Media summary: Variation in age at natural menopause might be best understood as a by-product of differing rates of somatic ageing.
1. Introduction
Menopause, as per the World Health Organisation definition (NCC-WCH, 2015), refers to the permanent cessation of menstruation in human females. While natural menopause is a ubiquitous phenomenon of the human female ageing experience, there is considerable variation in the timing of menopause (or age at menopause), and how menopause is experienced both within, and between populations (Jasienska, Bribiescas, Furberg, Helle, & Núñez-de, Reference Jasienska, Bribiescas, Furberg, Helle and Núñez-de2017; Laisk et al., Reference Laisk, Tšuiko, Jatsenko, Hõrak, Otala, Lahdenperä and Tapanainen2018; Monteleone, Mascagni, Giannini, Genazzani, & Simoncini, Reference Monteleone, Mascagni, Giannini, Genazzani and Simoncini2018; Sievert, Reference Sievert2006). To date, most research into menopause focuses either on the evolutionary emergence of menopause as a Darwinian puzzle or on the proximate determinants of ovarian ageing. Surprisingly, there have been only limited attempts at understanding patterns of diversity in age at natural menopause. This paper aims to address this deficit by developing an interdisciplinary and multilevel framework combining proximate, biomedical understandings of menopause with an ultimate, evolutionary ecology perspective. Considering how diversity in age at menopause is produced at one level (i.e. physiological) can help generate new hypotheses at the evolutionary level (i.e. evolutionary and ecological drivers), and vice versa. Here we build on ovarian ageing research to uncover the evolutionary ecological determinants of variation in menopause timing. In turn, we hope to stimulate a new research programme investigating whether menopause timing is best predicted by ecological models of somatic ageing.
What is menopause?
In the biomedical and population health sciences, natural menopause is defined as an event reached when a woman has not had a menstrual cycle for the past 12 months (Hillard et al., Reference Hillard, Abernethy, Hamoda, Shaw, Everett, Ayres and Currie2017). Following this final menstrual period (FMP), a woman is considered to have experienced menopause. Natural menopause, which indicates the cessation of reproductive function, most often occurs in the fourth or fifth decade of a woman's life. While menopause itself is the complete cessation of periods, it is best understood as a process rather than an event (Sievert, Reference Sievert2006). Indeed, individuals will be peri-menopausal for some years before and after their FMP. Menopause is preceded by peri-menopause, a time period characterised by the irregularity of menstrual cycle length and frequency (Paramsothy et al., Reference Paramsothy, Harlow, Nan, Greendale, Santoro, Crawford and Randolph2017) and the potential to experience vasomotor symptoms (e.g. hot flushes/night sweats; Hillard et al., Reference Hillard, Abernethy, Hamoda, Shaw, Everett, Ayres and Currie2017), urogenital discomfort, anxiety, depression and joint aches (Hillard et al., Reference Hillard, Abernethy, Hamoda, Shaw, Everett, Ayres and Currie2017). Note that not all women experience natural menopause, as some women may experience menopause owing to a pathology of the reproductive system before the expected age of cessation of reproductive function, or accelerated surgical menopause owing to procedures such as bilateral oophorectomy, hysterectomy, chemotherapy or GnRH analogues (Hillard et al., Reference Hillard, Abernethy, Hamoda, Shaw, Everett, Ayres and Currie2017).
From an evolutionary standpoint, menopause is often equated with another feature of the female reproductive lifespan – age at last birth. Most evolutionary approaches investigating the origins of menopause focus on the cessation of fertility rather than the cessation of reproductive function per se, especially in non-menstruating species. While reproductive capacity may coincide with age of last birth in other species displaying early reproductive cessation, e.g. the killer whale (Orcinus orca; Cant & Croft, Reference Cant and Croft2019; Croft et al., Reference Croft, Johnstone, Ellis, Nattrass, Franks, Brent and Cant2017), menopause and age at last birth do not always coincide in humans: an individual becomes post-fertile following their last birth, but they are not necessarily post-reproductive if they are still cycling (Figure 1; Levitis, Burger, & Lackey, Reference Levitis, Burger and Lackey2013). Indeed, the two phases are reached on average 10 years apart in contemporary populations (Towner, Nenko, & Walton, Reference Towner, Nenko and Walton2016), and there is only limited correlation between age at last birth and age at menopause (reviewed in Towner et al., Reference Towner, Nenko and Walton2016). Thus, one cannot assume that the cessation of fertility is dependent solely on physiological reproductive decline. Rather, age at last birth is influenced by sociocultural and biological factors other than reproductive senescence (Bongaarts, Reference Bongaarts1978), including exposure factors (partner availability), deliberate fertility control factors (family planning, induced abortion) and natural fertility factors (lactational infecundability, frequency of intercourse, pathological sterility, spontaneous intrauterine mortality; Bongaarts, Reference Bongaarts1978).

Figure 1. Measuring post-reproductive lifespan: the differences between post-fertile lifespan (PFLS) and post-reproductive lifespan (PRLS). ALB, Age at last birth; AM, age at menopause; AD, age at death. Post-fertile lifespan is defined as the length of time between age at last birth, which typically occurs between 39 and 41 years (reviewed in Towner, Nenko, & Walton, Reference Towner, Nenko and Walton2016) and age at death. In contrast, post-reproductive lifespan is defined as the length of time between age at menopause and age at death. Reproductive senescence corresponds to fertility decline over age, which culminates in the age at menopause (AM). Note that this is not the same as age at last birth. Arguments regarding the evolution of menopause – focusing on age at last birth – may not hold for explaining diversity in the timing of menopause.
The extent to which age at menopause and age at last birth are determined by the same factors is unclear owing to a paucity of studies considering the two traits together and thus whether the same predictive framework should be applied to both age at last birth and age at FMP remains an open question. To address this gap, in the remainder of this paper, we focus on age at natural menopause, i.e. the age at FMP, rather than age at last birth.
Human variation in age at natural menopause
Self-reported age at menopause is variable, with mean age throughout the twentieth century being anywhere between 44.6 and 54.5 years of age across different geographical regions (Laisk et al., Reference Laisk, Tšuiko, Jatsenko, Hõrak, Otala, Lahdenperä and Tapanainen2018), and between 46 and 51.7 years of age in studies conducted between 1990 and 2010 (Figure 2). Temporal changes in age at FMP occur across different birth cohorts, with a cohort study in Sweden identifying a 1 month increase of menopausal age with each year of birth (Rodstrom et al., Reference Rodstrom, Bengtsson, Milsom, Lissner, Sundh and Bjourkelund2003). While there seems to be significant variation worldwide in age at menopause, the data underpinning this picture are somewhat problematic owing to methodological considerations, including an overrepresentation of clinical based studies in the global North, debatable inclusion/exclusion criteria, data binning bias and cross-cultural bias (Box 1). Thus, much of the literature reviewed in this paper is only approximating global variation in menopause timing.

Figure 2. Variation in final menstrual period (FMP). This map was replicated from Laisk et al. (Reference Laisk, Tšuiko, Jatsenko, Hõrak, Otala, Lahdenperä and Tapanainen2018), with additional segregation of data based on the decade in which it was collected. Variation in self-reported mean age at FMP is measured between 1990 and 2010 across countries. Broadly, mean age of menopause is higher in the Global North than in the Global South, but owing to the lack of measurement of age at menopause across populations, there is a sizeable uncertainty associated with this pattern. Additionally, the measurement of age at menopause in the studies included here may also be subject to limitations (discussed in Box 1). See Supplementary Information for references, sample sizes and years during which the data were collected.
Box 1: Methodological considerations when measuring age at menopause
Advancing knowledge of current patterns of diversity in menopause timing requires population-based studies to be conducted outside the Global North. In addition, there are several limitations to both measuring menopause (e.g. age at FMP) within populations and interpreting the results. Those issues, listed below, should be addressed in future studies:
(i) The measurement of FMP may only be confirmed retrospectively. This increases the difficulty of recruiting women who are newly post-menopausal for cross-sectional studies. Additionally, many current cohort studies use the midpoint between two cohort waves where menstruation is present and then absent as age at FMP. Such data are then often analyzed using discrete categories or ‘binning’ (eg. <45, 46–50, 51–55, 56+), which may obscure any smaller trends in age at menopause.
(ii) A woman's true age at menopause may be masked pharmaceutically (FSRH, 2017; Hillard et al., Reference Hillard, Abernethy, Hamoda, Shaw, Everett, Ayres and Currie2017).
