In 2020, the WHO reported that there were 727 million older adults worldwide. Over the next three decades, this number is projected to more than double(1). As the population ages, there is an increased demand for healthcare resources and costs, particularly for management of chronic conditions, disease prevention and providing appropriate treatment(2).
Older adults are frequently affected by malnutrition, which has a prevalence of 8·5–28·0 % depending on the type of healthcare setting(Reference Leij-Halfwerk, Verwijs and van Houdt3). Malnutrition is 1·2–2·3 times more prevalent in hospitalised elderly patients compared with patients aged < 65 years(Reference Dent, Hoogendijk and Visvanathan4). Malnutrition leads to adverse outcomes including decreased quality of life, increased hospitalisation, disability and higher mortality(Reference Griffin, O’Neill and O’Connor5,Reference Söderström, Rosenblad and Thors Adolfsson6) . The causes of inadequate nutrition in older adults can be physiologic, pathologic, sociologic or psychological. Malnutrition is often caused by one or more of the following factors: physiological changes in gastrointestinal and sensory functions, psychosocial factors such as loneliness and depression, sedentary behaviour and disease-related inflammation, respectively, diabetes mellitus(Reference Norman, Haß and Pirlich7).
Type 2 diabetes mellitus (T2DM) is a prevalent chronic disease among older adults(8) that considerably increases the risk of inadequate nourishment. Compared with older adults without T2DM, those with T2DM have a higher prevalence and risk of malnutrition(Reference Junaid, Ojo and Adejumo9,Reference Sanz París, Gercia and Gómez-Candela10) . This increased risk is attributed partly to T2DM-related autonomic neuropathy manifesting as anorexia, gastroparesis, effects of antihyperglycaemic drugs and extreme dietary restriction or low-energy intake, which aim at blood glucose control, that can also contribute to malnutrition(Reference Liu, Chen and Yang11–Reference Umphonsathien, Rattanasian and Lokattachariya13).
Blood glucose levels need to be kept under control in patients with T2DM to reduce their risk of developing diabetes-related complications, especially those with HbA1c levels of ≤ 6 %(14,Reference Ohkubo, Kishikawa and Araki15) . Older adults with T2DM should have adequate glycaemic controlled(Reference Arnold, Lipska and Wang16) which is defined as the blood sugar levels kept in HbA1c target by changing behaviour or dietary restriction(Reference Sellahewa, Khan and Lakkunarajah17). However, it is important to consider that targeted HbA1c levels for older adults are more flexible than in other age groups due to the possible risk of hypoglycaemia, frailty(18) or even malnutrition(Reference Vischer, Perrenoud and Genet19). According to previous studies, a decrease in blood HbA1c level has been associated with a subordinate nutritional status(Reference Vischer, Perrenoud and Genet19,Reference Uyar, Gorar and Kok20) . This is presumably caused by endothelial dysfunction and reduced nutrient intake due to excessive dietary restriction, resulting in lower blood glucose levels(Reference Al-Adwi, Al-Haswsa and Alhmmadi21). Based on this background, it is hypothesised that older adults with T2DM who have well glycaemic control may be at risk of malnutrition. Although there are conflicting reports about HbA1c levels, some studies show an association between decreased levels and malnutrition as mentioned, while others indicate a relationship with elevated levels(Reference Vischer, Perrenoud and Genet19,Reference Uyar, Gorar and Kok20,Reference Kaya, Demir and Cinemre22,Reference Atif, Saleem and Asghar23) . Moreover, the association between individualised glycaemic control and malnutrition remains unclear. Therefore, the primary aim of this study was to investigate the relationship between well-managed glycaemic control and malnutrition, and the secondary aim was to determine the other factors contributing to malnutrition among older adults with T2DM. Understanding this association is crucial to providing comprehensive care to this vulnerable population.
