Introduction
Lichens are often overlooked in ecological studies and biodiversity inventories and, consequently, they are poorly known or understood in many parts of the world. This is particularly true of crustose lichens that typically constitute around two thirds or more of all lichen biodiversity in an area (Lendemer et al. Reference Lendemer, Harris and Tripp2013; Spribille et al. Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020; Manzitto-Tripp et al. Reference Manzitto-Tripp, Lendemer and McCain2022; Vondrák et al. Reference Vondrák, Svoboda, Malíček, Palice, Kocourková, Knudsen, Mayrhofer, Thüs, Schultz and Košnar2022).
The lichen biodiversity of South Africa is reasonably well documented but, at the same time, poorly understood. The most recent checklist (Fryday Reference Fryday2015) listed 1748 taxa for mainland South Africa (i.e. excluding the Prince Edward Islands). The first supplement (Ahti et al. Reference Ahti, Mayrhofer, Schultz, Tehler and Fryday2016) made numerous additions and deletions for a net gain of three taxa. A subsequent publication (Medeiros & Lutzoni Reference Medeiros and Lutzoni2022) and a second supplement currently in preparation (I. D. Medeiros & A. M. Fryday, unpublished data) add a further 25, making a total of 1776 taxa. Estimates for the potential total number of taxa present in the country range from 2000 (Crous et al. Reference Crous, Rong, Wood, Lee, Glen, Botha, Slippers, de Beer, Wingfield and Hawksworth2006) to 3000 (Fryday Reference Fryday2015), which is well below the more than 21 000 vascular plants reported for the country (South African National Biodiversity Institute 2023). It is also comparatively low when compared with the 1838 lichen taxa reported from Great Britain and Ireland (Smith et al. Reference Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) or c. 2000 from Germany (Wirth et al. Reference Wirth, Schiefelbein, Litterski and Blanz2018); these are smaller countries (0.25 and 0.3 the size of South Africa, respectively) with a temperate climate, which is arguably less conducive to a diverse lichen biota than the subtropical–Mediterranean climate of South Africa.
Many South African lichens were first described in the late 19th and early 20th centuries and are frequently known only from their type collections, which have often not been studied since the species were described. Key among these are the taxa described by Ernestus Stizenberger in his Lichenaea Africana (Stizenberger Reference Stizenberger1890, Reference Stizenberger1891), but Nylander ((Reference Nylander1869) and in Crombie (Reference Crombie1876)), Vainio (Reference Vainio1926) and Zahlbruckner (Reference Zahlbruckner1926, Reference Zahlbruckner1932, Reference Zahlbruckner1936) also described many new species from the country. Unfortunately, as is the case for most descriptions from this period, they are mostly inadequate for assessing which species they represent under current taxonomic concepts. This can usually be done only by direct comparison with the type specimens, which are often poorly developed and invariably housed in European herbaria. This is a major impediment to the taxonomy and systematic study of lichens in South Africa and needs to be urgently addressed.
In the second half of the 20th and early 21st centuries, there was intermittent research on the lichen biota of South Africa, mainly by visiting European lichenologists (e.g. Almborn Reference Almborn1966, Reference Almborn1987; Schultz et al. Reference Schultz, Zedda and Rambold2009) but also by lichenologists resident in South Africa. The most notable of these was Franklin Brusse who, between 1985 and 1994, published over 30 papers describing new genera and species from the country and often included new reports of other species. However, all these researchers usually restricted themselves to describing new species and have not explored the systematic position or relationships of their discoveries (but see Kondratyuk et al. Reference Kondratyuk, Kärnefelt, Thell, Elix, Kim, Kondratiuk and Hur2015; Leavitt et al. Reference Leavitt, Kirika, Amo de Paz, Huang, Hur, Elix, Grewe, Divakar and Lumbsch2018a, Reference Leavitt, Westberg, Nelsen, Elix, Timdal, Sohrabi, St Clair, Williams, Wedin and Lumbschb; Aptroot et al. Reference Aptroot, Maphangwa, Zedda, Tekere, Alvarado and Sipman2019). A fuller history of lichenology in South Africa is provided by Fryday (Reference Fryday2015).