(a) If a woman is taking combined oral contraceptives or hormone replacement therapy, bleeds are not menstrual cycles but rather withdrawal bleeds under the control of medication (FSRH, 2017). Prescription guidelines advise a change away from combined oral contraceptives to a progesterone-based contraceptive over the age of 50 (or 35 for smokers or people with other risk factors; Hillard et al., Reference Hillard, Abernethy, Hamoda, Shaw, Everett, Ayres and Currie2017), given the high risk of thromboembolism (FSRH, 2017). After this, bleeding may stop, and the individual may be considered post-menopausal. However, any bleeding experienced while on oral contraception is a withdrawal bleed, and a woman's reproductive capacity may have ceased prior to stopping oral contraceptive usage.
(b) Similarly, the use of combined hormone replacement therapy (HRT) during the peri-menopausal stage can also produce withdrawal bleeds (Hillard et al., Reference Hillard, Abernethy, Hamoda, Shaw, Everett, Ayres and Currie2017). These examples highlight the importance of defining menopause as the cessation of menstrual cycles, rather than all forms of bleeding, as bleeding can also originate from the use of hormonal contraceptives and HRT.
(c) If a woman is using progesterone-only contraceptive methods, age at menopause may also be masked by amenorrhoea produced by contraceptive usage. This is potentially a frequent issue: the chance of amenorrheoa by 12 months using the Mirena/levonorgestrel releasing intra-uterine system (the hormonal coil) is 20–80%, and this form of contraception has the highest continuation rates in women aged 39–48 (Currie, Reference Currie2019).
(iii) A woman's true age at menopause can be difficult to identify. It is possible that during peri-menopause a woman may not have a period in 12 months, then experience bleeding. The irregularity of menstrual cycles may result in periods longer than 12 months where a woman appears to be anovulatory, especially towards the later peri-menopause when menstrual cycles tend to be longer (Harlow & Paramsothy, Reference Harlow and Paramsothy2011). While this bleeding may be considered a period, it may not be a menstrual cycle but the result of reproductive malignancies which can occur in the post-menopausal body (Hillard et al., Reference Hillard, Abernethy, Hamoda, Shaw, Everett, Ayres and Currie2017). Furthermore, menstrual cyclicity amongst women is often more variable than a consistent 28 day cycle throughout reproductive life (Gorrindo et al., Reference Gorrindo, Lu, Pincus, Riley, Simon, Singer and Weinstein2007; Kato et al., Reference Kato, Toniolo, Koenig, Shore, Zeleniuch-Jacquotte, Akhmedkhanov and Riboli1999). This is especially pertinent amongst contemporary natural fertility populations where menstrual cycles may be much less frequent than in contemporary Western populations (Strassmann, Reference Strassmann1997).
(iv) There is no clinical diagnostic tool able to discern menopausal status through measuring hormone levels. While follicle-stimulating hormone (FSH) levels may be diagnostic for cases of early menopause (<45), FSH levels are unreliable for assessing menopausal status owing to fluctuations in levels throughout peri-menopause (FSRH, 2017; NCC-WCH, 2015). Additionally, levels of the anti-mullerian hormone (AMH), even in multiple assessments, are unreliable for measuring ovarian reserve, owing to the wide variation in levels of AMH within populations, as well as lack of a uniform AMH decline (de Kat et al., Reference de Kat, Dam, Onland-Moret, Eijkemans, Broekmans and van der Schouw2017).
Epidemiological studies, for the most part conducted in high-income countries and based on clinical rather than population-based samples, shed some light on the macro-level determinants of menopause timing. First, genetic contribution to age at menopause appears modest: GWAS-identified loci only explain 2.5–4.1% of population variation in menopause timing (Stolk et al., Reference Stolk, Perry, Chasman, He, Mangino, Sulem and Study2012), suggesting that genetic diversity holds little explanatory power for understanding diversity in age at menopause (but see Section 3 on the genetics of longevity). Other studies identified mixed associations between menopausal age and reproductive life history, socioeconomic status and lifestyle factors. For instance, early age at menopause has been associated with early menarche (G. D. Mishra et al., Reference Mishra, Pandeya, Dobson, Chung, Anderson, Kuh and Weiderpass2017; Ruth et al., Reference Ruth, Perry, Henley, Melzer, Weedon and Murray2016) and nulliparity (Duarte, de Sousa, Cadarso-Suarez, Rodrigues, & Kneib, Reference Duarte, de Sousa, Cadarso-Suarez, Rodrigues and Kneib2014; G. D. Mishra et al., Reference Mishra, Pandeya, Dobson, Chung, Anderson, Kuh and Weiderpass2017), while increasing parity (number of pregnancies) is associated with later age at menopause (Duarte et al., Reference Duarte, de Sousa, Cadarso-Suarez, Rodrigues and Kneib2014; Gold et al., Reference Gold, Bromberger, Crawford, Samuels, Greendale, Harlow and Skurnick2001; G. Mishra, Hardy, & Kuh, Reference Mishra, Hardy and Kuh2007; G. D. Mishra et al., Reference Mishra, Pandeya, Dobson, Chung, Anderson, Kuh and Weiderpass2017). Various markers of lower socioeconomic status or indicators of stress both in early life (household crowding; father's social class; parental divorce, poor cognitive ability, maternal smoking; perception of being thin; R. Hardy & Kuh, Reference Hardy and Kuh2005; G. Mishra et al., Reference Mishra, Hardy and Kuh2007; Ruth et al., Reference Ruth, Perry, Henley, Melzer, Weedon and Murray2016) and in later life (educational status, regional purchasing power; Duarte et al., Reference Duarte, de Sousa, Cadarso-Suarez, Rodrigues and Kneib2014; Schoenaker, Jackson, Rowlands, & Mishra, Reference Schoenaker, Jackson, Rowlands and Mishra2014) are associated with an earlier age at menopause. Those relationships are not mediated by the correlation between age at menarche and poor early life conditions given that most studies control for age at menarche (Schoenaker et al., Reference Schoenaker, Jackson, Rowlands and Mishra2014). Age at menopause is consistently associated with lifestyle factors – smoking has a strong association with earlier age at menopause (Gold et al., Reference Gold, Bromberger, Crawford, Samuels, Greendale, Harlow and Skurnick2001, Reference Gold, Crawford, Avis, Crandall, Matthews, Waetjen and Harlow2013; R. Hardy & Kuh, Reference Hardy and Kuh2002; Laisk et al., Reference Laisk, Tšuiko, Jatsenko, Hõrak, Otala, Lahdenperä and Tapanainen2018; Ruth et al., Reference Ruth, Perry, Henley, Melzer, Weedon and Murray2016; Schoenaker et al., Reference Schoenaker, Jackson, Rowlands and Mishra2014), while there is a weak association between lower body mass index and earlier age at menopause (Henderson, Bernstein, Henderson, Kolonel, & Pike, Reference Henderson, Bernstein, Henderson, Kolonel and Pike2008). Whether these different associations are globally salient is unknown, however, given that most epidemiological data are derived from clinical studies conducted in high-income countries.
There is currently no overarching framework for explaining why age at menopause correlates with reproductive, socioeconomic and lifestyle factors. Further, knowledge and theories from physiology, epidemiology and evolutionary ecology are not usually considered together. However, if one is to understand both why and how menopause timing varies, it is necessary to account for determinants at multiple levels. To do this, a human evolutionary ecology approach, drawing on the Mayr–Tinbergen Framework accounting for both ultimate and proximate causes of diversity together, offers a promising avenue (Laland, Sterelny, Odling-Smee, Hoppitt, & Uller, Reference Laland, Sterelny, Odling-Smee, Hoppitt and Uller2011). In the next section we harness this model by considering both current knowledge of the physiology of menopause (proximate) and current evolutionary theories for the evolution of early reproductive cessation (ultimate) to propose a new understanding of diversity in menopause timing in humans, i.e. the age at FMP.
2. Integrating ultimate and proximate explanations
In this section, we seek to answer the ultimate question ‘Why does variation in age at natural menopause exist?’ together with the proximate question ‘How does variation in age at natural menopause occur?’ In other words, we aim to integrate proximate understandings of ovarian ageing with evolutionary, historical approaches to menopause timing, which include both adaptationist (i.e. menopause timing has fitness benefits) and by-product (i.e. menopause timing has no fitness benefits) hypotheses (Nesse et al., Reference Nesse, Bergstrom, Ellison, Flier, Gluckman, Govindaraju and Valle2010; Stearns, Reference Stearns2012). Recent studies on menopause timing view age at menopause as a facultative adaptation – i.e. menopausal age varies in response to ecology in a way that maximises fitness (Chan, Gomes, & Singh, Reference Chan, Gomes and Singh2020; Galbarczyk & Jasienska, Reference Galbarczyk and Jasienska2013; Skjaervo & Roskaft, Reference Skjaervo and Roskaft2013; Yang, Arnot, & Mace, Reference Yang, Arnot and Mace2019). Those studies are generally silent with regards to physiological understandings of ovarian ageing, however. In contrast, the hypothesis viewing human menopause as an evolutionary by-product of the selection for an elongated lifespan is consistent with the finding that ovarian ageing is constrained by somatic processes rather than triggered (Box 2). In this way, the determinants of age of natural menopause may be similar to the genetic, developmental and ecological determinants of somatic ageing.