Experimental methods study population and ethical approval
This study was cross-sectional and conducted at the primary care unit (PCU) and general practice (GP) clinic of Songklanagarind Hospital in Thailand. The study participants were outpatients who had appointments and follow-ups at both clinics between April and October 2022. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Ethics Committee of the Faculty of Medicine, Prince of Songkla University (REC65-167-9-4). Written informed consent was obtained from all subjects. The two independent proportion methods were used to estimate the sample size by the n4Studies application(Reference Ngamjarus24), with an α error of 0·05 and a β error of 0·2. The formula includes all variables that previous studies have reported the association to malnutrition, such as glycaemic control(Reference Atif, Saleem and Asghar23), age(Reference Saintrain, Sandrin and Bezerra25), sex(Reference Ghosh, Dasgupta and Paul26), income, multiple co-morbidities, oral problems, chronic pain(Reference Boulos, Salameh and Barberger-Gateau27), education, cognition, functional ability, polypharmacy(Reference Bakhtiari, Pourali and Omidvar28), living alone(Reference Alzahrani, El Sayed and Alshamrani29), unemployment, depression(Reference Abdu, Yimamu and Kahsay30), rural residency(Reference Hefnawy31), diabetes duration(Reference Ayub and Ismail32) and digestive problems(Reference Chang33). Totally, the largest sample size needed was 287 participants, which was calculated by substituting the marital status variable with values of 0·18, 0·34 and 0·36 for the proportion of malnutrition in married (p1), divorce/widow (p2) and ratio (r), respectively(Reference Shi, Duan and Deng34).
The inclusion criteria of the current study were (1) Thai patients aged ≥ 60 years who were diagnosed with T2DM, (2) had an HbA1c level evaluated within the past 1 week and (3) could communicate well in Thai. The exclusion criteria were (1) the following poor health status according to The American Diabetes Association (ADA) criteria(18): (a) patients with end-stage chronic illness (such as congestive heart failure stage 3–4, oxygen-dependent lung disease, chronic kidney disease treated with haemodialysis and metastatic cancer), (b) had activities of daily living (ADL) impairment (Barthel ADL index for impairment of more than two activities)(Reference Laohaprasitiporn, Jarusriwanna and Unnanuntana35) or (c) had severe cognitive impairment (Mini-Mental State Examination (MMSE)-Thai 2002 scores less than 15, 18 or 23 for those who could not read and write, graduated from elementary school level and attended higher than elementary school level, respectively)(Reference Kuha, Wanichwarot and Boonmeephiphit36), (2) had been diagnosed with various diseases that affect HbA1c levels (such as Fe deficiency anaemia, vitamin B12 deficiency, haemoglobinopathies, thalassaemia, chronic liver disease, chronic renal failure, post-splenectomy, rheumatoid arthritis) or (3) were currently taking drugs that affect HbA1c levels (such as erythropoietin, aspirin, vitamin B12-C-E, antiretrovirals, ribavirin, dapsone and Fe).
Measurement tools
Glycaemic control
Based on HbA1c levels in the previous week, there were two categories of glycaemic control: adequate and poor. Participants were considered to have adequate glycaemic control if they met the HbA1c target based on the 2022 ADA guidelines(18); participants with less than three co-morbidities, intact cognitive function and functional status (Barthel ADL and Chula ADL impairment less than or equal to 2 activities) have HbA1C less than 7·5 %, while those with more than three co-morbid, mild cognitive impairment or instrumental ADL impairment (Chula ADL impairment more than two activities) should have HbA1C less than 8·0 %. Poor glycaemic control is classified for participants who did not meet the above criteria.
Nutritional status
The Mini Nutritional Assessment (MNA) questionnaire(Reference Vellas, Guigoz and Garry37,Reference Nilmanat, Naka and Kongin38) was used in this study to assess nutritional status, with a sensitivity of 96 % and a specificity of 98 %. The Thai version of this tool demonstrated good reliability with a Cronbach’s α of 0·83. The MNA questionnaire comprises fifteen questions with a total score of 30. Participants were categorised into two groups based on their MNA scores: normal nutritional status (24–30 points) and poor nutritional status, indicating a risk of malnutrition (17–23·5 points) or malnourishment (< 17 points).
Health status
The term health status was composed of four components: general health factors, depression, cognitive function and functional ability. First, general health factors were assessed using face-to-face interviews with a standard questionnaire comprising questions such as the duration of T2DM, number of co-morbidities that have been diagnosed in the medical record, including non-communicable disease, digestive problems and chronic pain (disease-related pain that persists for longer than 3 months), oral problems (chewing or swallowing problems, dental problems), number of medications taken and hospitalisations during the past year.
