Introduction
African lion Panthera leo populations have declined significantly across their range (Bauer & van der Merwe, Reference Bauer and van der Merwe2004; Hunter, Reference Hunter2006). Concern over extirpation and lowered viability of most lion subpopulations has recently generated calls for more conservation action (Loveridge et al., Reference Loveridge, Lyman and Macdonald2005; Ray et al., Reference Ray, Redford, Steneck and Berger2005, Reference Ray, Hunter and Zigouris2007). For example, the Wildlife Conservation Society's Great Cats Program recently brought together experts from across Africa to consolidate population data and identify priority sites for action (Hunter et al., unpubl. data). One objective of such planning exercises is to identify regional strongholds for the species that have the highest probability of persistence in the long-term. The northern Albertine Rift of Uganda and the Democratic Republic of Congo (DRC) may be such a lion stronghold, deserving urgent conservation attention.
However, estimates of lion abundance are lacking for many portions of the northern Albertine Rift and conservation interventions are difficult to initiate in this region ravaged by war, refugees, disease and poverty. Therefore, we generated several predictions of potential lion abundance for the largest complex of protected areas in this region (the Queen Elizabeth National Park complex in Uganda and the adjoining Parc National des Virunga in DRC; Fig. 1) to help determine if conservation interventions are needed.
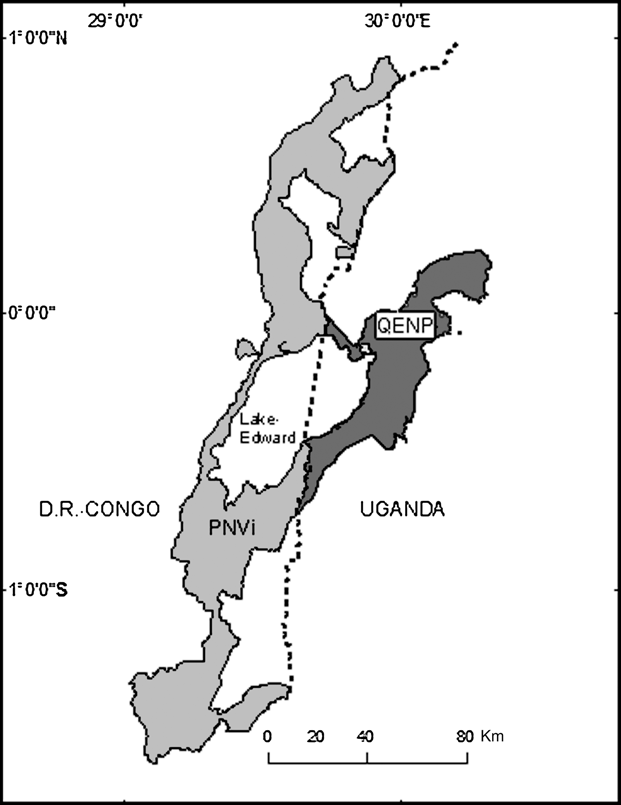
Fig. 1 Parc National des Virunga (PNVi) and Queen Elizabeth National Park (QENP) straddling Lake Edward and the national frontier (dotted north-south line) between Uganda and the Democratic Republic of Congo.
There are at least four accepted approaches to estimate large carnivore density of an area indirectly (van Orsdol et al., Reference van Orsdol, Hanby and Bygott1985; Gros et al., Reference Gros, Kelly and Caro1996; Bauer & van der Merwe, Reference Bauer and van der Merwe2004): (1) using interviews of visitors and residents to estimate total number; (2) estimating abundance based on average home range size of the carnivore measured at other sites, (3) estimating abundance for a new area based on average densities from other areas; (4) estimating abundance based on observed prey biomass. Gros et al. (Reference Gros, Kelly and Caro1996) found the interview method yielded the most accurate estimates for cheetah abundance in Kenya and Tanzania, albeit consistently underestimating an independent measure of abundance derived from actual counts. However, interview data were not available to us for the Albertine Rift lions, given the low rate of tourist visitation to Parc National des Virunga and sporadic access by park staff in this region of civil strife and armed insurgency (Plumptre et al., Reference Plumptre, Kujirakwinja and Kobusingye2003, Reference Plumptre, Kujirakwinja, Treves, Owiunji and Rainer2007). Gros et al. (Reference Gros, Kelly and Caro1996) reported the second most accurate, indirect method of estimating cheetah abundance was using the prey biomass method, which relates carnivore prey abundance to the number of carnivores that could be supported in the absence of other mortality causes. The prey biomass method correlated positively with the interview method for cheetahs, albeit being generally less accurate and consistently underestimating cheetah abundance (Gros et al., Reference Gros, Kelly and Caro1996). This approach has a long history in lion research (van Orsdol et al., Reference van Orsdol, Hanby and Bygott1985; Stander, Reference Stander1997).