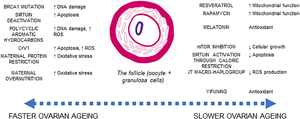
Box 2: Germ cells are well protected against ageing forces, but the somatic cells of the follicle are not
Perhaps one central issue to integrating ovarian ageing with somatic processes of ageing is that the oocyte itself is a germ cell. While the oocyte may possess multiple defence mechanisms against ageing, the somatic granulosa cells which surround the oocyte in the follicle are subject to somatic ageing. As the somatic granulosa cells decrease in quality, the quality of the overall follicle (including the oocyte itself) decreases and is at risk of undergoing apoptosis.
Ovarian ageing is often centred on the role of mitochondria, exploring the role of dysregulated respiration in the ageing process. Mitochondria are responsible for the energy production and also producing damaging reactive oxygen species (ROS) and reactive carbonyl species through respiration. Primordial follicles can be kept in a state of arrested prophase for upwards of 50 years and so there is potential during this arrest for damage to accumulate in the oocyte while it is quiescent (Hammond et al., Reference Hammond, Green, Shelling, Berg, Peek and Cree2016). However, the oocyte itself is well protected against oxidative damage, and it has been suggested that localised antioxidant production around the oocyte offers adaptive protection against DNA damage caused by ROS and during its suspended lifespan (Hammond et al., Reference Hammond, Green, Shelling, Berg, Peek and Cree2016; D. D. Zhang et al., Reference Zhang, Zhang, Zeng, Yuan, Liu, Yin and Liu2015). Localised production of melatonin in the ovary, which has antioxidant properties, also supports the presence of protective measures in the ovary against the impact of long-term exposure to ROS (Tamura et al., Reference Tamura, Kawamoto, Sato, Tamura, Maekawa, Taketani and Sugino2017). As such, mitochondrial DNA in the oocyte is not shown to accumulate mutations during ovarian ageing in the way predicted if there were no methods of oxidative shielding (Boucret et al., Reference Boucret, Bris, Seegers, Goudenege, Desquiret-Dumas, Domin-Bernhard and May-Panloup2017).
The granulosa cell therefore becomes the locus for attention on processes leading to follicular atresia, as well as the site of increasing genomic instability of ageing oocytes (Banerjee et al., Reference Banerjee, Banerjee, Saraswat, Bandyopadhyay and Kabir2014; Boucret et al., Reference Boucret, Bris, Seegers, Goudenege, Desquiret-Dumas, Domin-Bernhard and May-Panloup2017; May-Panloup et al., Reference May-Panloup, Boucret, de la Barca, Desquiret-Dumas, Ferre-L'Hotellier, Moriniere and Reynier2016; D. D. Zhang et al., Reference Zhang, Zhang, Zeng, Yuan, Liu, Yin and Liu2015). Follicular atresia is initiated through the granulosa cells, which accompany the oocyte from oogenesis to the creation of the antral follicle. Thus, the process of follicular atresia depends on the quality of the granulosa cells – not the oocytes. The senescence of the somatic cells in the ovarian microenvironment becomes the locus for studying determinants of ovarian ageing (Banerjee et al., Reference Banerjee, Banerjee, Saraswat, Bandyopadhyay and Kabir2014; Tatone & Amicarelli, Reference Tatone and Amicarelli2013).
Physiological understanding of menopause timing (proximate approach)
At the physiological level, the transition towards menopause is generally understood in terms of the processes of ovarian ageing and follicular atresia – the apoptosis (or programmed cell death) of oocytes (egg cells) (Narkwichean et al., Reference Narkwichean, Maalouf, Baumgarten, Polanski, Raine-Fenning, Campbell and Jayaprakasan2017). Ovarian ageing is the process whereby the ovaries decline in their ability to recruit and develop successful oocytes (Wang, Zhang, Jiang, & Seli, Reference Wang, Zhang, Jiang and Seli2017). Ovarian ageing adversely affects female fertility, reducing the probability of successful pregnancy owing to increasingly poor quality of follicles. The follicle is the cellular structure containing both the oocyte and surrounding granulosa cells and is recruited during the follicular phase of the menstrual cycle. If the follicle is of low quality, it will undergo atresia – programmed cell death – hypothesised to be under the control of the supporting granulosa cells (Banerjee, Banerjee, Saraswat, Bandyopadhyay, & Kabir, Reference Banerjee, Banerjee, Saraswat, Bandyopadhyay and Kabir2014; Tatone & Amicarelli, Reference Tatone and Amicarelli2013). As the ovary ages, both the quantity and the quality of follicles decreases (J. J. Zhang et al., Reference Zhang, Fang, Lu, Xiong, Wu, Shi and Wang2016), a process referred to as follicular depletion. At menarche the number of follicles is approximately 300,000–400,000 and reduces to below 1000 at menopause (Forman, Mangini, Thelus-Jean, & Hayward, Reference Forman, Mangini, Thelus-Jean and Hayward2013). The ovary loses follicles in two ways: ovulation and follicular atresia. As ~400 follicles are released through ovulation during the reproductive lifespan, the main source of follicle loss during the lifetime is atresia (Forman et al., Reference Forman, Mangini, Thelus-Jean and Hayward2013), with the rate of follicle loss also being influenced by multiple factors. Thus, while menopause is co-produced by ovarian ageing and follicular depletion together, ovarian ageing is constrained by somatic ageing of the follicle (Box 2).
Follicular depletion becomes implicated in determining the age at menopause when depletion causes the number of follicles to be below that required to support menstruation (Leidy, Godfrey, & Sutherland, Reference Leidy, Godfrey and Sutherland1998). At this point, menstrual cycles become dysregulated and ultimately cease. Conventional understandings of follicular atresia rates have considered the rate to be biphasic – with accelerated rates of atresia occurring beyond the age of 35 (Leidy et al., Reference Leidy, Godfrey and Sutherland1998). This, however, is shown to be the result of misinterpreting plots of follicular atresia rates (Leidy et al., Reference Leidy, Godfrey and Sutherland1998). Rather, accelerated rates of follicular atresia tend to occur much later, and are more likely within several years of the onset of menopause (Leidy et al., Reference Leidy, Godfrey and Sutherland1998). This suggests that processes underpinning the process of follicular atresia are key to the transition towards menopause.
The rate of follicular atresia is potentially influenced by the inflammatory profile of the menstrual cycle: ovulation is characterised by inflammation of the ovaries, while menstruation has been deemed a ‘massive inflammatory event’ (Alvergne & Högqvist Tabor, Reference Alvergne and Högqvist Tabor2018). Inflammation is a major determinant of the ageing process because it releases ROS, which are free radicals implicated in the aetiology of many non-communicable diseases through the promotion of cell senescence (Franceschi & Campisi, Reference Franceschi and Campisi2014). It remains to be investigated how repeated cycles of ovulation and menstruation influence ageing of the granulosa cells and thus follicular atresia. Diversity in cyclical life-history owing to anovulatory cycles, pregnancies or hormonal contraceptives is likely to be important for explaining patterns of reproductive senescence and the onset of menopause.
