Recognition of vitamin D deficiency early in life is important due to its effect on skeletal and non-skeletal health(Reference Wagner and Greer1, Reference Misra, Pacaud and Petryk2). Vitamin D is an essential micronutrient for good bone health, with low 25-hydroxyvitamin D (25OHD) levels linked to rickets in children, a resurgence of which has been reported across many countries(Reference Welch, Bergstrom and Tsang3). As vitamin D is important for the innate immune system, it may also have implications in neonatal sepsis(Reference Adams and Hewison4). Furthermore, vitamin D deficiency in infancy may be a risk factor for extra-skeletal chronic disease later in life such as autoimmune diseases like type 1 diabetes mellitus and atopy, although evidence to date is inconclusive(5). Preterm infants are at particular risk of vitamin D deficiency because they are dependent on maternal vitamin D status in utero (which is in turn dependent on both sunlight exposure and oral vitamin D intake) and on nutritional intake after birth for vitamin D supply(Reference Wagner and Greer1, Reference Misra, Pacaud and Petryk2). However, there is controversy about the adequate daily dose, duration and type of vitamin D supplementation.
The Institute of Medicine (IOM) recently revised the dietary reference intakes for the USA and Canada(5, Reference Ross, Manson and Abrams6). Using a risk assessment model, they specified the estimated average requirement that meets the average need for vitamin D and the recommended daily allowance that meets the need of 97·5 % of the population. Furthermore, accepting 25OHD as a biomarker of exposure but not as a biomarker of effect, they specified that a 25OHD level of 40 nmol/l corresponded to the estimated average requirement and that a level of 50 nmol/l corresponded to the recommended daily allowance. So, 25OHD is both a measure of the adequacy of supply and of risk for disease, but is not in itself a clinical outcome(Reference Aloia7).
For infants from birth to 1 year, the IOM does not specify an estimated average requirement or a recommended daily allowance for vitamin D due to lack of studies(5, 8). Instead, an adequate intake that is likely to meet the needs of most has been specified at 10 μg (400 IU) daily(Reference Wagner and Greer1, 5). There are even fewer data available to guide about nutritional intake in preterm infants. The IOM suggested that vitamin D intakes ranging from 4 μg (160 IU) to 10 μg (400 IU) daily seem adequate(5), while the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) advised a higher vitamin D intake of 20–25 μg (800–1000 IU) daily from a combination of feeds and supplements, and set a higher threshold level for serum 25OHD at 80 nmol/l(Reference Agostoni, Buonocore and Carnielli9).
As the adequacy of the above-mentioned recommendations in routine clinical practice in preterm infants has not been evaluated, we aimed to do so and to assess the response to long-term augmented vitamin D nutritional intake of ≥ 10 μg (400 IU) daily in those with a first serum 25OHD level < 50 nmol/l.
Methods
Patient population
A consecutive sample of stable preterm very low birth weight (VLBW) (gestation ≤ 32 weeks or birth weight ≤ 1·5 kg) infants, admitted to the Neonatal Unit over 26 months (June 2008–July 2010), was audited. This is a tertiary referral university centre, with approximately 10 000 deliveries and 160 VLBW admissions annually, of which approximately 50 % are transfers from other hospitals. All convalescent-stable VLBW infants were eligible for inclusion, but infants were excluded if they were transferred or died before meeting this criterion. Serum 25OHD level was first assessed once each infant was tolerating feeds. Demographic data gathered included gestational age, birth weight, sex, ethnicity, season of birth and chronological age at time of first serum 25OHD assessment. This was a prospective audit of routine clinical practice in our unit and did not involve either additional blood tests or alteration in the management of the infants concerned.