We estimated potential lion abundance in the two Parks using two variants of the prey biomass method. We validated our estimates for Uganda using ground-based survey data (Dricuru, Reference Dricuru1999; JZ, unpubl. data). Based on our estimates and validations, we make recommendations for conservation interventions in each Park.
Methods
Separate teams estimated large prey numbers by aerial survey over the whole of Queen Elizabeth National Park in 1999 and 2004 and over the northern and central portions of Parc National des Virunga in 2003 and 2006 (Table 1; Mushenzi et al., Reference Mushenzi, de Merode, Smith, Smith, Banza and Ndey2003; Rwetsiba, Reference Rwetsiba2005; A. Plumptre, D. Moyer, D. Kujirakwinja & N. Mushenzi, unpubl. data). In Dricuru's (Reference Dricuru1999) study area of 992 km2 in Queen Elizabeth National Park she counted at least 116 lions using the total count direct observation method, or 105 if one omits the seven that died during her fieldwork and four that were not associated with prides and may have been transients. We used these counts to validate our models based on lion prey in 1999. In 2005 and 2007 JZ (unpubl. data) used the same methods to count 88 lions and 59 lions, respectively, in a 641.9 km2 area of the same Park. We used his data to validate our estimates for the Park in 2004.
Table 1 Body mass of seven lion prey species and total counts, density and biomass density in Queen Elizabeth National Park complex, Uganda, in 1999 and 2004, and the adjoining Parc National des Virunga, DRC, in 2003 and 2006 (Fig. 1).
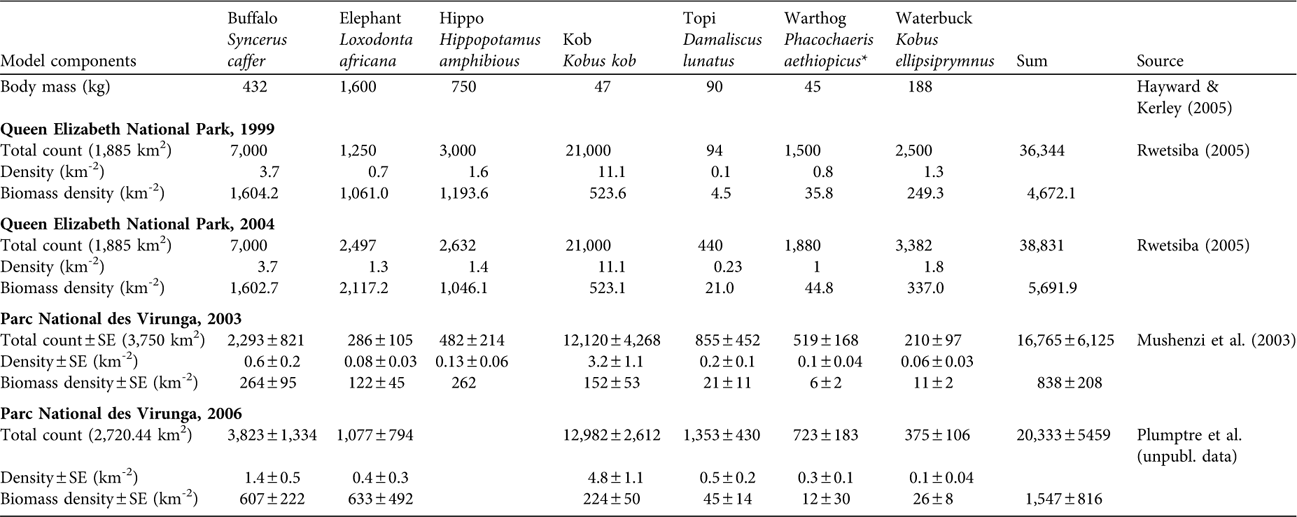
* Giant forest hogs Hylochoerus meinertzhageni included in warthog count
We had no estimates of prey availability specifically within the study areas of Dricuru (Reference Dricuru1999) or JZ (unpubl. data), so we interpolated simply from park-wide prey availability, although prey are not distributed evenly within the Park (Lamprey, Reference Lamprey2000; Lamprey et al., Reference Lamprey, Buhanga and Omoding2003). Aerial surveys cannot reliably detect lions because of their coloration and concealment but can detect their larger, open-country, ungulate prey (Hayward & Kerley, Reference Hayward and Kerley2005). Van Orsdol (Reference van Orsdol1984) reported that the lions of the Park ate buffalo Syncerus caffer, warthog Phacochaeris aethiopicus, waterbuck Kobus ellipsiprymnus, kob Kobus kob, topi Damaliscus lunatus and bushbuck Tragelaphs scripts most often. With the exception of bushbuck, these species are all readily detectable from aerial surveys. Aerial surveys underestimate the availability of small prey, such as bushbuck or warthogs, and hidden prey such as hippo Hippopotamus amphibious (Waser, Reference Waser1975; Norton-Griffiths, Reference Norton-Griffiths1978). We included Mushenzi et al.’s (Reference Mushenzi, de Merode, Smith, Smith, Banza and Ndey2003) aerial observations of hippo in Parc National des Virunga but we expect this underestimated hippo availability if the animals were underwater or otherwise concealed during an over-flight; similar data were not available for Queen Elizabeth National Park.
