INTRODUCTION
Diarrhoeal disease continues to be a global public health priority [1], especially in the context of climate change, global environmental change, and the globalization of travel and trade [Reference Costello2]. Endemic levels and outbreaks of foodborne, waterborne, and person-to-person transmission of acute gastrointestinal illness (AGI) contribute to considerable morbidity, mortality, and economic costs in developed countries [Reference Majowicz3–Reference Jones7], and could be particularly important to understand in high-risk populations [Reference Majowicz3–Reference Thomas5]. Therefore, environmental and public health practitioners continue to prioritize the surveillance of AGI to monitor the burden of illness, detect and control outbreaks, and evaluate control measures. Under-reporting of AGI is a limitation of surveillance data, especially when used to estimate the burden of illness [Reference MacDougall8]. Considering this high level of under-reporting, burden-of-illness studies that estimate rates and identify risk factors for AGI at the community-level are employed internationally [Reference Sargeant, Majowicz and Snelgrove4, Reference Scallan6, Reference Jones7, Reference Baumann-Popczyk9–Reference Thomas12].
While research on the burden of AGI is burgeoning, there is still a limited understanding of enteric illness in subsets of populations, including populations who could be the most vulnerable to enteric illness [Reference Cheng, McDonald and Thielman13–Reference Pardhan-Ali16]. For instance, although high-quality data are not currently available to accurately estimate the burden of AGI in Indigenous communities, research has documented environmental conditions that could increase the risk of AGI (e.g. overcrowding, limited accessibility and availability of safe drinking water) and some researchers have hypothesized that waterborne and foodborne disease is likely to be disproportionately higher in many Indigenous communities [Reference Hrudey17–Reference Harper20]. Indeed, some Indigenous populations in Canada, USA, and Australia live in substandard living conditions with more limited access to the quality and quantity of services and resources than other non-Indigenous citizens [Reference Adelson19]. These conditions contribute to disparities in several health outcomes between Indigenous people and non-Indigenous people living in the same country [Reference Adelson19, Reference King, Smith and Gracey21]. In addition, effective and timely public health surveillance for Indigenous populations can be challenging, particularly in rural or remote communities due to limited accessibility of healthcare services [Reference Adelson19, Reference Smylie and Anderson22]. This lack of access to these services can result in reduced care-seeking behaviour, which can compromise the quality and quantity of available surveillance data [Reference Adelson19, Reference Smylie and Anderson22–Reference Harper24].
Considering the possible increased vulnerability to AGI and the limitations of surveillance data in some Indigenous communities, there is a clear need for burden of AGI studies to estimate the incidence of, and identify potential risk factors for, AGI at the community level in these populations. In this context, the goal of this study was to investigate the burden of AGI in the Inuit communities of Rigolet, Nunatsiavut and Iqaluit, Nunavut, Canada. The specific objectives were to estimate the incidence and prevalence, and identify potential socioeconomic and environmental risk factors of AGI in each community in autumn (September) and spring (May) 2012–2013. The results of this study are important in identifying local risk factors that can point to possible intervention strategies, inform decision-making, and better understand the burden of AGI in the North.
METHODS
Study locations
The term Indigenous is an all-encompassing term that refers to Indigenous inhabitants of a country, including Inuit, First Nations, and Métis in Canada. Many Inuit live in Northern Canada in one of four Inuit settled land claim regions: Nunatsiavut, Nunavut, Nunavik and Inuvialuit. Inuit have developed a culture and lifestyle that is dependent upon cold climatic conditions, with the extensive ice and snow coverage providing transportation opportunities between communities, to hunting grounds, and to important cultural activities that are essential for health and wellbeing [Reference Cunsolo25–Reference Cunsolo27]. Canadian Inuit also face challenges regarding access to health services, low socioeconomic status compared to the national average, crowded and poor-quality housing, food insecurity, and concerns regarding basic services such as drinking-water quality and sanitation [Reference Adelson19].