Vitamin D nutritional strategy
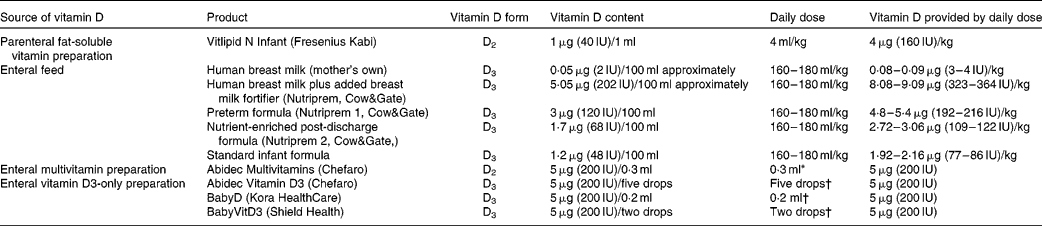
Vitamin D intake was augmented from birth according to our clinical practice guidelines, as outlined below, in order to achieve and maintain serum 25OHD levels ≥ 50 nmol/l. At first, infants received vitamin D2 from intravenous fat-soluble vitamin supplements (Vitlipid N, Fresenius Kabi), prescribed while receiving parenteral nutrition at a dose that provided 4 μg (160 IU)/kg body weight daily (Table 1). Infants then advanced to enteral feeds, which were expressed breast milk (vitamin D3 0·05 μg (2 IU)/100 ml)(Reference Misra, Pacaud and Petryk2) plus breast milk fortifier (vitamin D3 5·05 μg (202 IU)/100 ml), preterm formula milk (vitamin D3 3 μg (120 IU)/100 ml) or a combination of both. Finally, after establishment of full enteral feeds, vitamin supplements were commenced that provided an additional 5 μg (200 IU) vitamin D2 daily using Abidec Multivitamins (Chefaro®). We aimed to ensure a vitamin D intake of ≥ 10 μg (400 IU) daily, consisting of ≥ 5 μg (200 IU) vitamin D3 from enteral feeds and 5 μg (200 IU) vitamin D2 from supplements.
Table 1 Vitamin D content in different sources given to preterm infants

* This is the standard dose administered to all preterm inpatients. After discharge from hospital, if serum 25-hydroxyvitamin D < 50 nmol/l and infants were receiving predominantly breast milk, twice the standard dose was recommended; while infants receiving predominantly formula milk were recommended to continue the standard dose.
† A small number of patients (n 23) in our follow-up audit were given vitamin D3 as a supplement after discharge due to a new national policy in Ireland recommending vitamin D3 supplementation with 5 μg (200 IU) daily for all infants.
Audit of response to prolonged augmented vitamin D intake
In those infants with a first 25OHD level < 50 nmol/l, we aimed to audit their vitamin D status again at about 6 weeks corrected age (calculated by subtracting the number of weeks born before 40 weeks of gestation from the chronological age(Reference Engle10)). For these infants, we aimed to continue a daily vitamin D intake of ≥ 10 μg (400 IU) after discharge, in keeping with American Academy of Pediatrics' recommendations(Reference Wagner and Greer1). After discharge, breast milk fortifier was no longer added to breast milk, and formula milk was changed to a nutrient-enriched post-discharge formula (vitamin D3 1·7 μg (68 IU)/100 ml); later, this formula was changed to standard formula milk (vitamin D3 1·2 μg (48 IU)/100 ml) depending on the infant's growth. In order to continue a daily vitamin D intake of ≥ 10 μg (400 IU), for infants who were breastfed or partially breastfed, we recommended supplementation with 10 μg (400 IU) vitamin D2 daily, but for those predominately taking formula milk, we advised supplementation with 5 μg (200 IU) vitamin D2 daily. Near the end of our audit, the supplemental form of vitamin D was switched from vitamin D2 to D3, as a consequence of a national policy of infant vitamin D3 supplementation in Ireland (Table 1).