Second, depression was assessed using the Thai version of the Patient Health Questionnaire (PHQ)(Reference Kroenke, Spitzer and Williams39–Reference Piyaphat Dejphratham41), which includes the PHQ-9 and PHQ-2 versions. The Thai PHQ-9 has a sensitivity and specificity of 90 and 89 %, respectively. The Thai PHQ-2 version’s sensitivity and specificity are 80 and 73 %, respectively. This tool consists of nine questions, each scored from 0 to 3. The total scores range from 0 to 27. The PHQ-2 was used to initiate the examination; if the final score was ≥ 2, the PHQ-9 was assessed. If the patients’ PHQ-9 total score was ≥ 10, they were considered to have depression.
Third, cognitive function was investigated using the Thai version of the MMSE(Reference Kuha, Wanichwarot and Boonmeephiphit36) with a verified sensitivity and specificity of 86 and 92·6 %, respectively. This tool consists of eleven questions, each rated from 1 to 5. If the patients were unable to read and write, the assessments of items 4, 9 and 10 were omitted. The interpretation of these items depended on the level of education. Patients with cognitive impairment would receive 15–22 points if they are unable to read and write, 18–29 points for graduating from elementary school and 23–29 points for graduating from high school. Patients with intact cognitive function received maximum scores of 23 and 30 for those who were unable to read or write and graduated from elementary school or higher, respectively.
Functional ability was assessed using the Barthel ADL and Chula ADL indices(Reference Laohaprasitiporn, Jarusriwanna and Unnanuntana35,Reference Jitapankul42,Reference Jitapunkul, Kamolratanakul and Ebrahim43) . The inter- and intra-rater reliabilities for the Thai version of the Barthel ADL index were reported to be 0·714 and 0·968, respectively. This tool comprises ten items with scores ranging from 0 to 20. In this study, participants who got a full score of 20 were considered to have no impairment in basic ADL, while those who received a score of 0–19 were considered to have impaired basic ADL. The Chula ADL index had a kappa coefficient of 0·79. It consisted of five items. The total score ranged from 0 to 9 points. The cut-off points for scores were as follows: patients who score a full 9 points are good in instrumental ADL and score less than 9 are impaired in instrumental ADL.
Data collection
Patients meeting the eligibility criteria were selected from the medical records. Those willing to participate completed the questionnaires in a private room in the following order of items: general information, such as age, sex, monthly income, level of education, marital status, living alone, place of residence and employment; health status data, such as co-morbidities, medications, duration of diabetes, digestive problems, chewing and dental problems, chronic pain; Thai version Barthel ADL index; Chula ADL index; Thai version MMSE; PHQ2; PHQ9 and MNA. The HbA1c data were retrieved from the hospital information system. Based on these criteria, participants were categorised into adequate or poor glycaemic control groups(18).
Statistical analysis
Data were managed using R Studio Version 4.1.1 (Public Benefit Corporation). Descriptive analyses involved calculation of medians, interquartile ranges, means and percentages. The association between the glycaemic control groups and related factors among the nutritional status groups was assessed using the Wilcoxon rank-sum test, χ 2 test, Fisher’s exact test and t test. Differences in the MNA scores for each variable were evaluated using independent sample t tests, one-way analysis ANOVA, Mann–Whitney U-tests and Kruskal–Wallis tests. Spearman’s rank correlation coefficient was used to analyse the correlation between variables and MNA scores. Multiple logistic regression analysis was performed to examine the association between variables and poor nutritional status. Regression model 1 was utilised for analysing variables associated with nutritional status, including the variable of interest and glycaemic control. Subsequently, the variables identified as related factors in model 1 were examined in model 2, along with glycaemic control and co-morbidities, which have the possibility of impacting nutritional status(Reference Leij-Halfwerk, Verwijs and van Houdt3). Statistical significance was set at P < 0·05 for all analyses.
Results
Participant’s characteristic
Of the 287 participants, 63·4 % (n 182) were women. The median age was 64 (IQR 61–70) years. The median duration of T2DM was 4 (IQR 2, 9) years. According to the HbA1c levels, 83·6 % (n 240) patients had adequate glycaemic control, while 16·4 % (n 47) had poor glycaemic control. Multiple co-morbidities were present in 76 % of the participants (n 218); the most frequent co-morbidities were dyslipidaemia (95·8 %), hypertension (68·3 %) and musculoskeletal disease (15·0 %). Among these 36·9 % (n 106) who used polypharmacy, the most prescribed diabetes medications used in this study were biguanide (99·7 %), sulphonylurea (31·4 %) and thiazolidinedione (15·3 %), respectively. Based on the MNA scores, 85 % of the participants had a normal nutritional status and 14·6 % (n 42) were at risk of malnutrition. The two terms ‘malnutrition’ and ‘risk of malnutrition’ were combined into one term ‘poor nutritional status’ for analysis due to the small number of samples in both (Table 1).
