Introduction
Carrion is common in all ecosystems, occurring as a resource that is patchily distributed over large spatial scales and highly ephemeral in its availability as a food resource (Janzen Reference Janzen1977, Houston Reference Houston1985). Obligate scavengers feed exclusively on carrion, a foraging strategy that is rare among terrestrial vertebrates. Indeed, only a subset of the world’s vultures and condors can be classified as true obligate scavengers (Ruxton and Houston Reference Ruxton and Houston2004) although carrion is used by a wide range of animals with carnivorous diets (DeVault et al. Reference DeVault, Rhodes and Shivik2003, Wilson and Wolkovich Reference Wilson and Wolkovich2011; but see Moreno-Opo and Margalida Reference Moreno-Opo and Margalida2013). As obligate scavengers, vultures and condors exhibit specialised traits that allow them to move across large spatial scales to locate and consume carrion before it becomes inedible or is consumed by other scavengers (Janzen Reference Janzen1977, Shivik Reference Shivik2006). They are thought to have excellent eyesight and, in Cathartes vultures, well-developed olfaction (Houston Reference Houston1986), both of which aid them in detecting carrion from considerable distances (Mundy et al. Reference Mundy, Butchart, Ledger and Piper1992, Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). In addition, their large wingspans allow for use of energetically inexpensive soaring flight to move between widely separated foraging locations (Pennycuick Reference Pennycuick1969, Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). By locating carrion quickly and efficiently, these species have been able to specialise on a food resource to a degree that is not possible for non-volant animals (DeVault et al. Reference DeVault, Rhodes and Shivik2003, Ruxton and Houston Reference Ruxton and Houston2004, Shivik Reference Shivik2006). Nevertheless, obligate scavengers are one of the most imperilled avian functional groups in the world with many species formally listed as threatened or endangered due to a variety of factors (Sekercioglu et al. Reference Sekercioglu, Daily and Ehrlich2004, Gilbert et al. Reference Gilbert, Watson, Ahmed, Asim and Johnson2007, Pain et al. Reference Pain, Bowden, Cunningham, Cuthbert, Das, Gilbert, Jakati, Jhala, Khan, Naidoo, Oaks, Parry-Jones, Prakash, Rahmani, Ranade, Sagar Baral, Senacha, Saravanan, Shah, Swan, Swarup, Taggart, Watson, Virani, Wolter and Green2008, Margalida et al. Reference Margalida, Donázar, Carrete and Sánchez-Zapata2010, Ogada et al. Reference Ogada, Keesing and Virani2012). Therefore, understanding the spatial ecology of these species, especially which factors are linked to space use, is of critical importance for conservation efforts.
Many species of condors and vultures have highly evolved social structures, and some do not hold territories to defend food resources. Instead, they move over large areas and overlap substantially with conspecifics in their use of space throughout the annual cycle, although exceptions do occur during breeding (Mundy et al. Reference Mundy, Butchart, Ledger and Piper1992, Hertl Reference Hertl1994). Thus, quantifying space use by condors and vultures necessarily focuses on measuring the home range of individuals, which we define here as the spatial boundaries within which an individual normally spends its time (see Seaman and Powell Reference Seaman and Powell1996, Powell and Mitchell Reference Powell and Mitchell2012). Home ranges are ultimately determined by resource requirements, so accurate delineation of home ranges can provide insight into how home ranges fluctuate with the availability of resources throughout the year, how critical resources are distributed across the landscape, and the extent to which resource distribution constrains the number of individuals occupying a particular area (i.e. local carrying capacity; see Margalida et al. Reference Margalida, Colomer and Sanuy2011, Margalida and Colomer Reference Margalida and Colomer2012). Furthermore, home ranges may be influenced by other factors that pertain to intrinsic characteristics of animals such as the age, sex, or social class of an individual. Thus, a comprehensive assessment of an animal’s home range requires not only delineating the spatial boundaries of the home range in which an animal spends its time, but also documenting how these boundaries change over the annual cycle and how they are linked to factors that vary among individuals.