This study took place in two Inuit communities: Iqaluit, Nunavut and Rigolet, Nunatsiavut, Canada. Nunavut (‘Our Land’) is Canada's newest Territory spanning nearly 2 million km2, with 26 communities and a population of over 31000 residents (Fig. 1). Iqaluit is the capital of Nunavut with a population of 6699 people, 62% of whom identify as Aboriginal [28]. Nunatsiavut (‘Our Beautiful Land’) is Canada's most eastern Inuit region, with five remote communities and a growing population of 2617 [28]. The community of Rigolet is the most southern of five communities in Nunatsiavut (Fig. 1), with a population of 306, 94% of whom identify as Aboriginal [28].

Fig. 1. A map of Labrador and Nunavut, highlighting the communities in Nunavut and the Nunatsiavut Land Claim Settlement Region.
Study design and data collection tool
The National Studies on Acute Gastrointestinal Illness (NSAGI) study methodology used in Canada [Reference Thomas29] was adapted to reflect Inuit culture and the Northern context. Two retrospective cross-sectional surveys were conducted in person in the autumn and spring seasons. The autumn survey took place from 17 September to 3 October 2012 in Rigolet, and from 15 September to 5 October 2012 in Iqaluit. The spring survey took place from 22 May to 19 June 2013 in Rigolet, and from 18 May to 2 June 2013 in Iqaluit. Questionnaires previously used in the Canadian burden of AGI surveys [Reference Majowicz3, Reference Thomas5, Reference Majowicz, Horrocks and Bocking30], were modified for use in Rigolet and Iqaluit (questionnaires available from the corresponding author upon request). The primary outcome measure was self-reported AGI, which was captured by asking participants if they had experienced a new case of diarrhoea and/or vomiting in the past 14 and 28 days. The case definition used for AGI was self-reported vomiting and/or diarrhoea (any loose stool) in the past 14 days (September and May) as well as the past 28 days (May), excluding cases who reported vomiting or diarrhoea due to pregnancy, medication use, alcohol/drug use, or diagnosed chronic conditions (e.g. colitis, diverticulitis, Crohn's disease, irritable bowel syndrome, H. pylori, or other diagnosed chronic conditions) [Reference Majowicz, Horrocks and Bocking30]. In an attempt to capture incident cases, if the date of reported AGI symptom onset was prior to the recall period, the case was excluded. Data on potential risk factors were also gathered (Fig. 2) [Reference Majowicz3, Reference Thomas5]. The questionnaire was extensively pre-tested for content and context by academics, health practitioners, and Inuit community members.

Fig. 2. A summary of the information captured in the cross-sectional surveys in Rigolet, Nunatsiavut, and Iqaluit, Nunavut (2012, 2013).
Sample size, sampling framework, and survey administration
Rigolet, Nunatsiavut
Given the small population size of Rigolet (n = 306), a census sample was attempted; every individual in every household who was in the community during the sampling period was invited to participate. The private and confidential questionnaire was administered by trained community personnel using iPads in the language preferred by the participant, including English and Inuttitut; however, all participants chose English, which is their first language [28].
Iqaluit, Nunavut
A target sample size of 498 participants was calculated using Epi Info (Centers for Disease Control and Prevention, USA, 2000), to detect an expected 14-day period prevalence of 6% [Reference Majowicz3, Reference Thomas5]. with a 2% allowable error and a 95% confidence level in a population of 6184. A two-stage random sample approach was employed. The first stage involved proportionally, randomly selecting blocks of households in Iqaluit and a census of the block was attempted. The second stage involved randomly selecting a household member to participate in the survey using the last-birthday method [Reference Gaziano31], and all ages were eligible to participate. Participants had the option of conducting the interview in-person or scheduling a telephone interview. Contact with each house was attempted twice during different times of the day. The private and confidential questionnaire was administered by trained community personnel using iPads in the language preferred by the participant, including English, French, and Inuktitut.