Table 1. Characteristics of participants (Numbers and percentages; medians and interquartile ranges)

IQR, interquartile range; MMSE, Mini-Mental State Examination; bADL, basic activities of daily living; iADL, instrumental activities of daily living; HbA1c, glycated Hb; MNA, Mini Nutritional Assessment; PHQ, Patient Health Questionnaire.
Significant at P < 0·05.
* χ 2 test.
† Fisher’s exact test.
‡ Wilcoxon rank-sum test.
Glycaemic control and nutrition status
No significant association between adequate glycaemic control and poor nutritional status was found in this study (P = 0·67) (Table 1), similar to the absence of a correlation between HbA1c levels and MNA scores (r = 0·059, P = 0·318) (online Supplementary 2). Additionally, there were no significant differences in MNA scores between the groups with adequate and poor glycaemic control (P = 0·09) (online Supplementary 1).
Nutritional status and associated factors
Regarding socio-demographic variables, 31 % of participants aged > 75 years were malnourished or at risk of malnutrition, which was higher than those aged ≤ 65 years (8·1 %). Older adults with poor nutritional status were more likely to have incomes of less than £110 (5000 Baht) per month (67·4 %) and complete a lower level or equal elementary school (53·5 %). It demonstrates that those who are older, less educated and have lower incomes tend to have poor nutritional status. In terms of health variables, patients with poor nutritional status have a higher prevalence of cognitive impairment (14·0 %) than those with adequate nutrition status (3·7 %) (P = 0·014) (Table 1).
Spearman’s correlation revealed a positive correlation between the MMSE score and MNA score (r = 0·234, P < 0·001) and an inverse correlation between the number of medications and MNA score (r = −0·294, P < 0·001) (online Supplementary 2).
In multivariate logistic regression analysis, poor nutritional status has been found to be strongly associated with low monthly income (adjusted OR (AOR) 4·66, 95 % CI 1·28, 16·98 for income < £118 and AOR 7·80, 95 % CI 1·74, 34·89 for income £118–355). Additionally, poor nutrition was associated 4·23 times more with T2DM in older adults who were unemployed (AOR 4·23, 95 % CI 1·51, 11·85). Finally, it was revealed that there was a clear association between cognitive function and nutrition status; cognitive impairment raised the risk of poor nutrition by 5·28 times compared with normal cognition (AOR 5·28, 95 % CI 1·56, 17·93) (Table 2).
Table 2. Multivariate logistic regression model for the associating factor of poor nutrition (95 % confidence intervals)
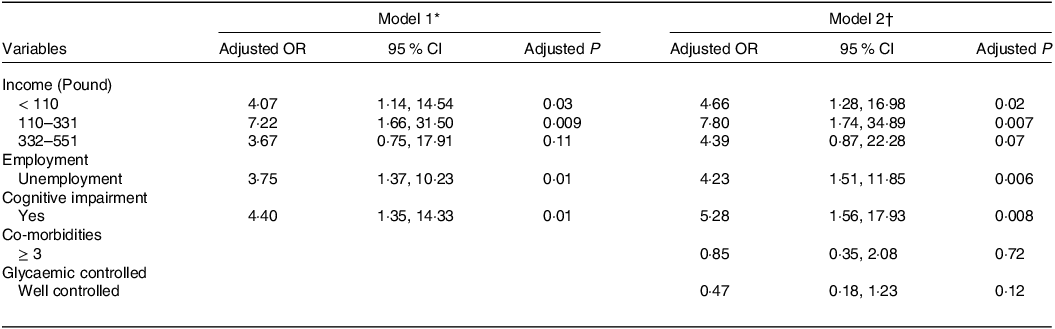
* Model 1: In multivariate logistic regression, forward (LR) variable selection method was used. Age, income, education, occupation, cognitive impairment, co-morbidities, polypharmacy, chronic pain, bADL impairment, iADL impairment and glycaemic control were adjusted for in the model.
† Model 2: The model used the variables selection method. Income, occupation, cognitive impairment, co-morbidities and glycaemic control were adjusted for in the model.