The California Condor Gymnogyps californianus (hereafter condor) is one of the most critically endangered birds in the world (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002), with a current population of c.400 individuals of which approximately half live as free-flying individuals in the wild (USFWS unpubl. data). The condor population underwent a severe decline due to several factors (e.g. shooting and poisoning; Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002) that led to all individuals being taken into captivity by 1987. However, since the early 1990s condors have been released into the wild with increasing frequency in three distinct areas: California, Arizona, and Baja, Mexico. As an obligate scavenger and the largest North American landbird (c.8.2 kg; Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002) with a wingspan that reaches 2.8 m, condors move over huge expanses of remote, rugged landscapes as they search for carrion (Meretsky and Snyder Reference Meretsky and Snyder1992, Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). Much of what is known about condor home ranges comes from previous studies that used re-sighting and radio telemetry data to delineate the movement of individuals (Snyder and Johnson Reference Snyder and Johnson1985, Meretsky and Snyder Reference Meretsky and Snyder1992, Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002).These studies were restricted in the extent and resolution of the spatial information they provided because they relied on visual observations, radio tracking of individuals, or both, leading to limited insights regarding the size of home ranges (see Meretsky and Snyder Reference Meretsky and Snyder1992). In contrast to earlier times, recent advances in global positioning system (GPS) technology now allow researchers to track animal movements in near real-time and at unprecedented spatial and temporal resolution (Tomkiewicz et al. Reference Tomkiewicz, Fuller, Kie and Bates2010). In this study, our objective was to use high-resolution GPS satellite telemetry data to quantify condor monthly home range characteristics in southern and central California to assess how individual characteristics (age, sex, and breeding status) and factors related to endangered species recovery program efforts (rearing method, release site) may be linked to variation in monthly home range size across the annual cycle. Because this knowledge is lacking in condors, the results from this investigation will serve as an important foundation for conservation and management decisions for this critically endangered species well into the future.
Methods
Study area and species
From July 2003 to December 2010 we studied the movements of California Condors that originated from three release sites located in the range of primary concern in California according the 1984 California Condor Recovery Plan (USFWS 1996) that encompassed habitats ranging from open grassland and desert scrubland to montane coniferous forests (Figure 1). The three release sites were: (1) Hopper Mountain National Wildlife Refuge Complex (34°28′N, 118°51′W), located in south-western California and operated by the US Fish and Wildlife Service (hereafter Hopper Mountain); (2) Pinnacles National Monument (36°29′N, 121°12′W), located in inland central California and operated by the National Park Service (hereafter Pinnacles), and (3) the Big Sur release site (36°17′N, 121°50′W), located in coastal central California and operated by the Ventana Wildlife Society (hereafter Big Sur). Although two of the release sites were established in previous years, individual condors with GPS transmitters were first released at Big Sur in 2003 and at Pinnacles and Hopper Mountain in 2004. The number of condors with GPS transmitters that originated from these release sites has increased over time as the population of free-flying individuals increased, from a low of two individuals in 2003 to a high of 50 individuals in 2010.

Figure 1. Map showing the range of primary concern (hatched area) according the 1984 California Condor Recovery Plan (USFWS 1996) and release sites (filled circles) in California used in this study. Note that Bitter Creek NWR and Hopper Mountain NWR are combined for analysis because both comprise the Hopper Mountain National Wildlife Refuge Complex.
Condors exemplify one extreme along the slow-fast life history continuum. Breeding females lay a single egg clutch in a sheltered location (typically an elevated cave or tree cavity), and both sexes provide similar parental care (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). Incubation lasts nearly two months, and offspring leave the nest after 5–6 months, even though they require additional care for at least four additional months after fledging. Thus, breeding pairs typically do not raise young in successive years unless they initiate nesting early in the first year of two successive breeding seasons. Condors retain immature plumage until six years of age, which coincides with the earliest age of breeding in the wild (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). To maintain the health of free-ranging individuals and to facilitate the transition of captive-reared condors to the wild, personnel at all three release sites regularly proffered lead-free carcasses throughout the year (Walters et al. Reference Walters, Derrickson, Fry, Haig, Marzluff and Wunderle2010). Although this important and necessary management tool has the potential to influence condor movement patterns, it was of critical importance for the recovery goals of this highly imperilled species. Unfortunately, data on the quantity and quality of proffered food is lacking and unavailable for incorporation in studies of condor spatial ecology and movement. Nevertheless, proffered food was available throughout the year and at all three release sites and therefore represented part of the food resource base that was available to free-ranging condors in the California population during this study.
