risk variant and depression (data not shown).
Meta-analyses of the main effects of the association between FTO and BMI
Fixed effects meta-analysis supports a significant association between rs9939609 polymorphism and log10(BMI) in the whole sample and in cases (whole sample: (β = 0.07, P = 1.2910−12; cases: (β = 0.12, P = 6.9210−12). There was no association between rs9939609 and log10(BMI) in controls (β = 0.02, P = 0.15).
No significant heterogeneity was detected among studies (whole sample: Q = 0.7, I 2 = 0; cases: Q > 0.05, I 2 = 56.88; controls: Q = 0.21, I 2 = 31.43). The results for the fixed effects meta-analyses in each group are shown in Table 2.
Interaction between FTO, BMI and depression
Original studies: Extended Radiant and PsyCoLaus
In the analysis of updated data from the original studies, we confirmed the significant interaction effect on log10(BMI) between rs9939609 genotype and depression that we had previously published (Radiant: (β = 0.18, P = 0.002; PsyCoLaus: (β = 0.12, P = 0.034) (Table 3). The P-value for the interaction results in the Radiant extended sample is lower than previously reported (P = 0.005). Reference Rivera, Cohen-Woods, Kapur, Breen, Ng and Butler19
Table 3 Interaction results between rs9939609 risk allele and depression on log10(BMI) in the five independent studies and fixed effects, random effects and Han/Eskin model meta-analyses

| Interaction | ||||
|---|---|---|---|---|
| n | β | s.e. | P | |
| Study | ||||
| Radiant | 3251 | 0.18 | 0.06 | 0.002 |
| PsyCoLaus | 2994 | 0.12 | 0.05 | 0.034 |
| GSK | 1677 | −0.09 | 0.07 | 0.168 |
| MARS | 1116 | 0.26 | 0.15 | 0.083 |
| NESDA/NTR | 4663 | 0.19 | 0.04 | 3.2 × 10−6 |
| Meta-analysis | ||||
| Fixed effects | ||||
| Replication studies a | 7456 | 0.12 | 0.03 | 2.7 × 10−4 |
| Replication studies (no GSK) | 5779 | 0.19 | 0.04 | 6.6 × 10−7 |
| All studies | 13 701 | 0.13 | 0.03 | 3.1 × 10−7 |
| All studies (no GSK) | 12 024 | 0.17 | 0.03 | 1.1 × 10−9 |
| Random effects | ||||
| Replication studies a | 7456 | 0.10 | 0.11 | 0.35 |
| Replication studies (no GSK) | 5779 | 0.19 | 0.04 | 6.6 × 10−7 |
| All studies | 13 701 | 0.12 | 0.05 | 0.02 |
| All studies (no GSK) | 12 024 | 0.17 | 0.03 | 1.110−9 |
| Han/Eskin model | ||||
| Replication studies a | 7456 | 0.10 | 0.11 | 1.410−5 |
| Replication studies (no GSK) | 5779 | 0.19 | 0.04 | 7.910−7 |
| All studies | 13 701 | 0.12 | 0.05 | 6.910−8 |
| All studies (no GSK) | 12 024 | 0.17 | 0.03 | 1.710−9 |
| Meta-analysis | ||||
| Replication studies | 7456 | 85.3 b | 13.614 c | 0.001 d |
| Replication studies (no GSK) | 5779 | 0 | 0.221 c | 0.638 d |
| All studies | 13 701 | 72.6 b | 14.608 c | 0.006 d |
| All studies (no GSK) | 12 024 | 0 | 1.828 c | 0.609 d |
BMI, body mass index; GSK, GlaxoSmithKline study; MARS, Munich Antidepressant Response Signature project; NESDA/NTR, Netherlands Study of Depression and Anxiety/Netherlands Twin Register.
a. Replication studies: GSK, MARS and NESDA/NTR.
b. I 2 statistic.
c. Q statistic.
d. P-value of Q.
Replication studies: GSK, MARS and NESDA/NTR
There was also no significant interaction effect in GSK (β = −0.09, P = 0.168) (Table 3). The interaction effect in MARS was consistent with NESDA/NTR (β = 0.26), but was not significant (P = 0.083), likely reflecting the smaller sample size in MARS. The NESDA/NTR studies showed a significant interaction between rs9939609 genotype and depression status in relation to log10(BMI) (NESDA/NTR: (β = 0.19, P = 3.22 × 10−6), replicating our previous findings. Reference Rivera, Cohen-Woods, Kapur, Breen, Ng and Butler19
Meta-analysis of the interaction effect between rs9939609 and depression in the three replication cohorts was also consistent with our earlier finding. In the fixed effects analysis there was directional consistency and a significant association, with a greater increase in BMI for individuals with depression carrying rs9939609 alleles (β = 0.12, P = 2.72 × 10−4). Owing to the opposite but non-significant interaction effect observed in GSK, a traditional random effects meta-analysis was not significant (β = 0.10, P = 0.35). However, the Han/Eskin random effects model, Reference Han and Eskin39 optimised to detect an effect in the presence of heterogeneity, was able to detect the interaction effect (P = 1.35 × 10−7) (Table 3).