Consent and ethics
Each participant was invited to watch an informational video and written (Rigolet) or oral (Iqaluit) informed consent was obtained. Participants aged <18 years required parental permission. For participants aged <12 years, the parent could act as a proxy respondent for the child. Compensation for the participant's time and information was provided following the recommendations of local project partners [32, Reference Pearce33]. The study protocol was approved by the Research Ethics Board at the University of Guelph and McGill University, Health Canada Research Ethics Board, and the Nunatsiavut Government Research Advisory Committee. A research license was obtained from Nunavut Research Institute under the Nunavut Scientist Act.
Data analysis
Data were exported from the iPad iSurveySoft© software into Excel (v. 14·2·5; Microsoft Corp., USA) and all analyses were conducted using Stata/IC 13.1 for Mac (StataCorp., USA), using a significance level of α = 0·05. Data from participants responding ‘unsure’ or ‘refused to answer’ were excluded from the analysis of that question. To examine the representativeness of the data, the sample population demographics were compared to census demographics [28] using Pearson χ 2 tests. The annual incidence rate and annual incidence proportion were estimated using equations (1) and (2), respectively [Reference Thomas11].
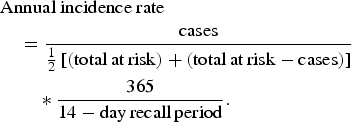 $$\eqalign{& {\rm Annual}\ {\rm} {\rm incidence}\ {\rm} {\rm rate}{\rm} \cr & \quad = \displaystyle{{{\rm cases}} \over {{1 \over 2}\left[ {\left( {{\rm total}\,{\rm at}\,{\rm risk}} \right) + \left( {{\rm total}\,{\rm at}\,{\rm risk} - {\rm cases}} \right)} \right]}} \cr & \quad \quad \ast \displaystyle{{365} \over {14-{\rm day}\,{\rm recall}\,{\rm period}}}.} $$
$$\eqalign{& {\rm Annual}\ {\rm} {\rm incidence}\ {\rm} {\rm rate}{\rm} \cr & \quad = \displaystyle{{{\rm cases}} \over {{1 \over 2}\left[ {\left( {{\rm total}\,{\rm at}\,{\rm risk}} \right) + \left( {{\rm total}\,{\rm at}\,{\rm risk} - {\rm cases}} \right)} \right]}} \cr & \quad \quad \ast \displaystyle{{365} \over {14-{\rm day}\,{\rm recall}\,{\rm period}}}.} $$
where x = (cases)/(total at risk).
Multivariable logistic models were built to examine potential risk factors for AGI, with a null hypothesis of no association between AGI (based on 14-day period prevalence) and potential risk factor variables of interest. There were four models; one for September and one for May in each community. For each model, a causal diagram was created to explore potential risk factors of interest based on peer-reviewed literature and biological plausibility. The assumption of linearity was assessed graphically by plotting the continuous variables against the log-odds of the outcome using lowess curves (i.e. locally weighted regression for smoothed scatterplots). To avoid collinearity issues, the correlation between predictor variables was assessed using Spearman's rank correlation analysis, using a cut-point value of 70%. If the correlation was above 70%, the most biologically plausible variable was used in the model-building process.
Since a census was attempted in Rigolet, a mixed multivariable logistic regression model with a random intercept to control for clustering by household was used for these data. To build the Rigolet models, a series of univariable (i.e. one fixed effect) logistic regression models with a random intercept for household were conducted to explore potential unconditional associations between predictor variables and the outcome variable. Then, a manual backward stepwise approach was used to build a mixed multivariable logistic regression model, considering all predictor variables with P < 0·20 in univariable analyses, with a significance level of α = 0·05 for risk factor variables to stay in the model. Full and reduced models were compared at each step using likelihood ratio tests; confounding was assessed at each step and if adding or removing a variable resulted in a 30% change in the model β-coefficients, the variable was considered a confounder and remained in the model regardless of statistical significance. All two-way interactions in predictors with P < 0·10 in the univariable analyses were assessed. Finally, the fit of the models was assessed via graphical examination of Pearson residuals, and assessment of the assumptions of normality and homogeneity of variance for the best linear unbiased predictors (BLUPs).