GPS tracking data and estimation of monthly home ranges
We used GPS transmitters (Argos/GPS PTT-100; Microwave Telemetry, Inc., Columbia, Maryland) to collect condor location data at hourly intervals from 05h00 to 20h00 PST each day. We were granted permits to capture and handle condors by appropriate institutional, state, and federal agencies, and our work was conducted in conformance with all applicable laws. Although management needs necessitated that we non-randomly assigned GPS transmitters to individuals, we attempted to assign them to different sexes and age classes in as balanced a manner as possible. Lead exposure is an important threat to free-ranging condors (Finkelstein et al. Reference Finkelstein, Doak, George, Burnett, Brandt, Church, Grantham and Smith2012, Rideout et al. Reference Rideout, Stalis, Papendick, Pessier, Puschner, Finkelstein, Smith, Johnson, Mace, Stroud, Brandt, Burnett, Parish, Petterson, Witte, Stringfield, Orr, Zuba, Wallace and Grantham2012), so individuals were regularly recaptured and blood-sampled to assess plasma lead levels. Condors with elevated lead levels were held in captivity for treatment, which led to gaps in movement data for some individuals. Therefore, we only estimated monthly home ranges of individuals for which we had a minimum of 100 GPS transmitter locations in each month, although the great majority of monthly home range estimates (1196/1378; 87%) were based on ≥ 200 GPS locations.
We estimated monthly home ranges using 99% fixed kernel density analysis (Silverman Reference Silverman1986, Horne and Garton Reference Horne and Garton2006). We initially evaluated two commonly used smoothing parameters to quantify condor monthly home ranges: the least squares cross-validation method (h lscv) and the reference method (h ref, Worton Reference Worton1989, Kernohan et al. Reference Kernohan, Gitzen, Millspaugh, Millspaugh and Marzluff2001). During our initial analysis we found that the algorithm used for h lscv failed because it was unable to minimise the mean integrated squared error function during the fixed-kernel density estimation procedure due to discretisation errors. We attempted to overcome this issue by (1) randomly shifting overlapping points by 100–300 m and (2) deleting overlapping points prior to running the algorithm. We found neither approach was successful, probably because condors are highly faithful in their use of perching and roosting sites throughout the annual cycle which leads to clustered GPS fixes at such locations. We therefore calculated monthly home range size by first estimating h ref with the Home Range Tools for ArcGIS (Rodgers et al. Reference Rodgers, Carr, Smith and Kie2005) and then using Hawth’s Analysis Tools for ArcGIS (Beyer Reference Beyer2005) to calculate 99% fixed-kernel monthly home ranges with a grid cell size of 100 m. We elected to use an ad hoc parameter (h adhoc) to choose the smallest increment of href that resulted in a contiguous 99% kernel polygon (i.e. 0.3*h ref = h adhoc) which can be used previously as an alternative method to h lscv which often fails with clustered GPS location data (Berger and Gese Reference Berger and Gese2007, Cline and Haig Reference Cline and Haig2011).
Statistical analysis
We used repeated-measures mixed linear modelling to examine the relationship between monthly home range size and age (two levels), sex (two levels), and breeding status of adults (two levels). We classified individuals as immature (comprising both juveniles [0-2 years] and sub-adults [3-5 years]) or adult (≥ 6 years) because breeding in the wild is rare for individuals before six years of age (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). We classified adults as breeding only if they inhabited a nesting site and were found to have laid an egg. We also investigated the influence of two management-based characteristics on monthly home range size: release site (three levels) and rearing method (two levels; wild vs. captive reared). We initially examined age as a continuous variable, but we do not include those results here because most age-related variation was due to differences between immature and adult birds (Rivers et al. unpubl. data). We used the PROC MIXED modeling function in SAS/STAT version 9.2 for Windows to account for the repeated nature of our measurements, with monthly home range sizes (on the logarithmic scale) as the repeated measure; age, sex, breeding status, release site, and rearing method as fixed categorical effects; and individual bird as a random effect. In our initial analysis we found that an autoregressive covariance structure outperformed a compound symmetrical covariance structure, so we retained the autoregressive covariance structure for all subsequent models and used the Kenward-Rogers method to calculate degrees of freedom for contrasts and estimates. Because of our unbalanced data, we obtained least-squares marginal means (LSMEANS) for effect sizes, with a Tukey-Kramer adjustment for all multiple comparisons. We report least squares marginal means and associated 95% confidence intervals (CIs) unless otherwise noted, with the alpha level for all tests set at P < 0.05.