Evolutionary understanding of menopause timing (ultimate approach)
Why menopause timing varies has attracted little research to date (but see (Laisk et al., Reference Laisk, Tšuiko, Jatsenko, Hõrak, Otala, Lahdenperä and Tapanainen2018) for a review), most research focusing on the question of why menopause – defined as the permanent cessation of fertility – exists at all in humans and in some other species. Adaptationist perspectives consider the paradoxical occurrence of fertility cessation to hold an adaptive benefit given females do not directly increase their fitness consistently throughout their adult life. In this framework, menopause – by enabling women to avoid later-life reproduction – reached fixation in humans because it conferred fitness benefits through increased alloparental care and decreased reproductive conflict and mortality risk (Cant & Johnstone, Reference Cant and Johnstone2008; Cant, Johnstone, & Russell, Reference Cant, Johnstone, Russell, Hager and Jones2009; Croft et al., Reference Croft, Johnstone, Ellis, Nattrass, Franks, Brent and Cant2017; Ellison, Reference Ellison2001; Hawkes & Coxworth, Reference Hawkes and Coxworth2013; Hawkes, O'Connell, Jones, Alvarez, & Charnov, Reference Hawkes, O'Connell, Jones, Alvarez and Charnov1998; Kirkwood & Shanley, Reference Kirkwood and Shanley2010; Packer, Reference Packer, Robine, Kirkwood and Allard2001; Peccei, Reference Peccei2001; Williams, Reference Williams1957). In contrast, by-product theories view the emergence of menopause as an epiphenomenon – a spandrel (Gould & Lewontin, Reference Gould and Lewontin1979) – co-produced by the finite nature of a female's oocyte supply and extended lifespan longevity, which allow females to outlive this supply (Cohen, Reference Cohen2004; Ellison, Reference Ellison2001; Peccei, Reference Peccei2001). In this view, menopause emerged in human females because somatic longevity increased, while reproductive longevity did not. Our purpose here is not to dispute which framework is more salient for understanding the emergence of menopause, as indeed processes underpinning the emergence and the maintenance of traits might differ. Rather, we use those hypotheses as a guiding framework for explaining why age at menopause varies.
Recent research into the timing of menopause has taken an adaptive stance. In this view, menopause is a facultative trait where menopause timing responds to ecological factors such as daughter's reproductive success, dispersal patterns and living in the matrilineal/patrilineal household (Cant & Johnstone, Reference Cant and Johnstone2008; Skjaervo & Roskaft, Reference Skjaervo and Roskaft2013; Yang et al., Reference Yang, Arnot and Mace2019). Studies have found little support for modification of menopausal age based on either mediating factor, nor have they given suggestions for physiological mechanisms to explain how age at menopause could be affected by factors such as dispersal and daughter's reproductive success. Additional adaptationist theories, such as the ‘shifting mate choice/shifting menopause’ hypothesis, posit that variation in age at natural menopause occurs in response to later age of reproduction, through the removal of deleterious alleles selecting for menopause, which have accumulated owing to male preference for younger mates (Chan et al., Reference Chan, Gomes and Singh2020). Fundamentally, adaptationist perspectives have not proposed or found a genetic or physiological pathway producing a cascade which triggers reproductive senescence during midlife and would allow menopause timing to be facultative.
Comparatively, menopause timing has seldom been explored from the premise that menopause is a by-product of selection on longevity, following the decoupling of somatic and reproductive lifespan in human females. This may be due to the unclear directionality of mechanisms considered to be involved in the decoupling of reproductive and somatic lifespan – a prerequisite for this hypothesis. Female reproductive skew, and the front loading of reproductive events, is invoked as a mechanism that could be the cause of the evolution of menopause as it would decrease selection on extended reproductive lifespan (Peccei, Reference Peccei2001). However, given that the preference for younger females is found in humans (Chan et al., Reference Chan, Gomes and Singh2020; Takahashi, Singh, & Stone, Reference Takahashi, Singh and Stone2017), but not particularly in chimpanzees (Takahashi et al., Reference Takahashi, Singh and Stone2017), the human male mate preference is likely a derived trait and may be the outcome, rather than the cause, of early reproductive cessation in women. Nevertheless, the length of the female reproductive lifespan in humans is comparable with that of other species of similar body sizes (Peccei, Reference Peccei2001), while the length of somatic lifespan is not, suggesting that extended longevity is a derived trait in humans, while the length of the reproductive lifespan is not. This raises the possibility that age at menopause (rather than age at last birth) is at least partly determined by processes underpinning somatic ageing. In this line, ageing of the human female reproductive capacity is constrained by somatic ageing of the follicles (Box 2), as measured by the rate of follicular atresia. The somatic cells supporting reproduction age faster than the oocyte and the ovary are because they are less well protected from oxidative damage. Thus, exposure to factors implicated in increasing longevity could increase reproductive lifespan.
Towards a multilevel framework
Patterns of diversity in age at menopause are poorly understood. To address this, we propose a multilevel, interdisciplinary framework, combining proximate, physiological understandings of ovarian ageing with ultimate, evolutionary ecological perspectives on ageing. We hypothesise that evolutionary ecological factors known to influence somatic ageing variation (the genetics of longevity, early life environments, infections) can also explain rates of ovarian ageing, follicular depletion and diversity in the onset of menopause.
Overall patterns of ageing and senescence are understood evolutionarily through the Disposable Soma hypothesis (Kirkwood, Reference Kirkwood1977, Reference Kirkwood1999), where the body's capacity to accumulate deleterious senescent cells is attributed to declining selection pressure of maintenance mechanisms as age increases, owing to increasing extrinsic mortality risk (Kirkwood, Reference Kirkwood1999). Through the evolutionary lens, age-related health decline results from accumulated damage and suboptimal functioning of bodily systems on the molecular, cellular and organ levels (Kirkwood, Reference Kirkwood1999). When menopause becomes conceptualised as the by-product of ageing of the reproductive system, by-product hypotheses of menopause are compatible with current physiological understandings of ageing and cellular senescence. Exploration into variation therefore allows overarching theories of ageing rate variation to be applied to the female reproductive system.
Rates of cellular senescence can vary depending on the interaction between an organism and ecological factors (e.g. food availability, stress, pathogen load), producing patterns of ageing rates which vary within and between populations. Ecological factors might also influence women's cyclical life-history, producing diversity in anovulatory cycles, pregnancies or hormonal contraceptives, which are likely to be important for explaining patterns of reproductive senescence and the onset of menopause. These ecological factors will be explored in the next section in relation to current epidemiological understandings of variation in age of natural menopause, and with suggestions for further research.
3. Understanding patterns of menopause timing
In this section, we review the role of genetic, environmental and reproductive factors in explaining diversity in somatic senescence rates – ecological interactions which influence somatic ageing. This follows from the previous section where we suggest how these might be applied to understanding diversity in ovarian ageing. We show that there are common genetic factors between extreme longevity and age at menopause with regards to genes mediating metabolic profiles, metabolism and oxidative shielding. Following research showing that the early life environment influences the pace of reproductive development and life-history ‘strategy’, we hypothesise that poor early life environment may result in lower embodied capital, and thus earlier age at menopause. Finally, we propose that women who experience a higher number of cumulative ovulatory menstrual cycles may experience earlier age at menopause through the cumulative exposure of localised inflammation in the female reproductive organs during ovulation. We show that the phenotype of age at menopause may be the result of an interaction between genetic, ecological factors and the cycling life-history.
Genetic factors
Genetic factors between ovarian ageing and overall somatic ageing show similarities in the biochemical pathways in which they are implicated. Human longevity is a complex biosocial trait, with genetics being highly context-dependent and rates of senescence resulting from a dynamic process (Giuliani, Garagnani, & Franceschi, Reference Giuliani, Garagnani and Franceschi2018). There are no genes which ‘code for’ longevity in humans (Giuliani et al., Reference Giuliani, Garagnani and Franceschi2018), and associations between alleles and longevity occur where such alleles produce a phenotype conducive for long life, especially amongst centenarians (individuals who have lived to age 100). Such phenotypes include metabolic profiles characterised by preserved glucose tolerance and insulin sensitivity; compressed morbidity and disability in later life, and general avoidance or postponement of age-related diseases; and decreased DNA methylation compared with others of the same chronological age (Giuliani et al., Reference Giuliani, Garagnani and Franceschi2018). Such phenotypes are conducive of reduced levels of accumulated damage contributing to the functioning of bodily systems on the molecular, cellular and organ levels. These phenotypes may therefore promote both somatic longevity and reproductive longevity, thus postponing age at menopause.
Genetic factors which have been identified as contributing to the phenotype of somatic longevity, reproductive longevity or both include the following:
• APOE: the APOE gene codes for apolipoprotein E, which helps maintain structural integrity and function of cholesterol rich lipoproteins. The protein structure of APOE varies and is found to exist in three different isoforms which alter its function. Isoforms APOEe2, APOEe3 and APOEe4 are positively associated, not associated or negatively associated with longevity, respectively (Abondio et al., Reference Abondio, Sazzini, Garagnani, Boattini, Monti, Franceschi and Giuliani2019). Regarding menopause, association between isoforms and reproductive longevity has been inconclusive. Heterozygous APOEe3/4 carriers show a delayed age at menopause compared with APOEe3/3 carriers in a Chinese population (Meng et al., Reference Meng, Wang, Liu, Zhao, Liu and Zhou2012). Both APOEe4 and APOEe2 isoforms have been associated with predicted earlier age at menopause amongst Iranian females and women of European descent, respectively (Koochmeshgi, Hosseini-Mazinani, Morteza Seifati, Hosein-Pur-Nobari, & Teimoori-Toolabi, Reference Koochmeshgi, Hosseini-Mazinani, Morteza Seifati, Hosein-Pur-Nobari and Teimoori-Toolabi2004; Tempfer et al., Reference Tempfer, Riener, Keck, Grimm, Heinze, Huber and Hefler2005).