For the Iqaluit data, multivariable exact logistic regression models were built since one individual per household was randomly selected and, thus, there was no household-level clustering for these data. Specifically, a series of univariable exact logistic regressions using conditional scores tests were conducted to explore potential unconditional associations between predictor variables and the outcome variable. A manual backward stepwise approach was used to build the multivariable models, considering all predictor variables with P < 0·20 in univariable analyses, with a significance level of α = 0·05 for risk factor variables to stay in the model regardless of statistical significance. Confounding was assessed at each step and if adding or removing a variable resulted in a 30% change in the model β-coefficients, the variable was considered a confounder and remained in the model. Finally, all two-way interactions in predictors with P < 0·10 in the univariable analyses were assessed. For Iqaluit and Rigolet models, if sex and age were not significant in the final model, we forced these variables into the model to examine any changes in predictor variable coefficients and confidence intervals; if little change was observed (i.e. <30%), the age and/or sex variable was not included the final model.
RESULTS
In Rigolet, a total of 245 people were in the community during the September study period, and 226 questionnaires were completed (response rate 92%); a total of 249 were in the community during the May study period and 236 questionnaires were completed (response rate 95%). In Iqaluit, a total of 532 questionnaires were completed in September (response rate 75%) and a total of 523 questionnaires were completed in May (response rate 55%). Each questionnaire took an average of 14 minutes to complete.
The demographics of September and May survey participants compared to the 2011 census of Rigolet and Iqaluit are shown in Table 1. Compared to the census demographics of Iqaluit, females, older people, and Indigenous people were over-represented in the September survey (P < 0·05), and females and older people were over-represented in the May survey (P < 0·05). In Rigolet, there were no significant differences in sex, age, and Indigenous identity between survey participants and the 2011 census (P > 0·05).
Table 1. Demographics of Rigolet and Iqaluit based on the 2011 Census, as well as the September 2012 and May 2013 survey respondents in Rigolet, Nunatsiavut, and Iqaluit, Nunavut, Canada in September 2012 and May 2013

* Proportion per category significantly different (P < 0·05) from all other categories combined using Pearson's χ 2 test. For age, we compared each category to all other categories collapsed.
AGI prevalence, incidence, and risk factors
In Rigolet, the estimated annual incidence rate was 3·8 and 3·9 episodes/person per year in September and May, respectively (Table 2; Fig. 3). In Iqaluit, the estimated annual incidence rate was 3·8 and 2·8 episodes/person per year in September and May, respectively. The 14-day and 28-day prevalence, estimated annual incidence, and estimated annual incidence proportion for Rigolet and Iqaluit are presented in Table 2. In the September final multivariable model for Rigolet, not visiting a cabin in the past month, increased spending on obtaining country food (i.e. locally harvested food including berries, duck eggs, fish, caribou, marine mammals, ducks), and a homeless person staying in the home (as defined by the Inuit Health Survey [Reference Saudny, Leggee and Egeland34]) increased the odds of AGI (Table 3). Final model results indicated no significant difference between models that did and did not account for clustering at the household level (Table 3); however, a random intercept was forced in the model to control for household-level clustering due to the structure of the Rigolet data, as well as similar variable coefficients, variable confidence intervals, and model fit (based on Akaike's Information Criterion) for models that did and did not control for clustering. In the May final multivariable model for Rigolet, age group (0–19, 20–55, >55 years), exposure to a puppy in the past month, and consumption of tap-water alternatives (e.g. bottled water and untreated brook water) in the past 2 weeks were significantly associated with increased odds of AGI (Table 4). Final model results indicated a significant difference between models that did and did not account for clustering at the household level; as such, the final mixed multivariable model is presented in Table 4.

Fig. 3. The estimated annual incidence of acute gastrointestinal illness in Hamilton [Reference Thomas29], British Columbia [Reference Thomas29], Ontario [Reference Sargeant, Majowicz and Snelgrove4], Rigolet, and Iqaluit, Canada, using a case definition of self-reported diarrhoea and/or vomiting in the past 28 days not due to pregnancy, medication, drugs/alcohol, or chronic conditions.