Results
We collected 444,808 GPS locations from 74 unique free-ranging condors (43 males, 31 females; 43 individuals assessed during immature stage, 45 individuals assessed during adult stage), allowing us to quantify a total of 1,357 monthly home ranges. Of the monitored individuals, 53% were released from Hopper Mountain, 27% from Big Sur, and 20% from Pinnacles. The mean number of monthly home ranges estimated per individual was 18 (range: 1–72 months) and the mean number of location fixes per month across all birds was 328(range: 100–517 fixes; see Table 1).

Figure 2. Median (± 95% CI) monthly home range size of immature (filled circles) and adult California Condors (open circles) across the annual cycle.
Table 1. Summary of GPS tracking data for 74 California Condors originating from three release sites with the recent historic condor range in California.

Mean monthly home range was significantly larger for adults (56,269 ha: 95% CI 42,719–74,124 ha) than for immatures (41,308 ha: 95% CI 30,019–56,835 ha; F 1,249 = 4.41, P = 0.037), and it varied significantly across the annual cycle (F 11, 1109 = 12.00, P < 0.001; Figure 2). However, we did not detect a significant difference in mean monthly home range size between sexes (males: 43,844ha: 95% CI 32,219–59,671 ha; females 53,013ha: 95% CI 39,478–71,190ha; F 1, 248 = 1.52, P = 0.219), nor did we find evidence of a month*sex interaction (F 11,1106 = 0.95, P = 0.492), an age*sex interaction (F 1, 257 = 3.26, P = 0.072), or a month*age interaction (F 11,1112 = 1.32, P = 0.208). Breeding adults (44,033 ha: 95% CI 31,518–61,519 ha) had mean monthly home ranges that were similar in size to non-breeding adults (52,786 ha: 95% CI 40,974–68,003 ha; F 1, 263 = 1.58, P = 0.209; Figure 3), and there were no breeder*sex (F 1, 323 = 2.47, P = 0.117) or breeder*month interactions (F 11,1109 = 0.57, P = 0.857). We found no difference in mean monthly home range size between condors reared in captivity (49,966 ha: 95% CI 42,100–59,302 ha; n = 67) and wild individuals (46,518ha: 95% CI 29,074–74,436 ha; n = 7; F 1, 215 = 0.09, P = 0.769; Figure 4). However, there were large differences in mean monthly home range size among the three release sites (Hopper Mountain: 89,581ha: 95% CI 70,130–114,429 ha; Pinnacles: 49,777ha; 95% CI 35,137–70,523 ha; Big Sur: 25,130ha; 95% CI 17,245–36,622 ha; F 2, 225 = 25.83, P < 0.001; Figure5).In addition, we found no evidence of a release site*age interaction (F 2, 253 = 0.79, P < 0.453) nor a release site*sex interaction (F 2, 214 = 1.44, P < 0.238).

Figure 3. Median (± 95% CI) monthly home range size of adult female and male California Condors relative to breeding status.

Figure 4. Median (± 95% CI) monthly home range size of California Condors that were raised in the wild by their genetic parents or reared in captivity.
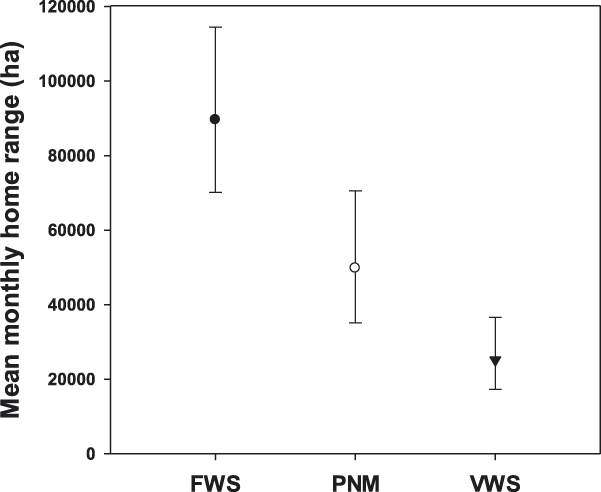
Figure 5. Median (± 95% CI) monthly home range size of California Condors from the three release sites in southern and central California (i.e., FWS = Hopper Mountain National Wildlife Refuge Complex) and central California (i.e., PNM = Pinnacles National Monument, VWS = Ventana Wildlife Society).