The first author of the current contribution, along with co-workers, recently described a new genus of crustose lichens in the Caliciaceae from Mpumalanga, South Africa (Fryday et al. Reference Fryday, Medeiros, Siebert, Pope and Rajakaruna2020). Subsequent fieldwork in other regions of South Africa by two of the current authors (AF, DAW) has revealed the presence of new species of crustose lichen that are known only from South Africa. This is the group of lichens that suffers from the greatest lack of attention within the South African lichen biota (Fryday Reference Fryday2015) and, although most require further study to clarify their systematic position, molecular data have been obtained for two of them. One occupies an isolated position within the Lichinomycetes and will be described elsewhere (A. Fryday et al., unpublished data), whereas the other occupies an unclear position within the Teloschistaceae and is described below as a new species.
Materials and Methods
This study is based upon specimens of crustose lichens collected by the first and fourth authors (AF, DAW) from the Tswalu Kalahari Reserve, Northern Cape, South Africa in April 2023.
Site description
Tswalu Kalahari Reserve (TKR; 27.20°S, 22.47°E, 102 000 ha) is located in the southern Kalahari Desert between the towns of Hotazel and Van Zylsrus in Northern Cape Province (Fig. 1) and has been developed into a high-end game lodge, nature reserve, and conservation-focused research facility. It ranges in altitude from 1020 m on the plains to 1586 m on the highest mountain peak and has a typically hot and arid climate, receiving an average of 286–318 mm of rain per year, 76% of which falls from mid- to late summer (December–April). The landscape is geomorphologically and ecologically heterogeneous, comprising plains, and fields of parallel dunes with the quartzitic Korannaberg Mountains extending from north to south through the eastern half of the reserve. Tswalu is centred within the Eastern Kalahari Bioregion, but the northern part extends into an outlier patch of the Kalahari Dunefield Bioregion. The included vegetation units comprise the northern tip of the Koranna Langeberg Mountain Bushveld, with Gordonia Duneveld to the north, Gordonia Plains Shrubveld to the west, Olifantshoek Plains Thornveld close to the mountains both to the east and west, and Kathu Bushveld to the east (Davis et al. Reference Davis, Scholtz, Kryger, Deschodt and Strümpher2010; Tokura et al. Reference Tokura, Jack, Anderson and Hoffman2018).