Discussion
This study is the first to investigate the association between poor nutritional status and glycaemic control using the concept of ‘individualised criteria’ based on standardised ADA guidelines that consider co-morbidities, functional abilities and cognitive functions. This study was conducted in a tertiary hospital, and the participants’ socio-demographic characteristics were similar to those of the 2021 Thai Elderly Health Survey(44). However, most participants in this study had a high school diploma or higher and lived in urban areas, unlike in previous studies with lower education levels and in rural locations(Reference Saritsiri45,46) . The study found relatively lower malnutrition among the participants based on the MNA compared with other studies(Reference Phodhichai, Satheannoppakao and Tipayamongkholgul47,Reference Chuansangeam, Wuthikraikun and Supapueng48) , possibly due to better access to medical services and health information among participants with higher education levels and urban residency(Reference Raghupathi and Raghupathi49,50) .
According to the primary objective, this study found no association between poor nutritional status and adequate glycaemic control. Furthermore, no correlation between the HbA1c levels and MNA scores was noticed, contradicting the results of previous studies. Vischer et al. (Reference Vischer, Perrenoud and Genet19) reported a positive association between MNA scores and HbA1c levels but focused on older patients with T2DM admitted to a geriatric hospital, unlike our outpatient clinic cohort. Junaid et al. (Reference Junaid, Ojo and Adejumo9) and Tasci et al. (Reference Tasci, Safer and Naharci51) showed that older adults with poorly controlled diabetes had an increased risk of poor nutritional status (50–88 %), whereas the present study found a lower prevalence of poor nutrition (15·0 %) and malnutrition (0·03 %). The main difference lies in the criteria for categorising patients into the adequate alchemically controlled group. Previous cohorts used HbA1c cut-off points alone(Reference Ayub and Ismail32,Reference Yildirim, Uzunlulu and Caklili52) , possibly overestimating well-controlled patients, whereas this study used the ADA guidelines with individualised criteria(18). These results align with those of Ayub et al. (Reference Ayub and Ismail32) and Yildirim et al. (Reference Yildirim, Uzunlulu and Caklili52), indicating that malnutrition is unrelated to blood glucose levels, particularly for diabetic control, in older adults (evaluated by an HbA1c cut-off point of 7 %). Therefore, in older patients with T2DM promoting optimal glycaemic control can prevent complications(Reference Atif, Saleem and Babar53) and improve lipid metabolism, resulting in lower total cholesterol, and higher levels of HDL(Reference Wang, Ji and Zhang54) can be achieved without concerns about an increased risk of malnutrition.
This study found an association between lower income and poor nutrition in older adults with T2DM. Those with monthly incomes of less than £355 had a 7 times higher risk of poor nutrition compared with the higher income groups. Similar findings were reported by Boulos et al. (Reference Boulos, Salameh and Barberger-Gateau27) and Abdu et al. (Reference Abdu, Yimamu and Kahsay30), in which low income or a lack of pension payments was linked to poor nutritional status. Lower income limits access to sufficient food with good nutritive value, particularly meat, fruits and vegetables. Unemployment has also been associated with poor nutrition in older adults with T2DM, as seen in studies by Poda et al. (Reference Poda, Hsu and Rau55) and Tamang et al. (Reference Tamang, Yadav and Hosseinzadeh56). Unemployment reduces social networking and the ability to afford healthy meals(Reference Smed, Tetens and Lund58) and may lead to decline in protein consumption(Reference Smed, Tetens and Lund58). Furthermore, this study found that cognitive impairment was linked to a 4-fold increased risk of poor nutritional status, which is consistent with the findings of Nguyen et al. (Reference Nguyen, Vu and Nguyen59) and Bakhtiari et al. (Reference Bakhtiari, Pourali and Omidvar28). Cognitive impairment can affect eating habits, food preparation and disorders of chewing or swallowing(Reference Fostinelli, De Amicis and Leone60). However, some studies did not find similar correlation because of variations in the levels of cognitive impairment and its effects on nutritional status(Reference Tasci, Safer and Naharci51,Reference Volkert, Chourdakis and Faxen-Irving61) .