We estimated lion abundance with two variations of the prey biomass method. The first uses an a priori theoretical relationship between prey numbers and predator numbers. Karanth et al. (Reference Karanth, Nichols, Kumar, Link and Hines2004) proposed a simple two-parameter, one-variable (prey abundance) model for predicting tiger Panthera tigris densities. It was supported well by empirical data. The second method, which is empirical, is to measure prey and carnivore density at several sites, test for a correlation, and use any detected regression relationship to extrapolate to other sites. The latter method indirectly adjusts for reduced lion abundance due to common factors other than prey (e.g. density-dependent mortality) by averaging across sites, whereas the first method assumes prey abundance alone dictates carnivore numbers.
Method 1
In theory, one can use prey biomass to predict potential lion biomass. However, the individual body mass and the edible biomass of ungulate prey are both variable and complex factors for lions (Schaller, Reference Schaller1972; van Orsdol, Reference van Orsdol1984), as with tigers (Karanth et al., Reference Karanth, Nichols, Kumar, Link and Hines2004). The body masses of individual prey taken by lions are 3–1,600 kg and, even restricted to preferred prey, the range is 190–550 kg (Hayward & Kerley, Reference Hayward and Kerley2005). Edible biomass varies similarly. In one study, individual prey edible biomass was 1.2 kg (guinea fowl)–619 kg (giraffe; n = 458; Hunter, Reference Hunter1998). However, Schaller (Reference Schaller1972) noted that the range of species taken by lions may be large but generally fewer than five medium to large ungulate species comprise c. 75% of items in a lion's diet. This pattern has since been demonstrated in many studies (Hunter, Reference Hunter1998). Calculation of edible biomass for top prey, corrected for age and sex, may not yield a net improvement in precision, given that aerial survey data do not specify age and sex of prey animals. Thus, we followed Karanth et al. (Reference Karanth, Nichols, Kumar, Link and Hines2004) and used the number of individuals of the top prey species to estimate the food available to lions and thereby the number of lions potentially using an area. Following Karanth et al. (Reference Karanth, Nichols, Kumar, Link and Hines2004) we expected lion density would follow
where L = lions per unit area, P = number of prey animals in the same area, A = the proportion of prey killed by each lion, and b (≤ 1.0) allows for a potential non-linear relationship between prey numbers and lion numbers. We excluded their scalar, random variable delta with mean one because we were not generating error estimates.
Karanth et al. (Reference Karanth, Nichols, Kumar, Link and Hines2004) assumed b = 1.0 for tigers (i.e. all prey are potentially eaten) but their field data later placed b closer to 0.514 (0.001–1.009). They did not propose an explanation for this discrepancy but we consider b to reflect intrinsic factors, such as the energetic efficiency with which prey can be converted to lions. Hence we propose the scaling factor b relates to the well-known scaling factor relating body mass to metabolic rate and energy intake (0.67–0.78: McNab, Reference McNab and Gittleman1989; White & Seymour, Reference White and Seymour2005; Carbone et al., Reference Carbone, Teacher and Rowcliffe2007). Because the precise value is disputed, we simply employed the median of 0.725.