Table 2. Estimates of acute gastrointestinal illness (AGI) estimated incidence in Rigolet, Nunatsiavut and Iqaluit, Nunavut, Canada in September 2012 and May 2013

* Study AGI case definition: any diarrhoea and/or vomiting not due to pregnancy, drugs or alcohol, medication or diagnosed chronic conditions.
† International AGI case definition: diarrhoea ⩾3 loose stools and/or vomiting not due to pregnancy, drugs or alcohol, medication or diagnosed chronic conditions [Reference Majowicz61].
‡ A 28-day recall period was not captured in the September 2012 questionnaire.
Table 3. Univariable exact logistic regression* and final multivariable logistic regression (controlling for household clustering) model† results, examining the effects of predictor variables on the odds of acute gastrointestinal illness in Rigolet, Nunatsiavut, in September 2012

OR, Odds ratio; CI, confidence interval.
* The results from the univariable analysis are presented for those variables with P < 0·20.
† Likelihood ratio test comparing the model with and without the household-level variable: variance (0·51, 95% CI 0·007–35·138, P = 0·300); intra-class correlation coefficient (0·14, 95% CI 0·002–0·914). Note that due to the structure of the Rigolet data, a random intercept was forced in the model to control for household-level clustering.
Table 4. Univariable exact logistic regression* and final multivariable logistic regression (controlling for household clustering) results†, examining the effects of predictor variables on the odds of acute gastrointestinal illness in Rigolet, Nunatsiavut, in May 2013

OR, Odds ratio; CI, confidence interval.
* The results from the univariable analysis are presented for those variables with P < 0·20.
† Likelihood ratio test comparing the model with and without the household-level variable: variance (2·54, 95% CI 0·55–11·65, P = 0·011); intra-class correlation coefficient (0·44, 95% CI 0·14–0·78).
In the September final multivariable model for Iqaluit, monthly frequency of eating country fish (i.e. locally harvested fish including salmon, char, trout), washing kitchen counter-tops and cutting boards with soap after preparing meat, employment status of the person responsible for food preparation, and cat exposure in the past month significantly affected the odds of AGI (Table 5). Finally, in the May final multivariable model for Iqaluit, a homeless person staying in the home, as well as an interaction between pet ownership and overcrowding affected the odds of AGI (Table 6).
Table 5. Univariable* and multivariable exact logistic regression model results, examining the effects of predictor variables on the odds of acute gastrointestinal illness in Iqaluit, Nunavut, in September 2012

OR, Odds ratio; CI, confidence interval.
* The results from the univariable analysis are presented for those variables with P < 0·10; however, those with P < 0·20 were considered in the multivariable model building process.
Table 6. Univariable exact logistic results (for those variables with P < 0·20) and final multivariable exact logistic regression model results, examining the effects of predictor variables on the odds of acute gastrointestinal illness in Iqaluit, Nunavut, in May 2013

OR, Odds ratio; CI, confidence interval.
DISCUSSION
The estimated annual incidence rates of AGI in Rigolet and Iqaluit were higher than those reported in other studies using similar methodology in Canada [Reference Majowicz3–5, 29, Reference Majowicz, Horrocks and Bocking30], New Zealand [Reference Adlam35], The Netherlands [Reference Doorduyn36], Italy [Reference Scavia10], Australia [Reference Hall37], Malta [Reference Gauci38], Cuba [Reference Prieto39], Poland [Reference Baumann-Popczyk9], Denmark [Reference Müller, Korsgaard and Ethelberg40], Argentina [Reference Thomas12], USA [Reference Jones7], and Hong Kong [Reference Ho41] (Table 2). It is important to note, however, that we sampled during potentially high-risk AGI seasons, which could result in overestimates. Nonetheless, the estimated annual incidence in the two communities was higher than that observed in Chile during high-risk seasons, using a similar design, case definition, and recall period [Reference Thomas11].