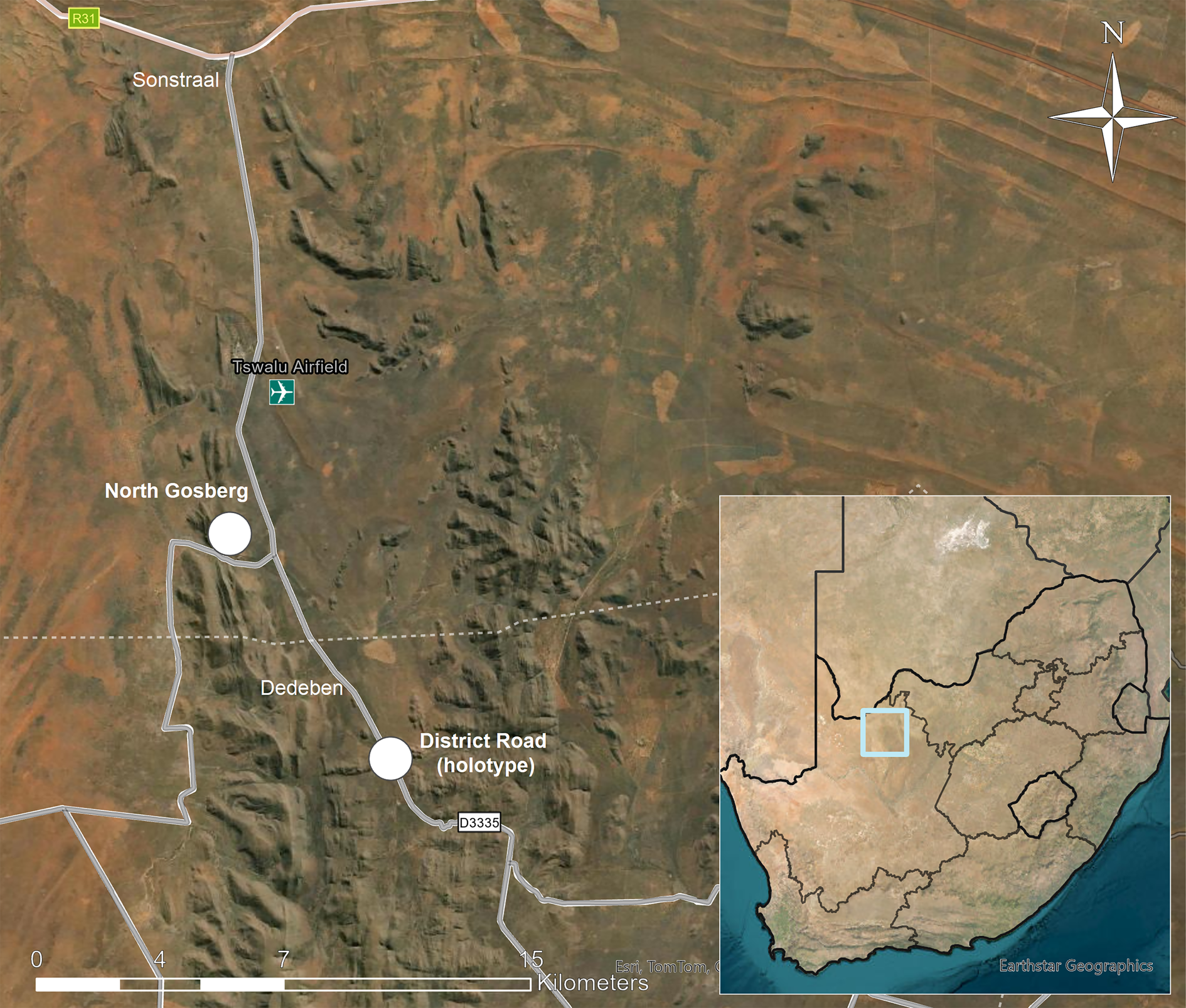
Figure 1. Map showing location of Tswalu Kalahari Reserve in South Africa (insert) and sites where Caloplaca tswaluensis was collected (white dots). In colour online.
Morphological methods
Apothecial characteristics were examined by light microscopy on hand-cut sections mounted in water, 10% KOH (K), 50% HNO3 (N) or Lugol's reagent (0.15% aqueous IKI). Thallus sections were investigated in water, K and lactophenol cotton blue. The ascus structure was studied in IKI, both without prior treatment and after pretreatment with K. Measurements of ascospores and paraphyses were made in water. Hamathecial filaments are referred to as ‘paraphyses’ regardless of their origin. Ascospore measurements are given as (minimum value–) mean ± standard deviation (–maximum value), n = number of measurements. Chlorinated anthraquinones were tested for in Teloschistaceae using a weak bleach solution (C) as described by Vondrák et al. (Reference Vondrák, Frolov, Arup and Khodosovtsev2013).
Molecular methods
DNA was extracted using a cetyltrimethylammonium bromide (CTAB)-based protocol (Aras & Cansaran Reference Aras and Cansaran2006). Two DNA loci were amplified: mtSSU and ITS. Polymerase chain reactions were performed in a reaction mixture containing 2.5 mmol/l MgCl2, 0.2 mmol/l of each dNTP, 0.3 μmol/l of each primer, 0.5 U Taq polymerase (TOP-Bio, Praha, Czech Republic) in the manufacturer's reaction buffer, and sterile water to make up a final volume of 10 μl. Details of sequenced loci, primers and PCR settings are given in Table 1. PCR products were enzymatically purified and sent for Sanger sequencing (GATC Biotech, Konstanz, Germany). Sequences were checked and edited using BioEdit v. 7.0.9.0 (Hall Reference Hall1999), and compared for similarity with the BLAST database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Datasets for mtSSU and ITS with relevant sequences were aligned using ClustalW multiple alignment in BioEdit, and manually adjusted. Both datasets were saved in nexus format and concatenated in SeaView v. 4.3.5 (Gouy et al. Reference Gouy, Guindon and Gascuel2010).