Five study meta-analysis of the interaction effect
Fixed effects meta-analysis in all five studies further confirmed the interaction between FTO, BMI and depression (β = 0.13, P = 3.1 × 10−7) (Table 3). A forest plot showing the interaction effect in each study as well as the fixed effects meta-analysis is shown in Fig. 1. The effect corresponds to an additional increase of 2.2% in BMI for subjects with depression for each risk allele, over and above the main effect of FTO. This translates to a 1.76 kg weight increase in an individual whose height and weight were 180 cm and 8 kg. Similar to meta-analysis in the three new cohorts, traditional random effects meta-analysis was only nominally significant (β = 0.12, P = 0.02), but highly significant based on the Han/Eskin model (P = 6.89 × 10−8) (Table 3).

Fig. 1 Forest plot showing interactions between FTO, depression and body mass index.
GSK, GiaxoSmithKline study; MARS, Munich Antidepressant Response Signature project; NESDA/NTR, Netherlands Study of Depression and Anxiety/Netherlands Twin Register; RE, risk estimate.
Heterogeneity and sensitivity analyses
Both the three-study replication meta-analysis and the full meta-analysis showed significant heterogeneity among studies, using both Cochran's Q statistic (P 3-way = 0.001, P 5-way = 0.006) and I 2 (3-way index 85.3%; 5-way index 72.6%). GSK accounted for the full extent of the heterogeneity. In sensitivity analyses of both the replication and full meta-analysis, removing GSK increased the significance of both fixed effects and random effects associations and eliminated all evidence of heterogeneity (Table 3).
Discussion
Main findings
The aim of this study was to confirm our previously reported interaction effect between FTO polymorphism and depression on BMI, conducting a replication meta-analysis in three new studies and a combined meta-analysis including 13 701 individuals from 5 studies. Reference Rivera, Cohen-Woods, Kapur, Breen, Ng and Butler19
The main effect meta-analysis showed a significant association between FTO and BMI in the whole sample. This association was attributed to the case group alone, with no association found in controls, replicating our previous findings. Reference Rivera, Cohen-Woods, Kapur, Breen, Ng and Butler19
In the Radiant, GSK and NESDA/NTR studies, patients with depression had higher BMI than controls. This could be attributable to a side-effect of antidepressant treatment or because individuals with depression are less physically active and/or have an increased food intake. In MARS, BMI in controls was higher than in patients with depression, and this could be because this study includes patients with untreated, first-episode depression. In the PsyCoLaus sample, BMI was not different between cases and controls. These differences possibly reflect the fact that PsyCoLaus participants were recruited from the community and were an arguably less severely affected group (18% were experiencing a current depressive episode and 82% were in remission), where only one episode was required for inclusion and only 37.5% of cases had ever received treatment with antidepressants.
In addition to this, the control samples were screened to have no history of psychiatric disorders. Previous studies investigating BMI, obesity and FTO have not taken this into account and it could further explain why there is no association observed in the control samples.
Another plausible explanation for the BMI differences across studies could be the clinical heterogeneity of depression. Evidence suggests that metabolic dysregulation may be more involved in one subtype of depression (atypical) than in another (melancholic). Reference Penninx, Milaneschi, Lamers and Vogelzangs40 Recently, it has been shown that obesity and increased appetite are more prevalent in atypical depression, whereas rates of obesity are similar or even lower compared with controls in melancholic (typical) depression. Reference Penninx, Milaneschi, Lamers and Vogelzangs40,Reference Lamers, Vogelzangs, Merikangas, de Jonge, Beekman and Penninx41 Therefore, when considering the overall depression diagnosis these differential effects are blurred.
Unfortunately, we could not include medication as a covariate in the analyses as this information was not available for all the studies. Therefore, we cannot exclude the possibility that the FTO effect that we have found is at least partly reflecting an increased susceptibility to the weight-inducing effects of medications. We also cannot exclude the possibility that psychiatric conditions that are frequently comorbid with depression play a part in modulating the effect of FTO.
The involvement of overlapping physiological mechanisms and shared genes between depression and obesity could support the hypothesis that the two disorders have shared genetic vulnerability. Reference Afari, Noonan, Goldberg, Roy-Byrne, Schur and Golnari3 In 2010, a systematic review and meta-analysis on the longitudinal relationship between depression, overweight and obesity confirmed a bidirectional association between depression and obesity. Reference Luppino, de Wit, Bouvy, Stijnen, Cuijpers and Penninx4 Several lines of evidence support the possibility of a biological pathway. Metabolic, immune–inflammatory and hypothalamic–pituitary–adrenal (HPA) axis dysregulations could be mediators of the reported association as they have a role in both depression and obesity. Reference Penninx, Milaneschi, Lamers and Vogelzangs40
Psychological factors such as body dissatisfaction, low self-esteem, stigmatisation and eating patterns should also be considered in addition to the biological mechanisms and could further contribute to the observed association between depression and obesity. Reference Green, Scott, Cross, Liao, Hallengren and Davids42,Reference Friedman, Reichmann, Costanzo, Zelli, Ashmore and Musante43 Reduced physical activity, sedentary lifestyle and/or unhealthy dietary choices as well as antidepressant treatment could be additional risk factors that induce weight gain in individuals with depression who are genetically predisposed to the disorder.