Discussion
Relationship between monthly home range size and age class
We found that mean monthly home range size of adult condors was significantly larger than that of immature birds, and this pattern persisted throughout most of the annual cycle. Our results are in accordance with previous work that found immature condors typically spend the first two years of life near natal areas even after achieving independence from their parents (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). As they age, condors gradually increase the size of their monthly home ranges, ultimately travelling throughout historic foraging ranges by the time they reach sexual maturity (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). Thus, it may be that increasing monthly home range size of immature condors as they age is due to learning from adults about historic foraging ranges either directly, when parents guide offspring to new areas during the course of post-fledging care, or indirectly, when independent immature birds follow older condors to foraging sites (indirect facilitation; see Houston Reference Houston1974, Jackson et al. Reference Jackson, Ruxton and Houston2008). If true, this highlights the importance of having experienced adults available to mentor younger birds in the wild population, a factor that has been shown to be a critically important tool for teaching appropriate social behaviour to young birds prior to their release into the wild (Utt et al. Reference Utt, Harvey, Hayes and Carter2008, Walters et al. Reference Walters, Derrickson, Fry, Haig, Marzluff and Wunderle2010). Of note, condors that are raised in captivity and then released into the wild as juveniles often appear dependent on proffered food in their first year after release (Burnett and Brandt pers. obs.), so this factor may also contribute to the smaller monthly home ranges of immature individuals. Unlike our results with California Condors, Bamford et al. (2007) reported that home range size of adults in the Cape Vulture Gyps coprotheres in Namibia was an order of magnitude smaller than that of immature individuals throughout the year. Although their sample sizes were modest and their analysis was considered over various temporal scales, these authors attributed the difference to strong competition for food between adult and immature birds, a result also reported by Mundy et al. (Reference Mundy, Butchart, Ledger and Piper1992) for the same species; a similar result was found for Cape Vultures in southern Africa (Phipps et al. Reference Phipps, Wolter, Michael, MacTavish and Yarnell2013). These studies represent an interesting contrast with the California Condor, as individual condors compete for food resources at the site of carcasses (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). Unfortunately, comparative data from other vultures are unavailable, so care should be taken when trying to generalise about age-dependent variation in home range size among obligate vertebrate scavengers.
Variation in monthly home range size across the annual cycle
We found clear differences in mean monthly home range size across the annual cycle, as monthly home range sizes during the late autumn and early winter (November–March) were up to 5–6 times smaller than monthly home range sizes observed during the late summer and early autumn months (July–October). Condors exhibited smaller monthly home ranges during the breeding season, when adults are potentially restricted in their movements because of parental duties (egg incubation and chick feeding). Nevertheless, variation in monthly home range size does not appear to be linked to breeding behaviour because we observed similar variation in immature birds which do not exhibit breeding behaviour, and because breeding and non-breeding adults did not differ in monthly home range size. If breeding status did not drive variation in monthly home range size over the course of the annual cycle, which factor(s) might have done so?
Temporal variation in atmospheric conditions across the landscape is one factor that likely mediates variation in condor monthly home range size during the annual cycle, as these conditions vary in both space and time. Condors, like other vultures, use soaring and gliding flight when searching for food and making long-distance movements (Pennycuick Reference Pennycuick1972, Hedenstrom Reference Hedenstrom1993, Ruxton and Houston Reference Ruxton and Houston2004, Duerr et al. Reference Duerr, Miller, Lanzone, Brandes, Cooper, O’Malley, Maisonneuve, Tremblay and Katzner2012). Soaring and gliding flight necessitates the use of buoyant, vertically-moving air currents via (1) convective thermals that form during the day when the earth’s surface is heated by solar radiation (thermal soaring; Pennycuick and Scholey Reference Pennycuick and Scholey1984, Spaar and Bruderer Reference Spaar and Bruderer1996, Pennycuick Reference Pennycuick1998) and (2) vertical lift that is created when horizontal winds rise vertically when forced over pronounced landscape features (slope soaring; Bildstein et al. Reference Bildstein, Bechard, Farmer and Newcomb2009, Mandel et al. Reference Mandel, Bohrer, Winkler, Barber, Houston and Bildstein2011, Bohrer et al. Reference Bohrer, Brandes, Mandel, Bildstein, Miller, Lanzone, Katzner, Maisonneuve and Tremblay2012). Several factors can influence the suitability of convective thermals used for soaring flight (Leshem and Yom-Tov 1996, Shamoun-Baranes et al. Reference Shamoun-Baranes, Leshem, Yom-Tov and Liechti2003, Mandel et al. Reference Mandel, Bildstein, Bohrer and Winkler2008, Bohrer et al. Reference Bohrer, Brandes, Mandel, Bildstein, Miller, Lanzone, Katzner, Maisonneuve and Tremblay2012), including variation in the amount of solar radiation hitting the earth’s surface (Stull Reference Stull1988). Thus, the lower levels of solar radiation in the winter months of November–March likely reduced thermal strength and, in turn, condor monthly home range size compared to late summer and early fall months, when atmospheric conditions were more favourable for thermal production (Stull Reference Stull1988).