Laboratory methods
Serum total alkaline phosphatase (ALP), total corrected Ca and P were measured using standard automated techniques. Serum total ALP was missing in eleven patients, and both serum-corrected Ca and serum P were missing in one patient. Serum 25OHD levels were measured by competitive RIA (Immunodiagnostic Systems Limited), as previously described(Reference Lonergan, Kinsella and Fitzpatrick11). We participate in the Vitamin D External Quality Assessment Scheme(Reference Carter, Berry and Gunter12).
Statistics
Descriptive statistics are presented as medians and interquartile ranges (IQR), means and standard deviations or numbers and percentages. The total group was divided into three sub-groups based on IOM thresholds for 25OHD (Tables 2 and 3). Differences between mean values for continuous variables were tested by one-way ANOVA, with Bonferroni correction for post hoc comparisons. Differences between categorical variables were tested by χ2 test. Bivariate associations were assessed by Pearson's correlation coefficients or Spearman's ρ coefficients. We conducted a forward multiple linear regression analysis in order to identify independent associations of individual factors in determining serum 25OHD levels at first assessment. We selected independent variables for the regression model if bivariate analysis was P< 0·20 and if there was no evidence of multicollinearity, as judged by correlation coefficient P< 0·8(Reference Katz13). Results with P< 0·05 were considered statistically significant. Statistical analysis was performed using SPSS for Windows version 20.0 (SPSS, Inc.).
Table 2 Categorical variables of the total group and divided according to first vitamin D status

25OHD, 25-hydroxyvitamin D.
* Value was significantly different between the three groups.
Table 3 Continuous variables of the total group and divided according to first vitamin D status† (Mean values and standard deviations)

25OHD, 25-hydroxyvitamin D; ALP, alkaline phosphatase.
* Mean values were significantly different between the three groups.
† Total number = 274, except for serum total ALP (n 263), serum Ca (n 273) and serum P (n 273). Laboratory serum reference values: Ca, 1·8–2·7 mmol/l; P, 1·62–2·52 mmol/l; total ALP 3·65–25 μg (146–1000 IU)/l.
Results
Descriptive statistics
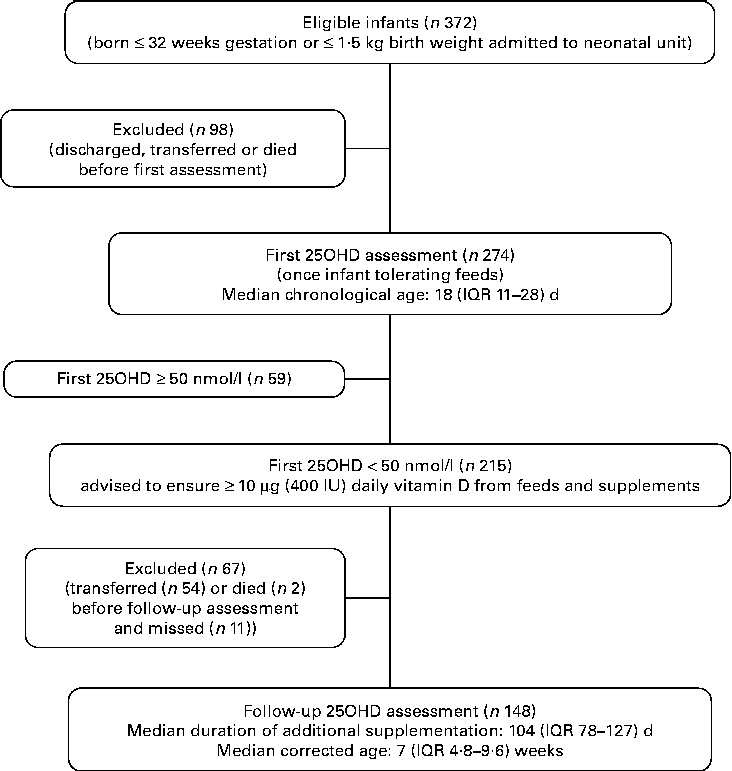
We evaluated 372 preterm VLBW infants who were admitted to the Neonatal Unit during the audit period; 274 infants were entered into the clinical audit and had blood drawn for serum 25OHD assessment and the remaining ninety-eight infants were discharged, transferred to another hospital or died prior to assessment (Fig. 1). Comparing those studied and those not, there was no statistical difference with respect to gestational age at birth, birth weight, prevalence of infants with birth weight below the 9th centile and sex.