Hunting, trapping, fishing, gathering, and sharing country food are still practised in Rigolet and Iqaluit, and are important aspects of Inuit livelihoods, health, and wellbeing [Reference Cunsolo27]. In Rigolet and Iqaluit, some factors related to country food (consumption of country fish and amount of money spent obtaining country foods) increased the odds of AGI in the statistical model. Other research has suggested that some country foods and country food preparation in the North could increase the risk of AGI [Reference Parkinson and Butler42, Reference Pufall43]. The routes of contamination, as well as preparation methods for country meat in the North are different than retail meats available for purchase, and these transmission pathways are not well understood. Nonetheless, in this study it was not possible to determine whether country food-related factors were direct contributors to AGI, or if these variables were proxy indicators for other risk factors. For instance, in Rigolet, households who spend more money on obtaining country foods might also spend more time hunting, trapping, fishing, and gathering foods, and some research has linked AGI with reduced hygiene practices during camping [Reference Boulware44]. More targeted research should explore more specifically how harvesting practices and country food consumption may affect exposure to, and infection by, agents of AGI in the Arctic, especially in the context of climatic change.
In Rigolet, not visiting a cabin recently significantly increased the odds of AGI in the statistical models, which is likely a proxy indicator for a related risk factor. For many Inuit communities, visiting cabins includes permanent or semi-permanent structures (e.g. small buildings that can be dissembled and relocated, as well as tents, snow-houses) that are located on land typically not owned by an individual and not accessible by road and thus reached by boat or snowmobile. Factors that impact on an individual's access to a cabin include having someone in the home that has the skills to navigate the land and ice, weather conditions (as well as climate changes), fuel and maintenance costs for boats or snowmobiles, and geographical location of the cabin. Past research in the Arctic has documented the importance of going to cabins to connect with the land as critical to good physical and emotional health [Reference Cunsolo26, Reference Cunsolo27]. Several studies have described a relationship between stress and decreased gastrointestinal health [Reference Drossman45, Reference Bhatia and Tandon46], which could perhaps also extend to AGI outcomes. Due to the cross-sectional nature of our study, however, we cannot assess the temporality of this statistically significant finding, nor did we attempt to measure stress in participants. It is possible that people who visit a cabin more frequently have lower levels of stress and thus reduced susceptibility to AGI outcomes; however, this finding could also suggest that those who had AGI were not able to visit the cabin because of their illness. Therefore, along with studies to define the health risks associated with land-based activities, Indigenous public health planning and programming would benefit from more empirical research to examine the physical health and wellbeing benefits of visiting cabins and connecting with the land.
In Rigolet and Iqaluit, exposure to cats and puppies was statistically associated with AGI outcomes, which was also reported in a similar study in Canada [Reference Majowicz3–5, 29, Reference Majowicz, Horrocks and Bocking30] and Chile [Reference Thomas11]. Cats and dogs are known to sometimes asymptomatically carry several pathogens that could cause AGI symptoms in humans [Reference Procter47–Reference Lefebvre49]. We did not test cats or dogs in this study for faecal shedding of AGI pathogens, nor were data on cat and dog exposure captured in other burden of AGI studies in North America [Reference Majowicz3, Reference Sargeant, Majowicz and Snelgrove4, Reference Jones7, Reference Thomas29, Reference Febriani50]. Therefore, we do not know whether cats and dogs were actually involved in exposure of participants of this study to agents of AGI, or if this exposure factor is a surrogate for other risk factors. Further research should examine the potential for cats, dogs, and other domestic pets to be reservoirs of agents of human AGI-related infection in Inuit communities, and also in North America in general.