Table 1. Details of loci sequenced in this study including primers, PCR settings and references

Phylogenetic analyses
A BLAST search with the ITS region of our new species showed it to belong to the subfamily Teloschistoideae. In order to reveal its systematic position, we used specimens of Teloschistoideae that had been sequenced for both ITS and mtSSU to produce a reasonable concatenated phylogenetic reconstruction covering most known genera in that subfamily of Teloschistaceae. The outgroup was selected from Caloplacoideae to be outside the target subfamily, but within the Teloschistaceae. Using the Smart Model Selection program (http://www.atgc-montpellier.fr/sms/), we selected GTR + Г + I as the best-fitting model for subsequent analyses. Phylogenetic analyses were carried out in MrBayes v. 3.2.7a (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001) for 10 million generations. Average standard deviations of split frequencies were 0.048859. Maximum likelihood analysis was performed in PhyML v. 2.4.3 (Guindon & Gascuel Reference Guindon and Gascuel2003), with 1000 non-parametric bootstrap replicates. Final trees were visualized using TreeView v. 1.6.6 (Page Reference Page1996), and graphically adjusted in Inkscape v. 0.92 (https://www.inkscape.org). Lichen voucher specimens, and existing and new accession numbers from these vouchers in NCBI databases, are given in Supplementary Material Table S1 (available online).
Results
Microscopic examination of the unidentified specimens of crustose lichens collected from Tswalu Kalahari Reserve revealed one to have quadrilocular ascospores and anthraquinones in both thallus and apothecia. It clearly belonged to Teloschistaceae but did not correspond to the descriptions of any previously described crustose Teloschistaceae with plurilocular ascospores (Hafellner & Poelt Reference Hafellner and Poelt1979; Wetmore Reference Wetmore1999). Consequently, DNA sequences of the ITS and mtSSU regions were obtained from the specimen and its placement in Teloschistaceae subfamily Teloschistoideae was confirmed by a concatenated dataset of ITS and mtSSU sequences (Fig. 2). The closest sequenced specimen available in GenBank was an epiphytic lichen from Mexico (ITS: KT291446, mtSSU: KT291484; Gaya et al. Reference Gaya, Fernandez-Brime, Vargas, Lachlan, Gueidan, Ramirez-Mejia and Lutzoni2015) identified