For A, Karanth et al. (Reference Karanth, Nichols, Kumar, Link and Hines2004) divided the number of prey killed by each tiger annually (50) by the proportion of available prey tigers annually removed (10%). We depart from this procedure because we believe the 10% rule incorporates the biological constraint discussed above. Instead, we set A as a fraction estimated from the number of prey eaten per lion per year, from data collected by Hunter (Reference Hunter1998) at the 170 km2 enclosed private reserve of Phinda, South Africa, a site with a similar assemblage of ungulate prey. Thanks to all lions being radio-collared and intensive and extensive coverage of the small area, Hunter (Reference Hunter1998) recorded virtually all medium- to large-bodied prey consumed by all lions over 40 months. On average, Phinda contained 9.7 lioness-equivalents (an index of the number of all ages and sexes standardized to lioness body mass); they consumed 529 prey animals (417 killed, scavenged, or unclear provenance + 112 presumed carcasses inferred from full stomachs, independent of the 417). He also measured availability of eight ungulate species comprising 394 (94.5%) of the carcasses consumed by lions (Hunter, Reference Hunter1998). If we assume these eight species also represented 94.5% of the unobserved kills/carcasses, the lions of Phinda would have consumed 500 individuals of the eight species in 40 months. If we include the 5.5% of other species killed, the 9.7 lioness-equivalents killed 527.8 prey animals or 16.3 per lioness-equivalent annually. This annual value falls in the low end of the range (16–32) estimated by Schaller (Reference Schaller1972) in the Serengeti. Our final model is therefore:
Method 2
Stander (Reference Stander1997) reviewed lion densities and prey biomass densities at 15 sites. He reported African lions ‘…occur at densities varying between 0.008–38 animals [per] 100 km2…' with a tight correlation to prey biomass density (r2 = 71%, an intercept at 0.002 and a slope of 0.003). No transformation was used and confidence limits on the slope were not provided. No methods were given on how biomass was calculated across studies, so we used prey body mass values in Hayward & Kerley (Reference Hayward and Kerley2005) and lion biomass as lioness-equivalents of 129 kg (Estes, Reference Estes1991). We used all prey for the biomass sum, although kob and warthog weigh < 60 kg (not included in Stander's (Reference Stander1997) regression). This yields
where L = number of lions per km2, and P is kg of prey per km2.
Although both equations 1 and 2 are expressed in lioness-equivalents, one should not misinterpret this to mean the prey base supports additional male lions. We use a single value to capture all lions whatever their mass, sex or age.
Results
Table 1 presents estimates for prey numbers and biomass from aerial surveys. Queen Elizabeth National Park had 4–5 times higher prey biomass than Parc National des Virunga. Prey in both parks increased over time but they remained low in Parc National des Virunga. Table 2 presents observed lion numbers for Queen Elizabeth National Park and predicted lion numbers based on prey for both parks. The park-wide prey values for Queen Elizabeth National Park in Table 1 were interpolated to the smaller study areas of Dricuru (Reference Dricuru1999) and JZ (unpubl. data) in Table 2. Our theoretical model (Equation 2) underestimated the observed numbers of lions in Queen Elizabeth National Park in 1999 by 26–33% and in 2005 by 32% (Table 2), as predicted by Gros et al. (Reference Gros, Kelly and Caro1996). By contrast, the empirical model (Equation 3) fell within the range of Dricuru's (Reference Dricuru1999) total lion count. Equation 3 predicted 132 lions in Queen Elizabeth National Park in 2004 (Table 2). By 2005 and 2007, the observed count of lions was 88 and 59 respectively (JZ, unpubl. data).
Table 2 Observed prey numbers, prey biomass density and lion abundance, and estimates of lion abundance predicted from prey numbers using equations 2 and 3 (see text for further details) in Queen Elizabeth National Park complex, Uganda, in 1999 and 2004, and the adjoining Parc National des Virunga, DRC, in 2003 and 2006 (Fig. 1).