In Iqaluit, not washing the kitchen counter-top with soap after preparing retail or country meat increased the odds of AGI. This finding is consistent with past research that identified kitchen counter-tops as an important source of foodborne illness [Reference Gorman, Bloomfield and Adley51, Reference Kendall52], which suggests that washing the counter-top after preparing meat reduces the risk of exposure to foodborne pathogens [Reference Gorman, Bloomfield and Adley51, Reference Medrano-Félix53]. Not washing the counter-top with soap might be explained by the transition in diet and food-handling practices in the Arctic. A rapid shift in diet from predominantly eating country foods (e.g. caribou, seal, walrus, whale) to food purchased at retail stores (e.g. chicken, beef, pork) has been documented in most Inuit communities [Reference Sharma54]. This transition in diet also requires a shift in food preparation and handling techniques. For example, in Iqaluit, country meats are commonly prepared on pieces of cardboard on the floor and consumed raw or frozen; after which, the cardboard is disposed of rather than washing and reusing it. Conversely, chicken, beef, and pork is prepared on the counter-top and eaten cooked. Further research is required to adequately understand the knowledge, attitudes, and behaviours regarding country and retail food handling and safety in the North to develop and implement effective public health planning and programming.
We also found that the odds of AGI significantly increased if the person responsible for food preparation in the house was employed (either part-time or full-time), which could be explained by employment limiting time available for proper food safety practices, higher income leading to more risky food consumption behaviours [Reference Yang55–Reference Altekruse57], or employment limiting time available to access the land for country food resulting in increased retail food consumption. Past research suggests that those with higher levels of income are less likely to wash counter-tops after preparing meat and practice other good food preparation techniques in the kitchen than those with lower levels of education and income [Reference Yang55–Reference Altekruse57]. These studies posit that those with higher levels of income are more likely to have higher knowledge regarding safe food-handling practices and behaviours, but still partake in the risky behaviour. The reasons for these trends might be due to cultural influences or social norms [Reference Yang55], but are not clear and warrant future research.
Several Inuit communities face water-related challenges in terms of the provision of safe municipal drinking water [Reference Martin58], effects of climate change and high impact weather events on drinking-water safety [Reference Harper20, Reference White59], and the preference of many residents to seek alternative drinking-water sources including brooks, streams, ice, and snow [Reference Martin58]. In using these alternative sources of drinking water, residents could be at higher risk for AGI, which could explain why drinking tap water in Rigolet was associated with significantly reduced odds of AGI. Alternatively, residents in Rigolet have reported perceived serious concerns about the safety of tap water [Reference Goldhar, Bell and Wolf60]; thus, this finding could be explained by AGI cases perceiving tap water as the source of their illness, and thus choosing not to drink tap water after being ill. Further research should specifically investigate the fraction of AGI attributable to drinking water, as well as the temporal relationship (both seasonally and interannually) between weather patterns, water quality, and AGI in Northern communities.
As discussed by other researchers, this study had several limitations that are not uncommon in burden of AGI studies [Reference Scallan6, Reference Thomas11, Reference Thomas12, Reference Majowicz61]. First, this study was conducted in only two Inuit communities, and Inuit communities are very heterogeneous in culture, language, mobility, and geographical locations. While we attempted to sample two communities to capture information from a small and large Inuit settlement, urban and remote settings, and from two Inuit regions, caution should be exercised in generalizing the results to the Nunatsiavut region, Nunavut Territory, or Inuit communities in general. Second, this study was conducted at two points in time during the year, which could result in an over- or under-estimate of the annual incidence of AGI. Collecting data longitudinally over an entire year or multiple years would enable more accurate estimates of AGI incidence. Third, while we excluded cases of AGI that were due to diagnosed chronic conditions, those cases with undiagnosed AGI-related chronic conditions might have been misclassified as a case. Fourth, considering the structure of the data and environmental modes of AGI transmission, in Rigolet we were surprised to find no significant clustering at the household level in the September survey; this warrants further research. Fifth, the outcome measure in this study used a self-reported case definition; pathogen testing was beyond the scope of this study. Our case definition was non-specific; however, we chose this outcome measure and case definition to match other Canadian studies to facilitate comparisons and inform decision-making processes that are based on national surveillance systems, as well as to capture community-level burden of illness (compared to burdens estimated from surveillance data). There are biases inherent in self-reported case definitions, including recall bias, interviewer bias, and misclassification bias. We have assumed that these biases are similar between Rigolet and Iqaluit, and with other burden of AGI studies using similar methods. Finally, while we attempted a census sample in Rigolet, the sample size was still relatively small for statistical modelling. The high response rate and no significant differences between demographics between the survey population and the Canadian census suggests a representative sample, which is unique and an important contribution as representative samples are not common in Indigenous health research, and can be challenging to achieve in many Indigenous communities [Reference Ford62].