as Solitaria chrysophthalma (Degel.) Arup et al. The Mexican specimen, presumably assigned to Solitaria chrysophthalma on the basis of morphological similarity, is fairly close to the Tswalu collection (94% identical in ITS and 99.5% in mtSSU) and the specimens can be considered congeneric. However, because Gaya et al. (Reference Gaya, Fernandez-Brime, Vargas, Lachlan, Gueidan, Ramirez-Mejia and Lutzoni2015) recovered the Mexican collection in Teloschistoideae, it is almost certainly misidentified. The type specimen of the basionym of S. chrysophthalma (Caloplaca chrysophthalma Degel.) is from Europe (Switzerland), and Arup et al. (Reference Arup, Søchting and Frödén2013) sequenced a European collection of S. chrysophthalma (Sweden) and showed that it belonged in the subfamily Xanthorioideae. As demonstrated in the phylogenetic tree, the likely congeneric South African and Mexican specimens do not belong to any known genus in Teloschistoideae; sequences of related genera (e.g. Catenarina, Cinnabaria, Scutaria and Wetmoreana) are up to 89% identical in ITS and 98% in mtSSU. Unfortunately, the Mexican collection was not available for us to study at this time.
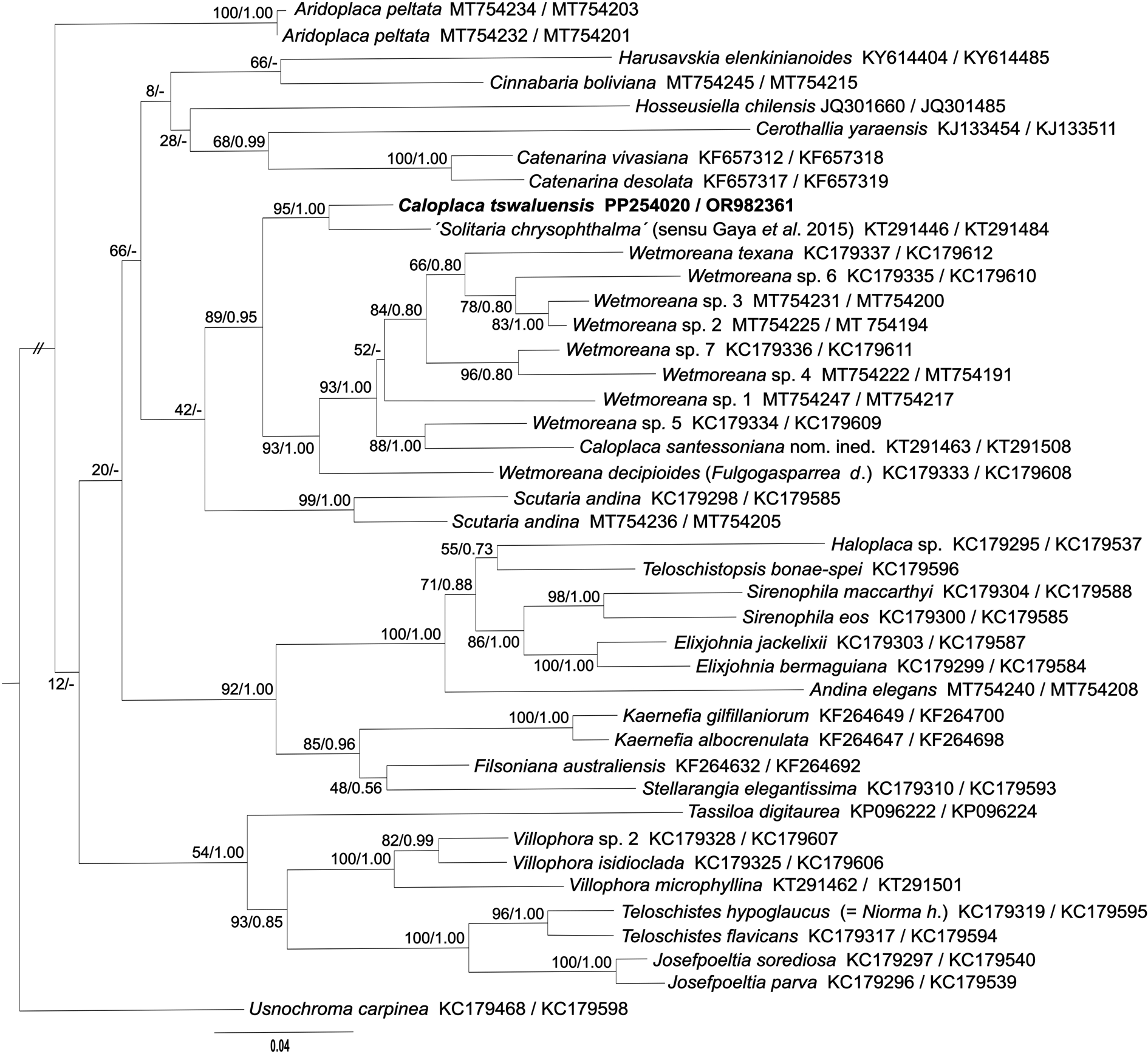
Figure 2. Phylogenetic relationships of the Teloschistoideae inferred by the maximum likelihood (ML) analysis of the concatenated datasets of ITS and mtSSU sequences. Numbers at the nodes show bootstrap values derived from ML analysis/posterior probabilities under the Bayesian inference (BI) analysis. When branching calculated in the ML analysis was not present in the BI analysis, the node was marked with a dash (-). Each original sample code consists of the species name and the GenBank Accession number (ITS/mtSSU).
Taxonomy
Caloplaca tswaluensis Fryday, S. Svoboda & D. A. Ward sp. nov.
MycoBank No.: MB 853830
Separated from all other crustose Teloschistaceae species by the quadrilocular ascospores, corticolous habit, presence of soredia and by its molecular sequence data.
Type: South Africa, Northern Cape, Tswalu Kalahari Reserve, 3.25 km south of Dedeben, 27.31084°S, 22.50910°E, 1224 m, on trunk of Vachellia erioloba (Fabaceae) in Acacia thicket (Olifantshoek Plains Thornveld) beside District Road, 24 April 2023, A. M. Fryday (11785), D. A. Ward & D. Smith (PRE—holotype; MSC—isotype). GenBank nos: PP254020 (ITS), OR982361 (mtSSU).