Fig. 1 Flow-chart outlining first and follow-up audit. 25OHD, 25-hydroxyvitamin D; IQR, interquartile range.
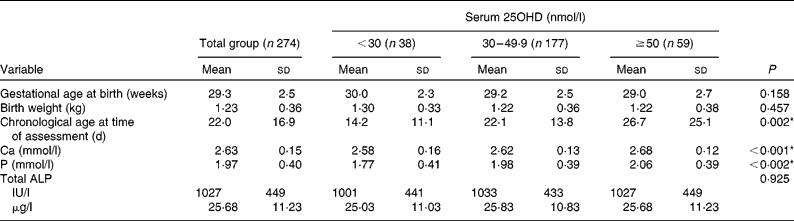
The mean for gestational age at birth was 29·3 (sd 2·5) weeks and for birth weight was 1·23 (sd 0·36) kg. There were 156 (57 %) male and 118 (43 %) female infants; 245 (89 %) were Caucasian and twenty-nine (11 %) non-Caucasian; and sixty-nine (25 %) had a birth weight < 9th centile (Tables 2 and 3). The median for chronological age at time of first serum 25OHD level assessment was 18 (IQR 11–28) d. Of the 274 infants who had a first assessment, 170 (62 %) were assessed prior to initiation of additional 5 μg (200 IU) vitamin D2 supplements and 104 (38 %) were assessed afterwards. In the latter group, the median of duration of additional vitamin D supplementation prior to assessment of serum 25OHD level was 12 (IQR 5–20) d.
Vitamin D status
The median for first serum 25OHD was 39·4 (absolute range 20·1–116·0) nmol/l. We interpreted the vitamin D status with respect to the IOM report that was published after completion of our first data collection. According to the IOM estimate of serum 25OHD status that corresponded to the IOM's specification on the distribution of vitamin D requirement(5), the following prevalence figures were noted: thirty-eight (14 %) below the level associated with increased risk of rickets ( < 30 nmol/l); 177 (65 %) within the IOM range of adequacy (30–49·9 nmol/l); and fifty-nine (22 %) above the IOM target level that is associated with sufficiency in 97·5 % of the population(5, Reference Aloia7) ( ≥ 50 nmol/l). In those assessed prior to receiving additional vitamin D supplementation (n 170), compared with those who had started additional supplementation at the time of assessment (n 104), the respective prevalence figures were as follows: serum 25OHD levels below 30 nmol/l (19 v. 5 %); between 30 and 49 nmol/l (61 v. 70 %); and ≥ 50 nmol/l (19 v. 25 %) (P= 0·002).
Relationship between vitamin D status and clinical measurements
Higher first serum 25OHD levels were associated significantly with greater chronological age at the time of assessment (P= 0·002) and having started additional vitamin D supplementation at time of assessment (P= 0·003) (Tables 2 and 3). Serum 25OHD levels correlated in order of significance with the following variables: duration of vitamin D supplementation (r 0·456; P< 0·001); chronological age at the time of assessment (r 0·368; P< 0·001); having started additional vitamin D supplementation at the time of assessment (ρ = 0·245; P< 0·001); and gestational age at birth (r − 0·187; P= 0·002); but not with ethnicity, sex, season of birth or birth weight. After adjusting for those who received additional vitamin D supplementation and the duration of administration prior to assessment, serum 25OHD levels still correlated with chronological age at the time of assessment (r 0·190; P= 0·002), indicating a positive relationship between duration of enteral feeding and vitamin D status irrespective of additional supplementation.