Unfortunately, measures of important confounding factors such as smoking, alcohol consumption or socioeconomic status, which might influence the association between higher BMI and MDD, were not available for all the studies.
The results from the meta-analysis of the interaction effect suggest a genetic mechanism by which individuals who have depression are at increased risk for obesity. Our results demonstrate that depression enhances the effect of FTO variants on BMI, such that individuals with depression have an additional 2.2% increase in BMI for each rs9939609 risk allele (A) compared with psychiatrically healthy controls.
Limitations and conclusions
The main limitation of this study is that the inclusion criteria for participants, study design, recruitment and sample composition vary across the studies. This could explain the significant heterogeneity found between studies.
To our knowledge this is the largest and most comprehensive study and meta-analysis investigating the interaction between FTO, BMI and depression concurrently. The overall interaction meta-analysis results suggest that having depression moderates the effect of FTO on BMI, such that the BMI-increasing effect is significantly enlarged. This meta-analysis demonstrates a modest but a consistent effect of the interaction between FTO, depression and BMI.
Although our analyses cannot infer causality or directionality about the relationship between obesity and depression, this study provides additional evidence that shared genetic factors between depression and obesity do exist. Furthermore, it is evident that FTO gene, BMI and depression influence brain structure. Reference Cole, Boyle, Simmons, Cohen-Woods, Rivera and McGuffin17,Reference Ho, Stein, Hua, Lee, Hibar and Leow18 Altogether, our results indicate that depression-related alterations in key biological processes may interact with the FTO risk allele to increase BMI or obesity risk. Future studies that include samples followed longitudinally will be crucial to better understand the nature and direction of this association.
Overall, our findings provide evidence that FTO is involved in the association between obesity and depression. Although FTO genotyping has modest implications for predicting which patients with depression are at risk of BMI-related disorders, the findings provide a useful starting point in understanding the biological mechanism involved in the association between obesity and depression. The identification of such mechanisms should in turn lead to better understanding of the development of comorbid states and eventually contribute to prevention of obesity-related disorders that are currently overrepresented among patients with depression. Reference Farmer, Korszun, Owen, Craddock, Jones and Jones2
Funding
This study was funded by the Medical Research Council, UK, and GlaxoSmithKline (). The GENDEP study was funded by a European Commission Framework 6 grant, EC Contract Ref.: LSHB-CT-2003-503428. M.R. was supported by a fellowship from the Marie Curie Research Grants Scheme (). J.R. was supported by a fellowship from the Wellcome Trust (). K.J.A. holds an Alberta Centennial Addiction and Mental Health Research Chair funded by the Government of Alberta. This work was funded in part by the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King's College London. This article presents independent research in part funded by the NIHR. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The CoLaus/PsyCoLaus was funded by four grants from the Swiss National Science Foundation (, , and ), the Faculty of Biology and Medicine of Lausanne and two grants from GlaxoSmithKline Clinical Genetics. The MARS project was funded by the Max Planck Society and in part by a research grant from the German Federal Ministry for Education and Research (BMBF) in the framework of the National Genome Research Network (NGFN2 and NGFN-Plus, ) and by the BMBF Programme ‘Molecular Diagnostics: Validation of Biomarkers for Diagnosis and Outcome in Major Depression’ (). Netherland Twin Register and Netherlands Study of Depression and Anxiety (NESDA): funding was obtained from the Netherlands Organization for Scientific Research (NWO) and MagW/ZonMW grants , , Geestkracht programme of the Netherlands Organization for Health Research and Development (), Center for Medical Systems Biology (CSMB, NWO Genomics), Genetic influences on stability and change in psychopathology from childhood to young adulthood (), , Biobanking and Biomolecular Resources Research Infrastructure (), VU University's Institute for Health and Care Research (EMGO+) and Neuroscience Campus Amsterdam (NCA); and the European Science Council (ERC Advanced, ). Part of the genotyping and analyses were funded by the Genetic Association Information Network (GAIN) of the Foundation for the National Institutes of Health, Rutgers University Cell and DNA Repository (), the Avera institute, Sioux Falls, South Dakota, USA, and the National Institutes of Health (, , Grand Opportunity grants and ).
Acknowledgements
The authors thank those who agreed to participate in the studies and the many colleagues who contributed to collection and phenotypic characterisation of the clinical samples, to genotyping and to statistical analyses.







eLetters
No eLetters have been published for this article.