In addition to atmospheric properties, variation in monthly home range size also may be linked to changes in photoperiod across the annual cycle. Photoperiod in our study area is reduced during the winter months by c.5 hours and reduces the time available for condors to undertake long-distance movements relative to summer months. Food availability may also play a role in influencing condor monthly home range throughout the annual cycle as it does in other obligate scavengers (Houston Reference Houston1990, Olea and Mateo-Tomás Reference Olea and Mateo-Tomás2009), as such changes may reflect the need for individuals to travel greater distances in search of food resources during certain portions of the year. In particular, the autumn deer hunting season is likely to provide additional food resources to condors in the form of offal and unretrieved animals (Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2010). The relative role of each of these resources in shaping condor spatial ecology is unknown, but ongoing research is currently examining the importance of atmospheric conditions and food availability in relation to condor space use (Rivers et al. unpubl. data, D’Elia et al. unpubl. data). Nevertheless, large gaps remain in our understanding of how spatial and temporal variation in these and other resources influence condor behaviour patterns, so additional research examining condor resource selection throughout the annual cycle are warranted.
Variation in monthly home range size as a function of release site
Monthly home range sizes varied among the three release sites: the largest mean monthly home range size was observed in individuals released from Hopper Mountain, followed by individuals released from Pinnacles, with the smallest monthly home range occurring in condors released from Big Sur. In addition to obvious differences that arise among sites due to their geographic location, a more proximate explanation for this pattern is that food availability in Southern California may differ relative to the other two release sites, both of which are located in the central part of the state. Condors in Southern California are currently restricted to terrestrial sources for carrion, which include both domestic and wild vertebrates (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002, Chamberlain et al. Reference Chamberlain, Waldbauer, Fox-Dobbs, Newsome, Koch, Smith, Church, Chamberlain, Sorenson and Risebrough2005). In contrast, condors in central California have both terrestrial and marine resources available to them, and individuals from both central California release sites have been observed foraging on carcasses of whales, seals, and sea lions (Burnett et al. Reference Burnett, Sorenson, Brandt, Sandhaus, Ciani, Clark, David, Theule, Kasielke and Risebrough2013). Indeed, use of marine resources by condors may be extensive because there is now evidence that condors are exposed to persistent environmental contaminants that bio-accumulate in marine mammals (Burnett et al Reference Burnett, Sorenson, Brandt, Sandhaus, Ciani, Clark, David, Theule, Kasielke and Risebrough2013). However, quantitative information about food availability among the California release sites is currently lacking and is an important factor that is being examined with ongoing studies (D’Elia pers. comm.).