The multivariable analysis of first serum 25OHD levels included the following four independent variables that meet the selection criteria: gestational age at birth, chronological age at time of serum 25OHD assessment, having started additional supplementation at the time of assessment and duration of additional vitamin D supplementation prior to assessment. In the forward linear regression model, the predictors of serum 25OHD levels were duration of vitamin D supplementation prior to assessment and gestational age at birth (adjusted r 2 0·215; P< 0·001).
Relationship between vitamin D status and secondary indices of vitamin D deficiency
The relationship between first serum 25OHD levels and secondary indices was examined by correlation analysis. Following adjustment for chronological age at time of assessment, birth weight and gestational age at birth, serum 25OHD levels correlated negatively with serum total ALP (r − 0·123; P= 0·047) and positively with both serum Ca (r 0·226; P< 0·001) and serum P (r 0·263; P< 0·001). Serum P correlated with both serum Ca (r 0·174; P= 0·004) and with serum total ALP (r − 0·321; P< 0·001); serum Ca correlated with serum total ALP (r − 0·150; P= 0·015).
Follow-up assessment in infants with first serum 25-hydroxyvitamin D levels <50 nmol/l
First serum 25OHD levels were < 50 nmol/l in 215 infants, of which follow-up serum 25OHD level was assessed in 148. Of the remaining sixty-seven infants, fifty-four had been transferred to other hospitals and results were not available, two had died and eleven missed testing. Comparing those assessed (n 148) with those not (n 67), there was no significant difference with respect to first serum 25OHD level, ethnicity or sex.
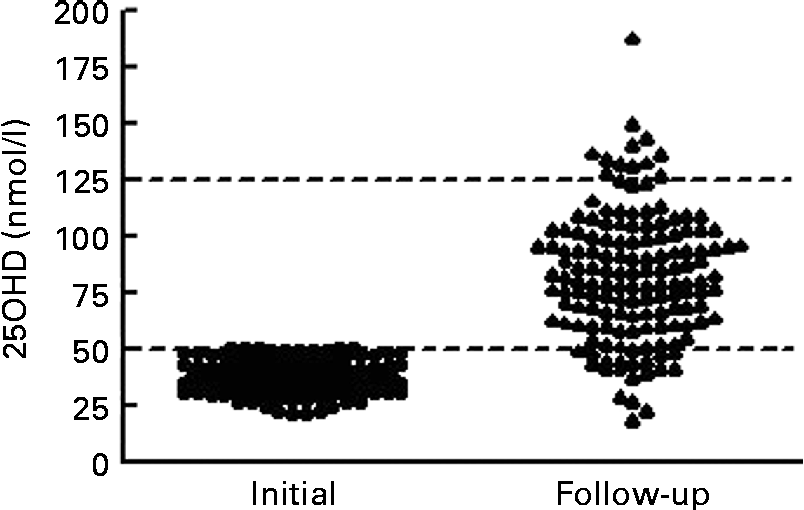
At the time of follow-up assessment, the median for duration of supplementation was 104 (IQR 78–127) d. The median for corrected age was 7·0 (IQR 4·8–9·6) weeks. Mean serum 25OHD levels increased significantly from 36·7 (sd 7·2) nmol/l to 82·7 (sd 28·6) nmol/l (t= 19·5; P< 0·001). The median increase in 25OHD was 44·3 (IQR 29·2–63·4) nmol/l. Only four infants (3 %) had serum 25OHD levels < 30 nmol/l, sixteen (11 %) were between 30 and 49·9 nmol/l and 128 (87 %) were ≥ 50 nmol/l. We noted that twelve (8 %) infants achieved serum 25OHD levels that exceeded 125 nmol/l (Fig. 2). On post hoc analysis, we defined a poor response as failure to achieve serum 25OHD level ≥ 50 nmol/l coupled with an increment of < 10 nmol/l; these criteria were met in fifteen of 148 infants (10 %). Of the fifteen infants, five had necrotising enterocolitis: one infant had stage III necrotising enterocolitis with a gastrointestinal perforation; four had stage I or II necrotising enterocolitis, not requiring surgical intervention; but all made a full recovery and tolerated feeds subsequently. Regarding the infant with the highest level (187·9 nmol/l) at follow-up assessment, this infant had a first serum 25OHD level of 43·6 nmol/l. We contacted the parent and found that the infant was receiving a nutrient-enriched formula (vitamin D3 1·7 μg (68 IU)/100 ml) and twice the recommended supplementation dose of vitamin D (10 μg (400 IU) vitamin D3 daily) at the time of follow-up assessment (after 114 d and at 7 weeks corrected age). This provided approximately 21·25 μg (850 IU) vitamin D3 daily (11·25 μg (450 IU) daily from feeds plus 10 μg (400 IU) daily from supplements). They were advised to cease supplementation and the nutrient-enriched formula, and to use instead standard formula milk (1·2 μg (48 IU)/100 ml vitamin D3). This reduced their intake to approximately 8 μg (320 IU) vitamin D3 daily. After 10 weeks, at 17 weeks corrected age, the serum 25OHD level was 133·9 nmol/l.