• Sirtuins: Sirtuins are proteins which modulate metabolism, cell proliferation and genome stability. Regulation of several sirtuin genes – SIRT5 and SIRT7 – has been found to have a positive association with longevity, while a minor SIRT6 homologous allele, affecting its function, has been associated with decreased lifespan (Giuliani et al., Reference Giuliani, Garagnani and Franceschi2018). Variation in sirtuin regulation has been linked to reproductive longevity, with downregulation of SIRT1, SIRT3 and SIRT6 being linked to an increased rate of ovarian ageing (J. J. Zhang et al., Reference Zhang, Fang, Lu, Xiong, Wu, Shi and Wang2016).
• Mitochondrial Haplotype J: Mitochondrial DNA Haplotype J is hypothesised to reduce the output of both ATP (the product of respiration) and ROS. The mtDNA J haplotype has been positively associated with somatic longevity in European populations (Giuliani et al., Reference Giuliani, Garagnani and Franceschi2018), and was underrepresented amongst French women with depleted ovarian reserves undergoing fertility treatment (May-Panloup et al., Reference May-Panloup, Desquiret, Moriniere, Ferre-L'Hotellier, Lemerle, Boucret and Reynier2014), suggesting it plays a role in reproductive longevity.
• FOXO3: FOXO3 is a gene which downregulates activity on the IGF1 pathway, helping to maintain a metabolomic profile conducive to longevity (Giuliani et al., Reference Giuliani, Garagnani and Franceschi2018). Associations between expression of FOXO3 and reproductive longevity are unknown.
• IL6: Modulation of interleukin 6, a multifunctional cytokine associated with inflammatory responses by a minor allele has also been associated with longevity and the aetiology of age-related disease (Giuliani et al., Reference Giuliani, Garagnani and Franceschi2018). Associations between IL6 modulation and reproductive longevity are unknown.
Additional single nucleotide polymorphisms associated with age at menopause have been linked to genes involved in hormonal regulation, immune function and DNA repair pathways (Stolk et al., Reference Stolk, Perry, Chasman, He, Mangino, Sulem and Study2012). A candidate gene located on the Human Leukocyte Antigen transcript has been associated with age at menopause as well as Type-1 diabetes and rheumatoid arthritis (Stolk et al., Reference Stolk, Perry, Chasman, He, Mangino, Sulem and Study2012). Such a gene implicates a pro-inflammatory component to physiological pathways mediating rates of ovarian ageing (Stolk et al., Reference Stolk, Perry, Chasman, He, Mangino, Sulem and Study2012). BRCA1 mutations also confer an increased rate of ovarian ageing, hypothesised to be due to increased rates of double-strand DNA breaks in follicles, causing subsequent increase in the rate of follicular atresia (Box 2, Figure 3; Lin, Titus, Moy, Ginsburg, & Oktay, Reference Lin, Titus, Moy, Ginsburg and Oktay2017).
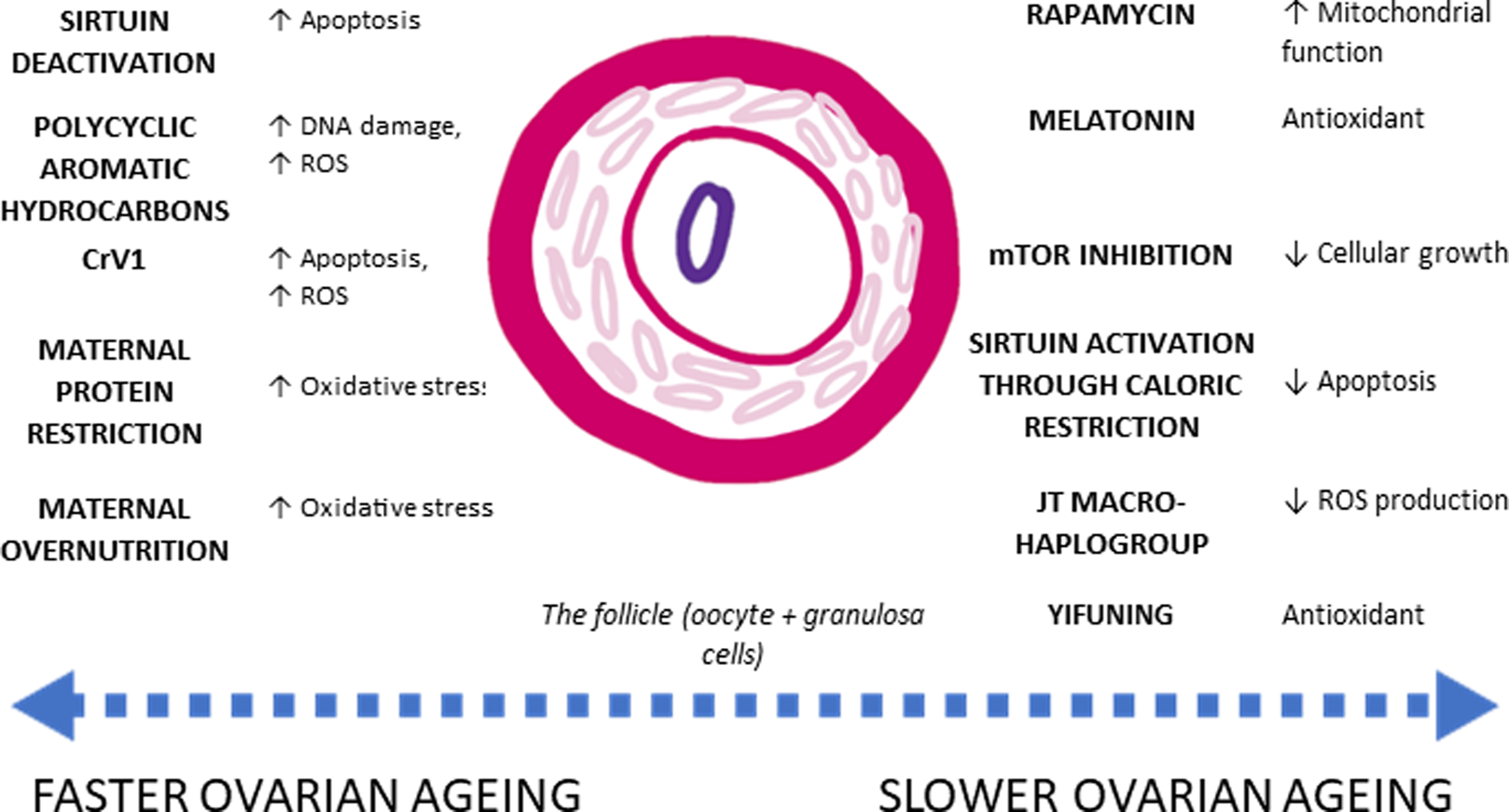
Figure 3. Agents which influence ovarian ageing. Agents with their respective effect on rates of ovarian ageing. ROS (reactive 2oxidative species) produce oxidative stress, which contributes to cellular senescence and cell apoptosis. Conversely, agents which contain antioxidants improve overall mitochondrial function, slowing down the rate of cellular senescence. However, while some physiological processes are known, there has been no ecological study accounting for fast or slow ovarian ageing. Lists of references are provided in the Supporting Information.
Determinants of longevity and somatic senescence are hugely complex, with genetic factors only explaining a small proportion of variation in longevity (Giuliani et al., Reference Giuliani, Garagnani and Franceschi2018). GWAS-identified loci and their related function only explain 2.5–4.1% of population variation in the age at menopause (Stolk et al., Reference Stolk, Perry, Chasman, He, Mangino, Sulem and Study2012). The genetic contribution to age at menopause, and overall senescence rates may be overpowered by ecological and environmental factors and so must be considered in relation to other exogenous factors. Despite the low contribution genetic variation makes, these studies indicate that processes of non-communicable diseases and ovarian ageing are underpinned by similar metabolic and inflammatory processes.