CONCLUSION
This study estimated AGI incidence at the community level in Rigolet and Iqaluit, and found a higher estimated incidence of AGI in these communities compared to other similar studies in Canada and abroad. Several factors were associated with increased odds of AGI, including food sources, water sources, animal exposure, overcrowding, and food safety practices. Information on this increased incidence of AGI and these potential AGI risk factors offers important information for public health planning, prioritization, and programming in Inuit regions. While this research estimates the incidence and potential risk factors of AGI in Rigolet and Iqaluit, the improved understanding of AGI experienced at the community-level in a Canadian Indigenous community sheds light on the need to better understand the burden of AGI in subsets of the population that might be at higher risk of AGI, including Indigenous populations.
APPENDIX
Indigenous Health Adaptation to Climate Change Research Group
Lea Berrang-Ford, Cesar Carcamo, Alejandro Llanos, Shuaib Lwasa, Didacus Bambaiha Namanya.
ACKNOWLEDGEMENTS
First and foremost, we thank the residents in Iqaluit and Rigolet for welcoming us into their community, engaging with us throughout the project, and contributing to the larger IHACC research project. We are grateful to all the surveyors in Rigolet (M. Baikie, I. Shiwak, D. Wolfrey) and Iqaluit (M. Naglingniq, S. Kownirk, G. Akpik, M. Atagoyuk, N. Atagoyuk, T. Atagoyuk, J. Campbell, N. Campbell, P. Davidee, A. Kilabuk-Degrasse, N. Lewis, S. Lewis, L. Mark, D. McGlade, E. Nevin, O. Nowdlak, I. Oopakak, M. Petooloosie, M. Pillaktuaq, M. Unahah). This survey would not have been possible without their diligent work and expertise. We also thank M. Baikie, P. Workman, K. Hutchinson, W. Joy, and many others in the Department of Health and Social Services, Government of Nunavut; J. Shirley and M. E. Thomas and others at the Nunavut Research Institute; M. Wood, G. Turner, M. Kinney, T. Buckle and others at the Department of Health and Social Development, Nunatsiavut Government; M. LeBlanc-Havard, C. Brice-Bennett, G. Elliott and others at the Labrador Grenfell Health Authority; C. Wolfrey, S. Blake, S. Wolfrey, and M. Williams at the Rigolet Inuit Community Government; and S. Maclachlan, N. Markwick, C. Huet, J. Ostapchuk, M. E. MacDonald, Y. Guo, and J. Fraser at the University of Guelph. Thanks are due to S. Majowicz for her assistance and burden of AGI expertise. Particular thanks go to A. Bunce and P. Arpin for their dedication throughout the project. These people played instrumental roles in contributing to the study design, data collection, data interpretation, and ultimately the success of this project. This research is part of an international project entitled the ‘Indigenous Health Adaptation to Climate Change’ project, with parallel field study sites in Uganda and Peru. The study design and conduct were independent of the funding source. This work was supported by the Public Health Agency of Canada; the Nasivvik Centre for Inuit Health and Changing Environments; the International Research Initiative on Adaptation to Climate Change (IRIACC) funded by the International Development Research Centre (IDRC), Canadian Institutes for Health Research (CIHR), Social Sciences and Humanities Research Council (SSHRC), and National Science and Engineering Research Council (NSERC); and a Vanier Canada Graduate Scholarship to S. Harper (CIHR).
DECLARATION OF INTEREST
None.