Fig. 2 Response to vitamin D (intake ≥ 10 μg (400 IU)) in infants with first serum 25-hydroxyvitamin D (25OHD) below 50 nmol/l (n 148). Median duration of augmented vitamin D intake was 104 (interquartile range 78–127) d. Lines are drawn at the Institute of Medicine thresholds for recommended daily allowance at 50 nmol/l and for risk of toxicity at 125 nmol/l.
Vitamin D2 and D3
A small number of patients (n 23) in our follow-up audit were given vitamin D3 rather than D2 as a supplement after discharge, due to a new national policy in Ireland recommending supplementation with 5 μg (200 IU) vitamin D3 daily for all infants from birth to 12 months. The mean serum 25OHD was 25 % higher in those who received vitamin D3 compared with vitamin D2 (99·8 (sd 37·8) v. 79·8 (sd 25·5) nmol/l; P= 0·023).
We found a significant correlation between change in 25OHD and duration of supplementation after correction for form of vitamin D administered, sex, ethnicity and first 25OHD level (r 0·192; P= 0·021).
Discussion
The primacy of nutritional vitamin D intake over sunlight exposure in the prevention and correction of vitamin D deficiency is long established(Reference McKenna14), and is never as germane as in preterm infants. In this prospective audit of a representative sample of preterm or VLBW infants at a median of 3 weeks chronological age, 78 % had a serum 25OHD level below our target of 50 nmol/l; however, only 14 % had a level < 30 nmol/l, reflecting the benefit of our policy of augmented vitamin D intake from birth. The principal determinant of serum 25OHD in the linear regression model was duration of additional vitamin D supplementation. The fact that first serum 25OHD correlated, weakly but significantly, with secondary indices, namely serum total ALP, Ca and P, may indicate that low serum 25OHD levels in these preterm infants already has had some adverse metabolic effect. In the present follow-up audit of infants with first serum 25OHD levels < 50 nmol/l, after a median duration of additional supplementation of 104 d, 87 % achieved levels ≥ 50 nmol/l. The outcome may have been even better, because in 10 % of infants we noted poor response based on our conservative criteria, suggesting either poor adherence or clinical conditions that affected response. Conversely, overcorrection was noted in 8 %, in that serum 25OHD exceeded 125 nmol/l; this was identified by the IOM as potentially indicating risk of harm(5). On the basis of the present findings, we suggest that a vitamin D nutritional intake of 10 μg (400 IU) daily, which is combined from feeds and supplements, achieves 25OHD levels ≥ 50 nmol/l in the majority. In the long term, once target levels have been achieved, a lower vitamin D intake for preterm infants may be adequate to maintain levels and obviate overcorrection, but strategies for compliance need to be addressed.