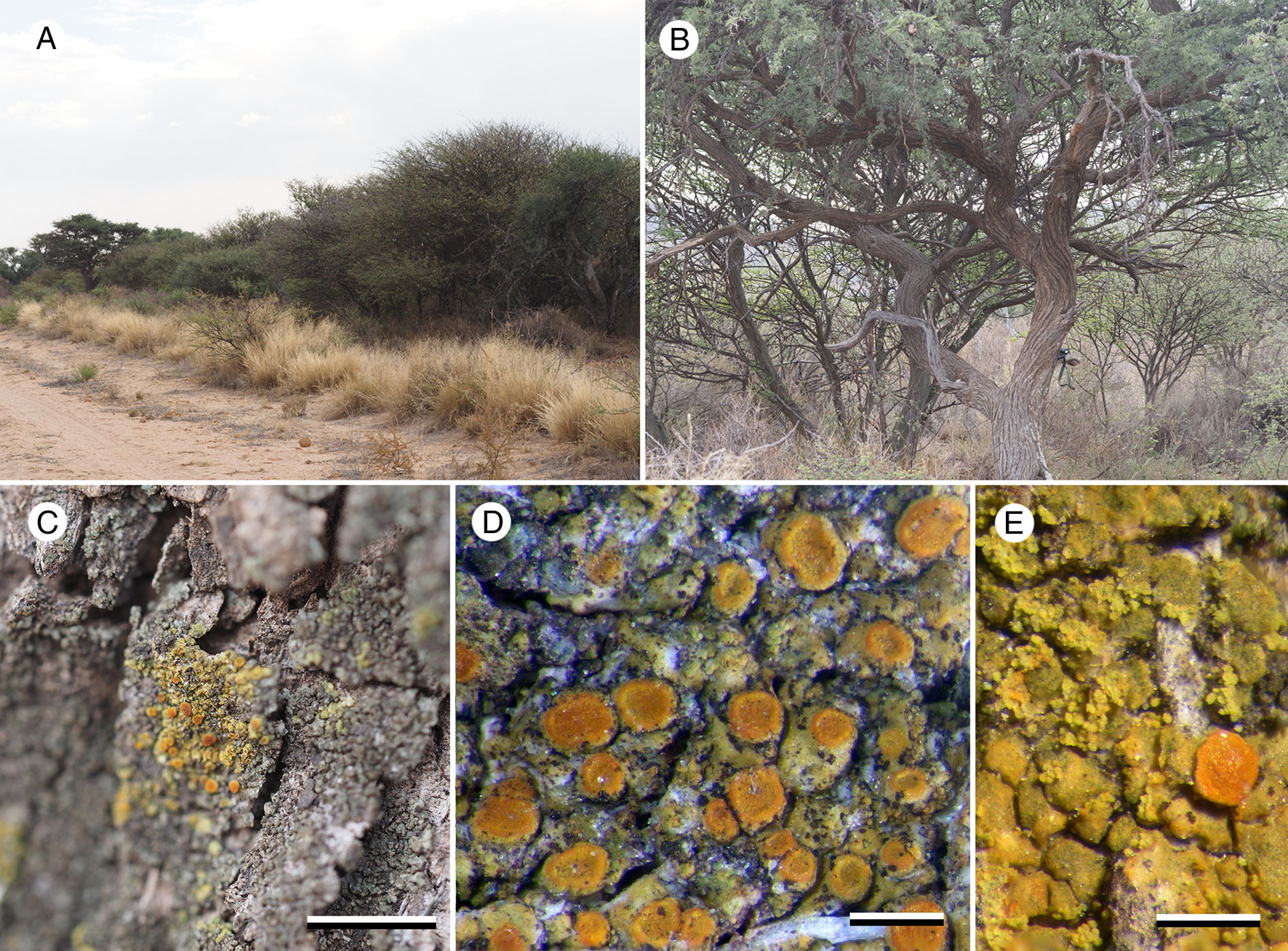
Figure 3. Caloplaca tswaluensis (A–D, Fryday 11785—holotype; E, Fryday 11762). A, the type locality, in Tswalu Kalahari Reserve. B, Vachellia erioloba (camelthorn) tree at the type locality. C, habit of Caloplaca tswaluensis among foliose Physciaceae. D, C. tswaluensis (fertile). E, C. tswaluensis (sorediate). Scales: C = 5 mm; D & E = 0.5 mm. In colour online.