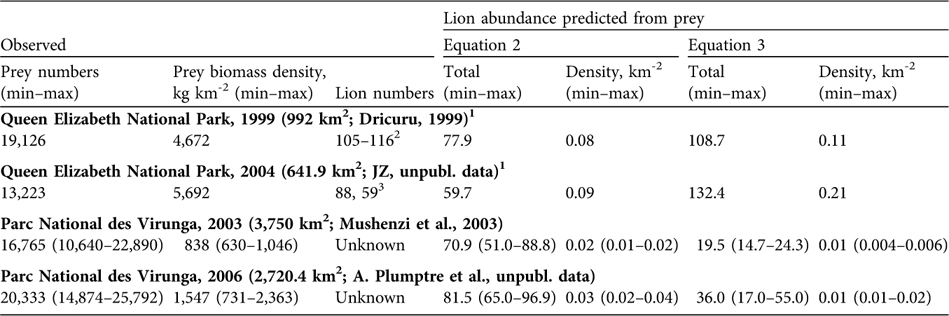
1 We assumed uniform distribution of prey from Table 1
2 Dricuru (Reference Dricuru1999) total count, less 4 loners and 7 that died during the study
3 JZ (unpubl. data) conducted two surveys of the same area, in 2005 and 2007
Given lower prey numbers in Parc National des Virunga's northern and central portions, predicted lion abundance was lower than in Queen Elizabeth National Park (Table 2). From Equation 2, we expect Parc National des Virunga's northern and central portions could have held 51–89 lions in 2003 and 65–97 lions in 2006. From Equation 3, which performed better for Queen Elizabeth National Park in 1999 (prior to lion-specific declines), we expect Parc National des Virunga's northern and central portions could have held 15–24 lions in 2003 and 17–55 in 2006, based only on prey numbers.
If prey in the northern and central sectors of Parc National des Virunga recover and the Park can sustain similar densities as in Queen Elizabeth National Park in 2004, then the combined areas of Queen Elizabeth National Park and Parc National des Virunga's northern and central portions could potentially contain 905 lions (adding the potential abundance in Queen Elizabeth National Park in 2004 to that same lion density of 0.206 lions per km2 multiplied by the Parc National des Virunga aerial survey area of 3,750 km2).
Discussion
Decades of poaching and transformation of wild habitat by refugees and neighbouring landowners in western Uganda and eastern DRC have taken their toll on wildlife, including the prey of lions (Treves et al., Reference Treves, Andiamampianina, Didier, Gibson, Plumptre, Wilkie and Zahler2006; Plumptre et al., Reference Plumptre, Kujirakwinja, Treves, Owiunji and Rainer2007). The situation is particularly dire for DRC, now emerging from years of armed insecurity during which both rebels and army forces camped in some of the eastern national parks. The near eradication of lions in the 20th century in Uganda (Treves & Naughton-Treves, Reference Treves and Naughton-Treves1999) is another grim reminder of how quickly human retaliation against lions for predation on livestock coupled with human exploitation of lions for commercial purposes can push the species to the brink of extinction in central Africa, even in the absence of prey declines.
Medium- to large-bodied, open-country ungulates in the northern and central portions of Parc National des Virunga have increased since 2003 but remain low compared to adjoining Queen Elizabeth National Park with the same ungulate species and similar habitats (Table 1). Parc National des Virunga's lions may also need protection; even if they have escaped direct human causes of mortality, chronic shortages of prey would lead to migration or death of lions. Potential lion densities predicted from prey availability (Table 2) put Parc National des Virunga near the bottom of the range described by Stander (Reference Stander1997). Ugandan conservationists cannot relax either, as poachers can cross the frontier and Ugandan causes of lion mortality have increased (Thawite, Reference Thawite2007). Dricuru (Reference Dricuru1999) and JZ (unpubl. data) documented a 10-year 50% decline in lion numbers in Queen Elizabeth National Park, while prey numbers generally increased 7% over that period (Rwetsiba, Reference Rwetsiba2005).
Surveys are essential to detect significant threats to wildlife or substantial declines in abundance. We modelled potential lion abundance using two approaches based on aerial surveys of lion prey and validated our models with ground surveys of lions using the total count method. In areas where ground-based surveys of prey are not feasible, aerial surveys can support conservation efforts for lions. Lions and some of their prey are under-counted by aerial surveys but the medium- to large-bodied ungulates of open country that constitute the major prey of lions across sites (Schaller, Reference Schaller1972; Hunter, Reference Hunter1998; Hayward & Kerley, Reference Hayward and Kerley2005) can be counted accurately by aerial survey (Norton-Griffiths, Reference Norton-Griffiths1978). Nevertheless, our models ignore the role of baboon Papio cynocephalus anubis, bushbuck and hippo, none of which are usually counted accurately by aerial survey but all of which can form part of the lion diet in the two Parks and elsewhere (van Orsdol, Reference van Orsdol1984; Dricuru, Reference Dricuru1999; Hayward & Kerley, Reference Hayward and Kerley2005). In particular, the theoretical model that depends on prey numbers rather than biomass would generate higher predictions if we had reliable bushbuck and baboon counts because Queen Elizabeth National Park has a high density of the former and baboons appear numerous in both parks (Waser, Reference Waser1975; Plumptre et al., unpubl. data). The empirical model based on biomass is unlikely to be affected strongly by the light-weight species but hippos are massive, occasionally eaten by lions and have undergone some dramatic fluctuations in numbers (Rwetsiba, Reference Rwetsiba2005). However their aquatic habits complicate counts from the air. In summary, both models may systematically under-predict lion abundances.