Ecological factors
Rates of age-related health decline are in part mediated by an individual's ability to accrue somatic capital – a factor dependent on environmental constraints on energy available for their growth and development. Somatic capital can be understood as the energetic investments made by the body in growth and maintenance of tissue beds and organs (Kaplan, Lancaster, & Robson, Reference Kaplan, Lancaster and Robson2003), which will depreciate over time through wear and tear. As the body's ability to maintain cellular and tissue function decreases over time, mechanisms in the ageing body must rely on their existing somatic capital to ensure optimal function is maintained. Somatic capital accrual can be influenced by the life history strategy of the individual. Life history theory (Ellison, Reference Ellison2003; Gluckman, Beedle, & Hanson, Reference Gluckman, Beedle and Hanson2009) broadly describes patterns of growth, reproduction and mortality in an individual's life and in a given environment. One particularly influential concept in life-history evolution is that of the ‘fast–slow continuum’, which accounts for the fact that many life-history traits co-vary across and within species (Stearns, Reference Stearns1992). Age at menopause may therefore be understood as an outcome of a life-history strategy, itself contingent on the somatic capital of the female reproductive system, determined by ecological factors (e.g. food availability, stress, pathogen load). Using a life history theory approach allows investigating whether variation in age at menopause reflects overall rates of ageing in the body or is specific to reproductive senescence.
Extrinsic mortality
Life history theory posits that, in environments with high extrinsic mortality (i.e. mortality independent of an individual's phenotype), metabolic investment in reproduction is prioritised at the expense of other fitness components (somatic maintenance, growth; Stearns, Reference Stearns1992). This leads to the acceleration of an organism's life-history (hence a ‘fast life-history’ strategy; Hidaka & Boddy, Reference Hidaka and Boddy2016; Nettle, Reference Nettle2010; Stearns, Ackermann, Doebeli, & Kaiser, Reference Stearns, Ackermann, Doebeli and Kaiser2000) and is hypothesised to affect rates of ageing and the development of age-related diseases. In humans, age at first birth in England is younger in deprived areas compared with more affluent areas, which is interpreted as a response to the ecological context of poverty (Nettle, Reference Nettle2010), with girls from moderately stressful environments of nutritional inadequacy experiencing accelerated pubertal timing (Ellis, Reference Ellis2004). In turn, low embodied capital of the reproductive system may cause suboptimal tissue defence (Noguera, Reference Noguera2017) against the oxidative stress of menstruation and reproduction, increasing rates of follicular atresia. This may ultimately accelerate reproductive ageing towards menopause. In comparison, those living in energy-rich, low-mortality environments may accrue higher somatic capital owing to a slower life history strategy (Ellis, Reference Ellis2004). Higher socioeconomic living conditions may therefore be associated with later age at menopause given the prolonged ability for tissue maintenance in those with higher somatic capital.
It is important to clarify at this point that life history strategies are often used in a behavioural context, to explain patterns of behaviour – often related to reproduction (Nettle, Reference Nettle2010). Here, we use life history strategies to refer to the allocation of physiological resources, contributing to the embodied capital of the individual rather than in a more behavioural context.
Fast/slow life history theories as a predictive framework are in line with trends in epidemiological studies where earlier age at menopause is found amongst low/middle-income populations, as well as amongst those who were exposed to poor environmental conditions earlier in life (Duarte et al., Reference Duarte, de Sousa, Cadarso-Suarez, Rodrigues and Kneib2014; R. Hardy & Kuh, Reference Hardy and Kuh2005; G. Mishra et al., Reference Mishra, Hardy and Kuh2007; Ruth et al., Reference Ruth, Perry, Henley, Melzer, Weedon and Murray2016; Schoenaker et al., Reference Schoenaker, Jackson, Rowlands and Mishra2014). Furthermore, in Western populations, earlier age at menopause has been associated with an increased risk of cardiovascular diseases (CVD), atherosclerosis, stroke and osteoporosis (Forman et al., Reference Forman, Mangini, Thelus-Jean and Hayward2013; Schoenaker et al., Reference Schoenaker, Jackson, Rowlands and Mishra2014) while later menopause has been associated with both a reduced risk of CVD and all-cause mortality and an increased risk of breast and ovarian cancer and osteoporosis (Forman et al., Reference Forman, Mangini, Thelus-Jean and Hayward2013; Henderson et al., Reference Henderson, Bernstein, Henderson, Kolonel and Pike2008; Ossewaarde et al., Reference Ossewaarde, Bots, Verbeek, Peeters, van der Graaf, Grobbee and van der Schouw2005; Schoenaker et al., Reference Schoenaker, Jackson, Rowlands and Mishra2014). Finally, studies into oestrogen-receptor negative breast cancer rates suggest that a fast life history strategy may result in a higher incidence of breast cancer amongst women of lower socioeconomic status (Hidaka & Boddy, Reference Hidaka and Boddy2016).
Infectious diseases
Additional metabolic trade-offs between growth, maintenance and reproduction can occur in the presence of infectious disease where energy is allocated to the immune system at the expense of other bodily functions (Ellison, Reference Ellison2003). Sievert has previously explored the relationship between age at menopause and exposure to infectious diseases over the life course amongst Bangladeshi women living in London. They were found to have a significantly earlier age at menopause than other women living in London, with earlier age being strongly associated with a history of infectious disease exposure on multiple occasions (Sievert, Reference Sievert2014). As immune defences against pathogens are energetically costly, pathogen load may also contribute towards reducing bodily investment in the growth and maintenance of the body. Studies researching the effect of prolonged infection on age at menopause show a younger age at menopause amongst women with HIV compared with women without HIV in the Bronx (Schoenbaum et al., Reference Schoenbaum, Hartel, Lo, Howard, Floris-Moore, Arnsten and Santoro2005), although this result is not entirely consistent (Conde, Pinto-Neto, & Costa-Paiva, Reference Conde, Pinto-Neto and Costa-Paiva2008). There is potential for expanding research into the influence of infectious diseases on age at menopause by studying (a) the impact of infections earlier versus later in life, (b) population-level patterns where malaria is endemic and (c) and immunocompromised populations.
Cyclical reproductive life history
Variation in rates of ovarian ageing may result from the cumulative exposure of the female reproductive system to cyclical inflammation, which may vary across ecologies. Reproduction in human females is characterised by cyclical fertility, with menstrual cycles completed between approximately 24 and 38 days (Alvergne & Högqvist Tabor, Reference Alvergne and Högqvist Tabor2018), with the end of non-conceptive cycles characterised by menstruation, a massive inflammatory event. Localised inflammation also occurs in the ovaries during the inflammation-mediated repair of the corpus luteum immediately after ovulation (Alvergne & Högqvist Tabor, Reference Alvergne and Högqvist Tabor2018). Furthermore, the ovaries are the site of oestrogen production – hormones which can act as pro-inflammatory, depending on dose. Through menstrual cycling, cyclical, systematic inflammation may contribute to damage of the granulosa cells and ovarian microenvironment, resulting primarily in the accelerated senescence of the female reproductive function relative to other organs of the body.
There is some evidence that ovarian ageing rates may vary according to the total number of menstrual cycles experienced in a female's reproductive lifespan. First, high cumulative levels of oestrogen exposure are known to be a risk factor for the development of oestrogen receptor-positive breast, ovarian and endometrial cancers (Aktipis, Ellis, Nishimura, & Hiatt, Reference Aktipis, Ellis, Nishimura and Hiatt2014; Jasienska, Bribiescas, et al., Reference Jasienska, Bribiescas, Furberg, Helle and Núñez-de2017; Jasienska, Sherry, Holmes, & SpringerLink, Reference Jasienska, Sherry and Holmes2017; Strassmann, Reference Strassmann1999). Given tumorigenesis also operates through cellular damage and mutations, it is not implausible to consider the effect of concentrated cumulative oestrogen exposure on cellular senescence of the reproductive organs. Second, preliminary epidemiological data show that nulliparity (as a discrete entity) is significantly associated with earlier ages of menopause (Duarte et al., Reference Duarte, de Sousa, Cadarso-Suarez, Rodrigues and Kneib2014; G. D. Mishra et al., Reference Mishra, Pandeya, Dobson, Chung, Anderson, Kuh and Weiderpass2017). Normally cycling nulliparous women who are not taking any form of hormonal contraception do not experience the gaps in ovulation that occur during the gestation period and breastfeeding. This suggests that the female reproductive life history should be considered in its entirety – e.g. as total number of menstrual cycles experienced – rather than as a composite of discrete entities (e.g. age at menarche, parity, breastfeeding and use of hormonal contraception) as it is often approached within epidemiological studies. This approach has already been used in several epidemiological studies of breast cancer, where higher numbers of cumulative menstrual cycles have been associated with an increased risk of breast cancer (Chavez-MacGregor et al., Reference Chavez-MacGregor, Elias, Onland-Moret, van der Schouw, Van Gils, Monninkhof and Peeters2005; Clavel-Chapelon & Grp, Reference Clavel-Chapelon and Grp2002; Rautalahti et al., Reference Rautalahti, Albanes, Virtamo, Palmgren, Haukka and Heinonen1993).