The possible reasons for the low serum 25OHD levels in our preterm infants once established on full feeds include: probable low vitamin D status at birth, because transplacental transfer of vitamin D mainly occurs in the last trimester and poor maternal vitamin D status at the time of birth in view of the close correlation between maternal serum 25OHD and cord 25OHD levels(Reference Misra, Pacaud and Petryk2, Reference Viljakainen, Saarnio and Hytinantti15). Pregnant women in Ireland and Great Britain frequently have poor vitamin D status(Reference O'Riordan, Kiely and Higgins16, Reference Holmes, Barnes and Alexander17). Sub-optimal vitamin D status among Irish females of childbearing age and adolescents has also been reported similar to other northern European countries(Reference Hill, Cotter and Mitchell18–Reference O'Sullivan, Nic Suibhne and Cox23). Ireland is at latitude 51–55° North and there is no UV radiation of the appropriate wavelength above 42° latitude in winter(Reference Webb, Kline and Holick24). However, we did not find a relationship between season of birth and 25OHD levels. Poor vitamin D status is increasingly recognised in neonates born to mothers with either dark skin or concealing clothing(Reference Dijkstra, van Beek and Janssen25). However, we found no relationship between ethnicity and serum 25OHD. Of course, poor vitamin D status may be less relevant in preterm infants compared with term infants, because the former seem to rely on passive absorption of Ca and P, with absorption being maximised by both the use of fortified breast milk and special preterm formulas; it is not clear what role vitamin D has at this stage with respect to Ca homeostasis, notwithstanding its purported role with respect to non-calcaemic issues(Reference Bronner, Salle and Putet26, Reference Abrams, Fledman, Pike and Abrams27).
Serum 25OHD is both a measure of vitamin D supply and an indicator of risk of disease. There has been a trend over the past two decades to define vitamin D deficiency in terms of the measured level, with thresholds steadily increasing from 25 up to 75 nmol/l(Reference Holick, Binkley and Bischoff-Ferrari28). The IOM demonstrated that serum 25OHD is not a health outcome; it is a measure of risk of a health outcome such as rickets. The IOM shunned the term vitamin D insufficiency, and it demonstrated that the risk of adverse skeletal outcomes is virtually absent when the serum 25OHD level exceeds 50 nmol/l(5). The IOM specified an adequate intake of 10 μg (400 IU)/d for infants from birth to 1 year, but noted that adequate intake, not being a recommended daily allowance, may be an overestimate of requirement. For preterm infants, the IOM did not make any specifications, but suggested that the need for vitamin D may be less in preterm infants, in view of their dependence on passive Ca absorption, and that any need was probably 4–10 μg (160–400 IU)/d(5). We found that vitamin D nutritional intakes of ≥ 10 μg (400 IU) daily achieved serum 25OHD levels of ≥ 50 nmol/l in 87 %.
The next consideration is to determine the upper safe serum 25OHD level, which some experts have set at 250 nmol/l(Reference Holick, Binkley and Bischoff-Ferrari28). The IOM expressed concern about levels above 125 nmol/l, based on emerging evidence about risks that could not be defined in the usual terms of vitamin D toxicity(5). While there are widely different views on the definition of both sufficiency and toxicity, the IOM report surpasses all other reports in scientific rigour and probity and is now the standard regarding vitamin D requirement in all age groups(Reference Aloia7, Reference Rosen, Abrams and Aloia29). In the present follow-up audit after a median duration of 104 d, 8 % had levels >125 nmol/l on an estimated nutritional intake of ≥ 10 μg (400 IU) daily. We observed a very high serum 25OHD level of 187·9 nmol/l in one infant, whose vitamin D intake at 21·25 μg (850 IU)/d exceeded our recommendation. This led us to question if the ESPGHAN recommendation of 20–25 μg (800–1000 IU) daily for preterm infants may be excessive for routine clinical practice, and may seem to be justified for a limited duration. The evidence in term infants is that a daily dose of 6·25 μg (250 IU) is safe and effective in achieving and maintaining adequate vitamin D status(Reference Siafarikas, Piazena and Feister30).