To validate our models we used total count data from Queen Elizabeth National Park collected by Dricuru (Reference Dricuru1999) and JZ (unpubl. data). The theoretical model (Eq. 2) grossly under-estimated the observed lion abundance in Queen Elizabeth National Park in 1999, whereas the empirical model (Eq. 3) based on major lion prey biomass was accurate for Queen Elizabeth National Park in 1999, contrary to the expectations of Gros et al. (Reference Gros, Kelly and Caro1996) from cheetah research. However, the two models’ curves intersected at low prey biomasses (0.7–2.4 × 106 kg) and the theoretical model (Eq. 2) generated a tighter range of predictions (Table 2).
A weakness of the theoretical model (Eq. 2) is the uncertain use of the exponent b. Many factors may lower b; some intrinsic biological constrains (e.g. metabolic costs of search time, injury, social behaviour and conversion of carcasses into reproduction), and others extrinsic constraints affecting predators across sites (e.g. predator-specific mortality). The exponent can be conceptualized by comparing a lion foraging only on porcupines Hystrix spp. to one foraging on the same number of oribi Ourebia ourebi, an antelope of similar mass. The former should support fewer lions because of greater handling time and injuries (intrinsic costs). Likewise, poachers may reduce predator numbers or alter foraging behaviour (extrinsic factors). In both cases, b would vary across sites (Karanth et al., Reference Karanth, Nichols, Kumar, Link and Hines2004). Empirically, Karanth et al. ( Reference Karanth, Nichols, Kumar, Link and Hines2004) found b to be close to 0.51, whereas we found b closer to 0.76 by adjusting the exponent to equal the number of lions in Queen Elizabeth National Park in 1999 (Table 2). Such a value falls close to the daily, energy-intake, scaling factor of 0.79 ± SE 0.09 expected of large mammalian predators (Carbone et al., Reference Carbone, Teacher and Rowcliffe2007). The different scaling factors of tigers and lions could reflect differences between solitary and group hunting. Yet, we hesitate to recommend further research, given the utility of the empirical model (Eq. 3).
Our empirical model of potential lion abundance produced accurate predictions before the 2005 lion decline in Queen Elizabeth National Park; it predicted 132 lions could use Queen Elizabeth National Park in 2004. This potential depends on curbing current causes of lion mortality. Likewise, the potential number of lions in Parc National des Virunga (Table 2) assumes no lion-specific mortality has reduced their numbers even further than predicted from low prey numbers. The potential lion abundance in Parc National des Virunga (Table 2) will not be attained without protecting lions.
Concerted conservation action on both sides of the border, as envisioned by Plumptre et al. (Reference Plumptre, Kujirakwinja, Treves, Owiunji and Rainer2007), could dramatically improve the outlook for lions and their prey. We believe the two adjoining Parks could potentially host 905 lions, making this transfrontier area a potential regional stronghold for the species and a potentially valuable source of tourism revenue for both countries. However, a recent, one third decline in lion numbers in the Ugandan Park and pervasive threats to the Congolese Park lead us to recommend immediate conservation intervention for lions and their prey. In Uganda, we recommend focused action to protect lions from poaching and retaliation, whereas in Congo general enforcement of wildlife protection and a ground-based survey for lions are needed. Since this article went to press we know of no reason to adjust these recommendations.
Acknowledgements
We thank Kim Berger and Laurence Frank for helpful comments. Marie Vicksta and Kerry Martin helped prepare the map.
Biographical sketches
Adrian Treves heads the Carnivore Coexistence Laboratory at the University of Wisconsin, USA (http://www.nelson.wisc.edu/people/treves). He studies patterns of livestock predation by carnivores, methods of mitigating such conflicts, and attitudes to carnivore management. His recent research has focused mainly on wolves and bears but he is also working with graduate students on African cats and hyenas. Andrew Plumptre's research interests include methods for wildlife monitoring. Luke Hunter's current projects include assessing the effects of sport hunting and illegal persecution on leopards outside protected areas, developing a conservation strategy for lions across their African range, and the first intensive study of Persian leopards and the last surviving Asiatic cheetahs in Iran. Joel Ziwa is a veterinarian who has taken part in a number of wildlife health interventions. His interest is the conservation of wildlife, especially large predators.