This study found an inverse correlation between age and MNA scores, consistent with previous research(Reference Bakhtiari, Pourali and Omidvar28,Reference Mardani, Abbasnezhad and Rezapour62) , likely due to age-related physiological changes in gastrointestinal function(Reference Remond, Shahar and Gille63). However, no association was identified between age and poor nutritional status in this study, possibly because the majority of participants were aged ≤ 65 years and had fewer digestive problems. The number of co-morbidities and medications used also showed an inverse correlation with the MNA scores, as observed in previous studies, indicating that a higher Charlson Co-morbidity Index was associated with lower MNA scores(Reference Leão, Engedal and Monteiro-Junior64). However, functional ability (both basic and instrumental ADL) was correlated with MNA scores(Reference Petersen, Brooks and Titus65). However, unlike previous research(Reference Tasci, Safer and Naharci51,Reference Gupta and Singh66) , no association was found between these factors and poor nutrition in the current study. This disparity could be due to differences in the measures and tools used to assess functional capacity between this and previous studies. Additionally, the exclusion of participants with basic ADL impairment in more than two activities in this study may have contributed to the difference in results.
Contrary to the results of prior studies, poor nutritional status was not found to be associated with oral, digestive or chronic pain conditions in this study(Reference Ferdous, Kabir and Wahlin67,Reference Ballaziri, El Aziz and Chadli68) . The assessment of oral problems and chronic pain relied on self-reports, and the severity of these issues was not determined, which possibly affected the observed effects on nutritional status(Reference Razak, Richard and Thankachan69). Depression was also not independently associated with poor nutrition in this study, unlike previous studies that reported a 1·7–1·9 fold increased risk of poor nutrition in older adults with depression(Reference Boulos, Salameh and Barberger-Gateau27,Reference Yoshimura, Yamada and Kajiwara70) . Because the screening tool used in this study to define depression was used and the prevalence of depression was low (2 %), this potentially affected the ability to detect significant associations. Finally, this study did not find an association between the duration of diabetes and poor nutrition, because the majority of study participants had their disieases for lower than 5 years (51.2 %) which less than previous studies. A prior study reported that the disease duration of 15 years or longer in hospitalised older adults with T2DM had an increased risk of poor nutrition compared with a shorter disease duration(Reference Yildirim, Uzunlulu and Caklili52).
Based on the study’s findings, older adults with T2DM should prioritise achieving optimal glycaemic control in order to lower their chance of developing diabetes complications. This can be done without concern that lowering blood sugar will raise their risk of malnutrition. In addition, regular nutritional screening and appropriate assessment should be provided during follow-up treatment, especially for older adults with cognitive impairment or economic challenges, including those with low incomes and unemployment.
Future studies should consider including more patients with nutritional problems from various settings, such as the community and hospitalised patients. Different study methods, including case–control and cohort study, can determine the direction of the correlation between glycaemic level and nutritional status. The strengths of this study include the use of standardised criteria for glycaemic control, considering individual factors for more accurate stratification, and being the first in Thailand to investigate the relationship between glycaemic control and malnutrition in older adults with T2DM. However, this study had limitations, including a relatively low prevalence of malnutrition compared with previous studies, a lack of severity categorisation for certain factors, an absence in evaluating frailty due to the controversial background of standard assessment tools, especially in the Thai version(Reference Lee, Lee and Jang71), and being unable to determine cause-and-effect relationships between the identified factors because it was a cross-sectional study.
Conclusion
In conclusion, this study found no association between adequate glycaemic control and poor nutrition in older adults with T2DM. Consequently, healthcare providers should be informed about the importance of glycaemic control without concern for its effect on poor nutritional status. However, annual nutritional monitoring is recommended, particularly for those who have low income, unemployment and cognitive impairment. According to the relatively small prevalence of malnutrition in this study, the generalisability of the results should be applied in similar settings.
Acknowledgements
The authors would like to express their gratitude to all the volunteers who participated in the study and healthcare workers in PCU and GP clinics who facilitated the data collection process in this study.
The Faculty of Medicine at the Prince of Songkla University, Thailand, provided full financial support for this study. Study method, publication choice and article preparation were performed independently of the funders. This study was approved by the Ethics Committee of the Faculty of Medicine, Prince of Songkla University (REC65-167-9-4).
R. T. formulated the research questions, designed and carried out the study, analysed the data, interpreted the findings and wrote the article. N. K. formulated the research questions, designed and carried out the study, analysed the data, interpreted the findings and wrote the article. N. B. analysed the data, interpreted the findings and wrote the article. S. S. interpreted the findings and wrote the manuscript.
The authors declare no potential conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114524000175