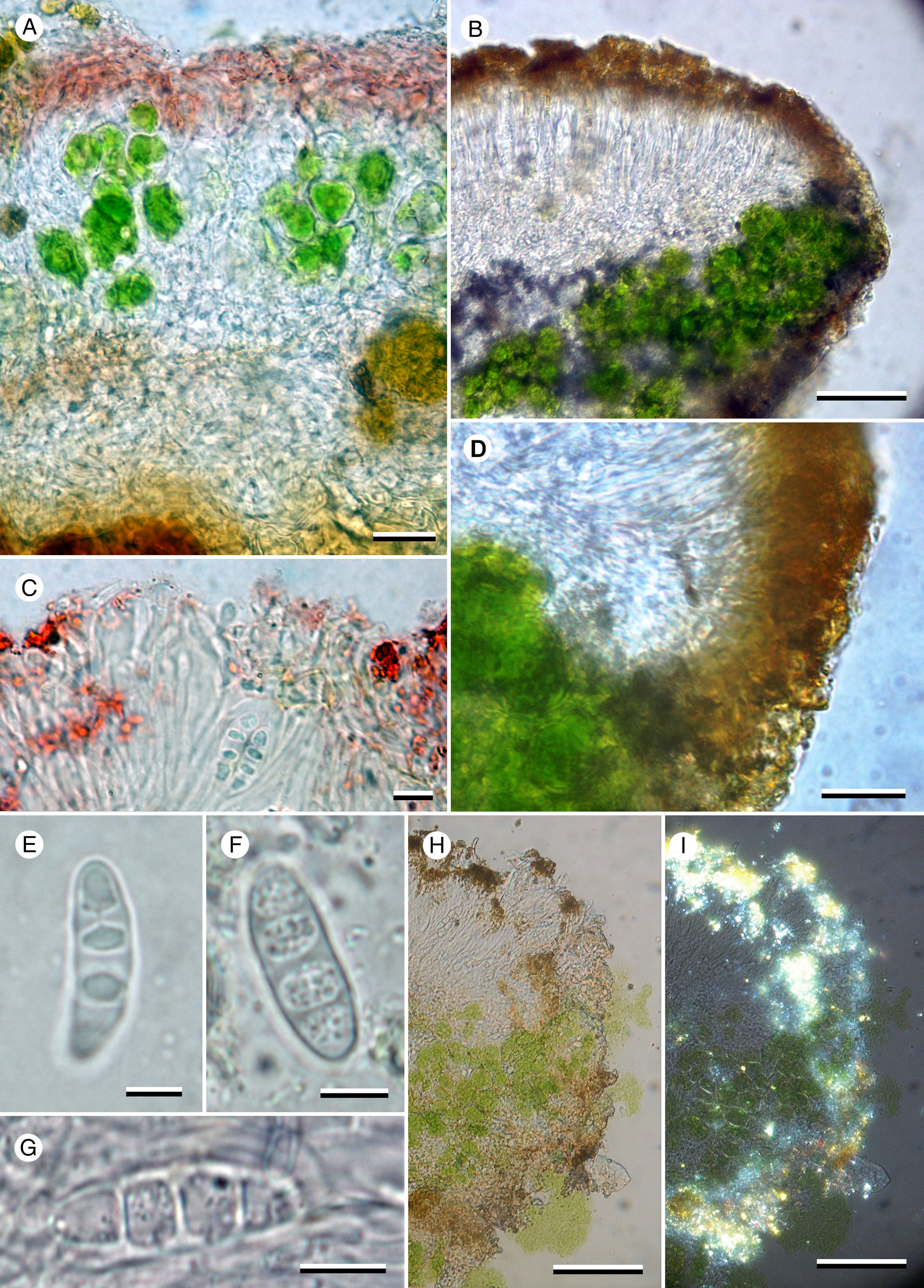
Figure 4. Caloplaca tswaluensis (Fryday 11785—holotype). A, section of thallus in K showing K+ purple cortex and interrupted photobiont layer. B, section of exciple showing photobiont cells in thalline exciple. C, section of apothecium in K showing paraphyses with swollen tips. D, section of exciple showing radiating hyphae in proper exciple. E–G, ascospores; mounted in KOH (E) and water (F & G). H, exciple and epihymenium under normal light. I, exciple and epihymenium under polarized light showing location of crystals. Scales: A = 20 μm; B, H & I = 50 μm; C & E–G = 5 μm; D = 10 μm. In colour online.