The use of proffered food at release sites for condors is similar to “vulture restaurants” that are common in many areas and serve as an important means by which uncontaminated carrion can be obtained by imperilled vultures (Gilbert et al. Reference Gilbert, Watson, Ahmed, Asim and Johnson2007, Deygout et al. Reference Deygout, Gault, Sarrazin and Bessa-Gomes2009, Cortés-Avizanda et al. Reference Cortés-Avizanda, Carrete and Donázar2010). Recent work has found that space use of the White-rumped Vulture Gyps bengalensis was shaped by human-provisioned food at such sites; satellite-derived home range estimates decreased by 23–59% during a period of experimental food supplementation (Gilbert et al. Reference Gilbert, Watson, Ahmed, Asim and Johnson2007). Those data were collected from male vultures only and it is unclear how representative these results are of the population, but they do support idea that supplemental food can alter movement patterns and space use of obligate scavenging birds. Additional evidence of how feeding is linked to space use comes from an introduced population of the Eurasian Griffon Vulture Gyps fulvus in France. Despite the presence of feeding stations, vultures were found to maintain natural foraging on unpredictable resources (Monsarrat et al. Reference Monsarrat, Benhamou, Sarrazin, Bessa-Gomes, Bouten and Duriez2013), especially when natural food abundance was high in the landscape. This is also true for condors, which use natural food sources regularly (Finkelstein et al. Reference Finkelstein, Doak, George, Burnett, Brandt, Church, Grantham and Smith2012, Burnett et al. Reference Burnett, Sorenson, Brandt, Sandhaus, Ciani, Clark, David, Theule, Kasielke and Risebrough2013), so careful documentation of the amount and consistency of supplemental feeding at condor release sites will allow for understanding how condors make use of natural and proffered food items and how human-provided food may influence space use by condors.
Factors not linked to variation in monthly home range size
Our analysis found that several factors were not associated with variation in condor monthly home range size including the sex, breeding status, and rearing style of individuals. It is not surprising that monthly home range size would not differ between males and females, as the two sexes are similar in body mass and parental roles (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002) and likely have similar energetic needs. Similarly, Carrete and Donázar (Reference Carrete and Donázar2005) found no sex-related differences in the home range size of the Cinereous Vulture Aegypius monachus when measured during both the breeding and non-breeding seasons. These authors also reported that home ranges of breeding Cinereous Vultures were larger than home ranges of non-breeding individuals (Carrete and Donázar Reference Carrete and Donázar2005), which contrasts with our finding that monthly home range size was similar in breeding and non-breeding condors. A clear distinction between these two species is that Cinereous Vultures breed in colonies of 45–100 pairs whereas condors select nest sites that are far from other condor nests and defend these sites from conspecifics (Snyder and Schmitt Reference Snyder, Schmitt, Poole and Gill2002). We also did not detect any difference in monthly home range size between captive and wild-reared condors suggesting that the current captive rearing programme does not appear to impact the spatial movements of young birds, at least in terms of monthly home range size. However, there are a limited number of wild-reared birds in the population so additional study of this topic is needed as the number of wild-reared condors continues to increase.
Ours is the first detailed study of monthly home range size of the critically endangered California Condor, and we found that several factors (age, release site, and month of the annual cycle) were linked to changes in monthly home range size, whereas others were not (sex, breeding status, and rearing style). Because the home range is a fundamental concept in the study of resource selection and space use, our findings should be useful for future studies that are focused on understanding movement ecology and resource selection of condors and other obligate vertebrate scavengers. Furthermore, our results can also be used to investigate topics such as how condors use space in locations which are of interest for wind energy development (Walters et al. Reference Walters, Derrickson, Fry, Haig, Marzluff and Wunderle2010) and how movement patterns of individuals may lead to variation in contaminant exposure (Rideout et al. Reference Rideout, Stalis, Papendick, Pessier, Puschner, Finkelstein, Smith, Johnson, Mace, Stroud, Brandt, Burnett, Parish, Petterson, Witte, Stringfield, Orr, Zuba, Wallace and Grantham2012, Finkelstein et al. Reference Finkelstein, Doak, George, Burnett, Brandt, Church, Grantham and Smith2012), two important pressures currently facing condors in California. In particular, additional studies are necessary to fill remaining gaps in our knowledge of condor spatial ecology which, in turn, should provide information that is vital for future conservation and management decisions regarding this critically imperilled species.
Acknowledgements
We thank the Bureau of Land Management; California Department of Fish and Game; National Parks Service, Pinnacles National Monument; USFWS, Condor Recovery Program; USFWS, Ventura Field Office; USGS Forest and Rangeland Ecosystem Science Center; and Ventana Wildlife Society for financial and logistical support. H. Beyer, J. Glendenning, P. Haggarty, D. Howard, K. Jenkins, J. Kern, B. Kertson, B. Massey, J. Marzluff, C. Phillips, A. Rodgers, N. Snyder, D. Wiens, and S. Wilbur provided for logistical support and feedback, and E. Forsman and A. Margalida provided helpful comments on the manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.