How ecology influences a woman's cumulative exposure to cyclical inflammation is poorly understood. A 1994 study estimate that women in contemporary Western populations experience up to 400 cycles during the lifetime, compared with a median of 94 within a contemporary natural fertility population (Strassmann, Reference Strassmann1997). In the absence of data on the cycling life-history, reproductive traits across the lifespan could be used as a proxy to estimate a woman's cumulative exposure to inflammatory menstrual cycles. Note that ideally, it is the number of ovulatory, as opposed to anovulatory, cycles that is the most relevant measure. Proximate determinants of the number of menstrual cycles might themselves be the outcome of life history strategies explored earlier (see Ellis, Reference Ellis2004), but similar life-history ‘strategies’ may have different impacts on the number of menstrual cycles depending on sociocultural contexts (i.e. availability of contraception, norms around breastfeeding etc.; Ellis, Reference Ellis2004), although these life-history strategies are not necessarily prescriptive (Nepomnaschy, Rowlands, Costa, & Salvante, Reference Nepomnaschy, Rowlands, Costa and Salvante2020; Sheppard & Van Winkle, Reference Sheppard and Van Winkle2020). Nevertheless, life-history and reproductive cyclicity approaches are not mutually exclusive.
Accounting for the cost of cumulative menstrual cycles may have implications for evolutionary models. First, it adds nuance to what may count as a ‘cost of reproduction’ – this is often referred to as the impact of reproduction and pregnancy on the female body, at the expense of physiological functioning (Ryan et al., Reference Ryan, Hayes, Lee, McDade, Jones, Kobor and Eisenberg2018). While pregnancy may incur a physiological cost to somatic functioning (Ryan et al., Reference Ryan, Hayes, Lee, McDade, Jones, Kobor and Eisenberg2018), it may also be protective over ovarian function with regards to the onset of menopause (Duarte et al., Reference Duarte, de Sousa, Cadarso-Suarez, Rodrigues and Kneib2014; G. D. Mishra et al., Reference Mishra, Pandeya, Dobson, Chung, Anderson, Kuh and Weiderpass2017). Thus, cyclical menstruation and pregnancy may be better considered as separate entities rather than falling under the all-encompassing ‘cost of reproduction’. Second, given the physiological processes of reproductive and somatic ageing are physiologically similar, reproduction might entail costs not only for somatic senescence, a trade-off often studied by evolutionary biologists (see T. B. L. Kirkwood & Westendorp, Reference Kirkwood, Westendorp, Robine, Kirkwood and Allard2001), but also for reproductive senescence. While cyclical inflammation confers fitness benefits early in life, more frequent cyclical ovulation in humans might directly influence the onset of menopause through the antagonistic pleiotropic effects of cyclical inflammation.
In this section, we have explored possible evolutionary ecological determinants of diversity in menopause timing. While much of the literature in this review comes from studies in high-income countries, the framework we have developed here may help formulate hypotheses for studies of populations in lower-income countries. Future research investigating how factors such as socioeconomic status, poverty, food insecurity and infectious diseases interact with life history and cyclical reproductive life histories may help expand understandings of variation in age of natural menopause within different populations.
4. Implications for public health
As ageing populations are perceived to present challenges to the maintenance of population health, healthcare provision, demographic structure and society, there is increasing importance placed on research aiming to understand and predict patterns of ageing (USC Programme on Global Ageing & Policy, 2018). However, current public health approaches towards understanding diversity in the experience of menopause (age and symptoms) and its impact on health and overall wellbeing are scarce. Here we show that an ecological approach to variation in menopause might help with (a) nuancing assumptions about the ‘normal’ menopause, (b) understanding the relationship between menopause and health decline, (c) interrogating whether earlier menopause and diseases of old age originate from the same ecological determinants of health and (d) how understanding variation in menopause experience can benefit wider studies into successful ageing.
Stimulating public health research into the diversity of menopausal experience
Despite a substantial focus within public health on ageing (Beard & Bloom, Reference Beard and Bloom2015), menopause as a facet of the female ageing experience is often excluded from research questions into ageing and subsequent public health interventions (e.g. breast cancer screening). For instance, out of the 15 ageing cohort studies found on the Gateway to Global Ageing Data (USC Programme on Global Ageing & Policy, 2018), a harmonised dataset aiming at providing resources to support cross-national research on ageing, only five studies collected any form of data on menopause from their female participants. The questions and cohort studies which did include menopause-related variables are found in Table 1. The observation that menopause is excluded from ageing cohort studies, which premise themselves on collecting data on the multifactorial nature of the ageing experience, reveals the absence of menopause from public health discourses of ageing, which suggests that its impact on the ageing experience is neglected. Any relationships existing between menopause and health are unable to be identified, allowing prevalent biomedical assumptions to prevail. Ignorance of menopause as a facet of female ageing creates a measurement trap, in which lack of information is both the cause and the effect of continuing exclusion (Graham, Reference Graham1998).
Table 1. Menopause-related variables in the Gateway to Global Aging Data, produced by the USC Program on Global Aging, Health & Policy, with funding from the National Institute on Aging
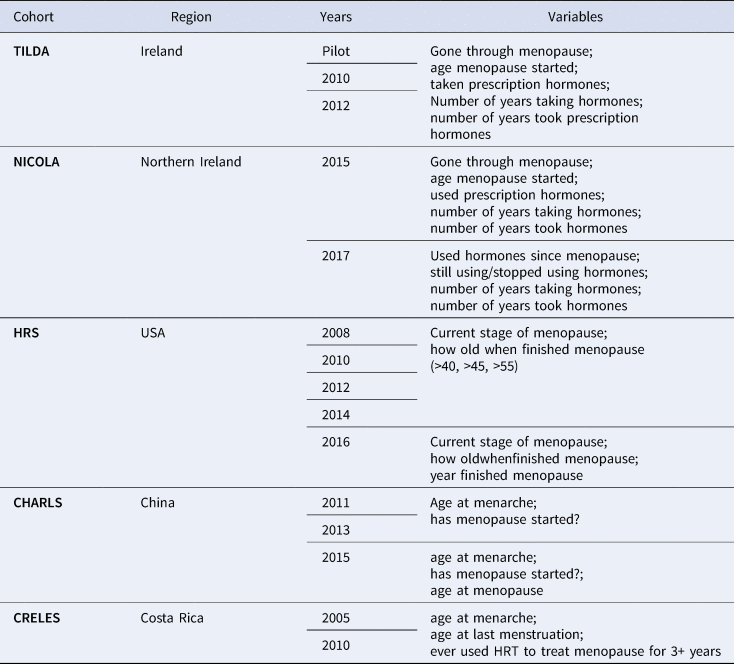
Since the 1990s, several longitudinal studies have been started, many with the specific aim of understanding the impact of HRT usage on later life health among post-menopausal women such as the Women's Health Initiative (Nabel, Reference Nabel2013; Rossouw, Anderson, & Oberman, Reference Rossouw, Anderson and Oberman2003) and Million Women Study (The Million Women Study Collaborative, 1999). Study of Women's Health Across the Nation and the International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events are currently collecting and synthetising health data on peri- and post-menopausal women. Inclusion of questions around the menopausal experience in ageing cohort studies, and expansion of menopause-related research questions beyond HRT and later-life health outcomes, will help to corroborate the data collected by the Study of Women's Health Across the Nation and International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events and improve the robustness of research into menopause.
Reframing the menopausal transition as normal
Understanding menopausal variation can help alleviate the assumptions still present within the biomedical approaches of menopause. Biomedical perspectives of menopause were for most of the twentieth century predicated on the assumption that menopause and the oestrogen-deficient body were inherently ‘risky’ (Harding, Reference Harding, Petersen and Bunton1997; Lock, Reference Lock1993), with this risk to be countered through the prescription of HRT during the post-menopausal life stage. While the Women's Health Initiative and Million Women Study revealed the health risks associated with indiscriminate long-term prescription of HRT (to the extent that the experimental studies had to be prematurely ended; Nabel, Reference Nabel2013), assumptions surrounding the causality of post-menopausal health issues as well as a lack of recognition of menopause experience variation may arguably still persist within Western biomedicine and public health.