The present audit has a number of limitations such as absence of data on maternal vitamin D status or maternal vitamin D intake. We did not formally evaluate a vitamin D dose response, in that infants were not tested prior to intervention and the exact intake for each infant was not measured, but rather we assessed the efficacy of the present protocol of vitamin D intake at achieving serum 25OHD levels ≥ 50 nmol/l. We could not control for other potential determinants of vitamin D status such as intercurrent illness, progression through steps of augmenting vitamin D intake and adherence to advice regarding supplementation following discharge.
We must also acknowledge two issues with respect to the use of vitamin D2 compared with vitamin D3. The first is assay methodology; the present assay detected 75 % of 25OHD2 and 100 % of 25OHD3. The second is comparative effectiveness of similar doses of the two forms of vitamin D in raising total 25OHD levels. Two studies using physiological doses of vitamin D of 25 μg (1000 IU) daily either show no difference in the rise in 25OHD levels(Reference Holick, Biancuzzo and Chen31) or a 30 % higher rise in serum 25OHD levels with vitamin D3 compared with vitamin D2, but without any difference in free serum 25OHD or serum parathyroid hormone levels(Reference Glendenning, Chew and Seymour32, Reference Chew, Inderjeeth and Glendenning33). Taking assay methodology and potential differences in serum 25OHD response to the administration of different forms of vitamin D, and given that our infants received about 50 % of their intake as vitamin D2, it is likely that we have underestimated their serum 25OHD levels by about 10–15 % at first assessment. In the follow-up audit, as some were taking the higher dose of vitamin D2 supplement, we may have underestimated levels by 20–30 %. We were able to confirm, in part, this underestimation, because a small number of patients (n 23) in the present follow-up audit were given vitamin D3 as a supplement after discharge due to a new national policy in Ireland recommending vitamin D3 supplementation with 5 μg (200 IU) daily for all infants. The mean serum 25OHD level was 25 % higher in those who received supplements with vitamin D3 compared with vitamin D2.
Conclusion
We conclude that poor vitamin D status is an issue for preterm infants, warranting early nutritional intervention to prevent and correct. Nutritional intake of 10 μg (400 IU) daily of vitamin D from all sources, starting as soon as possible from birth, achieves adequate vitamin D status. If serum 25OHD levels remain below 50 nmol/l, then adherence should be questioned. In the long term, once adequate status has been achieved, a lower vitamin D intake may be sufficient to maintain levels and obviate overcorrection. A long-term randomised controlled trial comparing different intakes of vitamin D and evaluating multi-organ outcomes is warranted to determine the desired level of serum 25OHD for optimal health in preterm infants and the nutritional intake that is needed to safely achieve this level.
Acknowledgements
Authors' contributions were as follows: R. A. M. had responsibility for patient recruitment, data collection and contributed to protocol development, data analysis and writing the manuscript; M. J. M. had responsibility for data analysis and contributed to protocol development and writing the manuscript; O. O. and O. U. contributed to patient recruitment; B. F. M., J. J. B. and M. T. K. contributed to laboratory assessments; J. F. M., A. T., C. P. O. and N. P. M. contributed to protocol development; and E. J. M. had responsibility for protocol development and contributed to data analysis and writing the manuscript. We also wish to acknowledge the assistance of C. McCafferty and C. Murphy (Neonatal Clinical Nurse Specialists) and L. Phillips (Administration). This was an audit of our hospital policy of augmented vitamin D intake to a level of intake that was in keeping with present guidelines. As it was an audit of a routine clinical practice, we did not seek formal Ethics Committee approval. There was no conflict of interest that inappropriately influenced the action of any of the authors and the present research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.