Further, public health research into menopause variation can primarily help nuance the designation of the menopausal transition as ‘normal’ or ‘pathological’. Current UK guidelines state that any woman entering menopause at age <40 is experiencing premature ovarian insufficiency while those entering menopause at age <45 are experiencing early menopause (NCC-WCH, 2015). As there is little consensus on hormonal diagnosis of ovarian ageing (Box 1) and given that variation in age at menopause exists within and between populations, normal ‘earlier’ menopause in some women may be accidentally pathologised, while abnormal but ‘later’ menopause may remain undiagnosed in others. Current biomedical understandings of ‘normal’ menopause are predicated on normative views of how a ‘normal’ body should behave (Wiley & Cullin, Reference Wiley and Cullin2020). Gathering data to explore the true variation of menopausal age within and between populations will allow this assumption to be challenged.
Rethinking menopause as the by-product rather than the catalyst of biological ageing
Age at menopause is associated with varying health outcomes, with earlier age at menopause being generally associated with increasing risk of all-cause mortality (Forman et al., Reference Forman, Mangini, Thelus-Jean and Hayward2013; Schoenaker et al., Reference Schoenaker, Jackson, Rowlands and Mishra2014). Thus, age at menopause can be used to identify at-risk groups of older women, who could then be targeted with preventative screening programmes and treatment against associated diseases such as cancers, CVD and osteoporosis prior to any manifestation of disease. However, risk factors for health and disease that accelerate biological ageing may also contribute to earlier age at menopause rather than menopause itself being the catalyst for biological ageing (Levine et al., Reference Levine, Lu, Chen, Hernandez, Singleton, Ferrucci and Horvath2016). For instance, menopause has been associated with epigenetic processes linked to cellular senescence and ageing when epigenetic biomarkers of methylation are compared with chronological age (Levine et al., Reference Levine, Lu, Chen, Hernandez, Singleton, Ferrucci and Horvath2016; USA & European populations, n = 3110). The epigenetic age at blood was found to have a negative correlation with age at menopause, which supports observational studies that found that for every one-year increase in age at menopause, the age-adjusted mortality rate decreases by 2% (Levine et al., Reference Levine, Lu, Chen, Hernandez, Singleton, Ferrucci and Horvath2016). In this study, there is a suggestion of directionality, with post-menopausal women who had late onset of menopause found to be epigenetically younger than women with early onset menopause. However, risk factors for health and disease that accelerate biological ageing may also contribute to earlier age at menopause rather than menopause itself being the catalyst for biological ageing (Levine et al., Reference Levine, Lu, Chen, Hernandez, Singleton, Ferrucci and Horvath2016). Such research nuances prevailing assumptions around menopause being the cause or catalyst of poor health and disease in later life.
Contrasting with contemporary biomedical perspectives, an ecological approach to understanding diversity in the onset of menopause may show that correlations between earlier menopause and diseases of old age originate from the same life history determinants of health, encompassing somatic capital and life history strategies and the wider sociocultural determinants of health. Such life course studies would fall into the emergent discipline of evolutionary public health (Wells, Nesse, Sear, Johnstone, & Stearns, Reference Wells, Nesse, Sear, Johnstone and Stearns2017), where both proximate and ultimate explanations into patterns of population health and disease are considered within the theoretical framework (Wells et al., Reference Wells, Nesse, Sear, Johnstone and Stearns2017). An understanding of how ecological and evolutionary contexts throughout life can help explain patterns of health in older age within and between socioeconomic strata, owing to developmentally and environmentally determined patterns of energy allocation (Wells et al., Reference Wells, Nesse, Sear, Johnstone and Stearns2017). Evolutionary public health allows the integration of menopause timing within overarching understandings of ageing and senescence in life history as well as its inclusion in public health data collection and approaches to ageing. This is not to say that menopause has no adverse impact on the health of ageing females, but its insertion into large-scale health data collection would allow any risk factors emerging from menopause to be identified and nuanced, combating the pathologisation of menopause as a whole.
Aside from evolutionary ecological approaches to menopause, there is also scope for integrating menopause into the wider evolutionary medicine paradigm. Reconceptualising health, from an evolutionary perspective, as a means to an end of reproductive success (Wells et al., Reference Wells, Nesse, Sear, Johnstone and Stearns2017) requires the recognition that reproductive function is intrinsically intertwined with ‘non-reproductive’ health. The peri- and post-menopausal body can be reconceptualised as the female body with minimal interaction between the reproductive system and other bodily systems. In doing so, there is incentive to study how the dysregulation and cessation of the menstrual cycle may impact the immune system (for review see Alvergne & Högqvist Tabor, Reference Alvergne and Högqvist Tabor2018), or the aetiologies of non-communicable diseases.
Diversity in menopausal experience and the capacity for successful ageing
While the study of variation may be useful in understanding disease risk, it may be equally important to consider how and why variation in age and experience affects an individual's capacity for ‘successful’ ageing (Rowe & Kahn, Reference Rowe and Kahn2015). There is an increasing awareness of ‘successful ageing’ in Public Health and Gerontology, which encompasses the social, cultural and psychological impact of growing older beyond the increasing health risks. In this view, the ageing experience is expanded beyond the disease risk and frailty to include facets of the ageing experience that are more important to the individual (Rowe & Kahn, Reference Rowe and Kahn2015). Therefore, approaches to menopause as a component of female ageing should also be expanded beyond focusing on health risks.
Facets of the menopausal experience and wider female ageing are already being studied and could benefit from taking the existence of variation into account. This includes areas such as: menopause in the workplace (C. Hardy, Reference Hardy2019); grandmothering, its impact on familial health and how menopause may affect the ability to alloparent (Sear, Reference Sear2016); female personhood during the life course (Pickard, Reference Pickard2019); menopause and sexuality; and more critical medical anthropological perspectives on menopause, biopower and pharmaceutical intervention (Harding, Reference Harding, Petersen and Bunton1997; Padamsee, Reference Padamsee2011). Expanding focus onto how diversity in the experience of menopause impacts the wider social and cultural experience of growing older will improve the robustness of public health perspectives on women's ageing, closer to actual lived experience.
5. Conclusion
The goal of this paper is to stimulate an interdisciplinary, multilevel framework for understanding the role of evolutionary and ecological factors in shaping diversity in age at natural menopause. By engaging with the definitions of menopause across disciplines, we can ensure that proximate and ultimate approaches to menopause are addressing the same phenomenon, i.e. the cessation of menstrual cycles, rather than broader features of the post-fertile lifespan. We have shown the compatibility of biomedical, physiological understandings of ovarian ageing with evolutionary theories viewing the emergence of menopause as a by-product of recent increases in longevity (e.g. the reproductive-somatic mismatch hypothesis; Cohen, Reference Cohen2004). This suggests that evolutionary hypotheses usually applied to somatic senescence (e.g. the Disposable Soma hypothesis, the antagonistic pleiotropy hypothesis, the embodied-capital theory) may also become fruitful for understanding patterns of diversity in menopausal traits.
A consistent theme throughout this paper has been to highlight potential areas where menopause research is lacking, and which can be expanded both in the medical sciences and in human ecological studies. We also suggest potential implications for approaches towards ageing women's health in public health and the wider medical sciences. We suggest that menopause is currently excluded from public health approaches to ageing and that its continued exclusion cannot be justified. Not only should menopause be measured in ageing cohort studies, but its measurement should be done with the methodological considerations outlined earlier in mind. We also posit that recognition of variation in menopause may help nuance assumptions surrounding normalcy and the menopause, and the clinical cut-offs made between ‘normal’ and ‘abnormal’ menopause. We further recognise that through the application of evolutionary theories of ageing towards menopause variation there is an opportunity to reconceptualise menopause as a process of ageing, rather than its cause. This might stimulate novel research questions into which processes underlay both reproductive and overall senescence. This also stands in contrast to the social construction of menopause as a pathology within Western biomedicine, and reaffirms the menopausal transition as normal, rather than inherently pathological.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ehs.2020.59
Acknowledgements
EW is funded by the Medical Research Council (MC_UU_12017/13) and the Chief Scientist Office, Scottish Government (SPHSU13). We thank Gabriella Kountourides and Rose Stevens, members of the Applied Evolutionary Anthropology Group at Oxford Anthropology, for providing useful feedbacks on the manuscript.
Author contributions
Article written by AF, with editing, feedback and guidance from AA, EW and CJ.
Financial support
EW is funded by the Medical Research Council (MC_UU_12017/13) and the Chief Scientist Office, Scottish Government (SPHSU13).
Conflict of interest
AA is on the editorial board of Evolutionary Human Sciences.