Thallus effuse, in small patches c. 0.3–1.0 cm wide, yellow, areolate, areoles flat to convex, 0.2–0.4 mm wide, becoming irregularly sorediate; soralia laminal, rarely marginal on areoles, initially ±orbicular (0.1–)0.15–0.2(–0.3) mm diam., but becoming confluent and irregular in outline; soredia granular, (25–)35–40(–50) μm diam.; in section areoles 0.12–0.14 mm thick; upper cortex alveolate, c. 20 μm thick, of two layers, upper layer composed of 3–5 layers of horizontally aligned hyphae 1.5–2 μm wide, with yellow pigment (K+ crimson), lower layer (and rest of the thallus) composed of hyaline ±spherical cells, 5–10 μm diam.; photobiont layer 40–70 μm thick, in columns mostly 20–30 μm (but occasionally up to 100 μm) wide, interrupted with columns of medulla cells 20–30 μm wide; medulla hyaline, 15–30 μm deep. Photobiont trebouxioid, cells ±spherical, 10–20 μm diam.
Apothecia 0.2–0.45 mm diam.; disc orange, flat with a rough surface; proper margin persistent, concolorous, 0.025 mm wide, slightly raised above the level of the disc; thalline margin usually visible at the base of the proper margin, especially in young apothecia, yellow, not corticate, clumps of algal cells usually visible at the periphery of the apothecia in surface view (especially when wet) but these appear to be different from those in the thallus and exciple. In section, proper exciple narrow at base, widening to 30 μm at the surface, composed of narrow vertical hyphae, algal layer up to 70 μm thick, with a narrow (10 μm) orange-brown cortex (K+ crimson) containing small crystals (POL+), photobiont layer ±continuous beneath the apothecia. Hymenium c. 70 μm tall, KI+ blue; paraphyses narrow, not branched or anastomosing except near the apex, upper 3–4 cells moniliform and widening to 4–5 μm; epihymenium 10–15 μm with numerous minute, golden brown crystals (POL+, K+ crimson). Ascus Teloschistes-type, cylindrical becoming somewhat clavate, 40–55 × 10–15 μm; ascospores 3- (rarely 4-) septate or quadrilocular (1-septate when immature), (13–)16.5 ± 1.9(–20) × (5–)5.4 ± 0.4(–6) μm; l/w ratio (2.5–)3.0 ± 0.2(–3.4), n = 20; cells lens-shaped when quadrilocular, especially in KOH. Hypothecium 70–80 μm tall at the centre of the section, narrowing to nothing at the exciple, hyphae predominately vertically aligned above and horizontal below.
Conidiomata not observed.
Chemistry
Upper cortex of thallus and epihymenium of apothecia K+ crimson, C−.
Distribution and ecology
The new species was collected at two localities within Tswalu Kalahari Reserve. At both locations it occurred on the trunks of Vachellia erioloba (E. Mey.) P. J. H. Hurter (camelthorn) trees in a corticolous lichen community dominated by Physciaceae species, with Phaeophyscia orbicularis (Neck.) Moberg as the dominant species. Corticolous communities were investigated at only three localities in Tswalu and C. tswaluensis was present at two of these, indicating that the species is probably frequent and widespread in the area. The trees in the localities where C. tswaluensis was collected were quite close together, whereas the locality where it was not observed consisted of large, isolated trees, suggesting that some shade was required for the lichen to be present. The disjunct distribution of C. tswaluensis and its closest relative from a similar habitat in Mexico suggests that this lineage has a transatlantic subtropical distribution in arid, partially wooded areas.
Remarks
Ascospores observed in water were 3-septate, without swelling of the septa or spore walls to form locules and with oil droplets visible inside the cells. However, on the addition of KOH the spores became quadrilocular and the oil droplets were no longer apparent, but were otherwise unchanged in shape or size. Further flushing of the section with water did not return the cells to their previous 3-septate state, indicating that the change was permanent.
Additional collections
South Africa: Northern Cape: Tswalu Kalahari Reserve, North Gosberg, 27.2501°S, 22.4657°E, 1267 m, on trunk of Vachellia erioloba in acacia thicket on south-facing slope, 2023, A. M. Fryday (11762), D. A. Ward & D. Smith (MSC, UNWH); ibid., 3.25 km south of Dedeben, 27.31084°S, 22.50910°E, 1224 m, acacia thicket beside District Road, on trunk of Vachellia erioloba, 2023, D. A. Ward (2076) & D. Rossouw (MSC, PRA—topotype).
Discussion
There are over 1000 species of crustose Teloschistaceae (Arup et al. Reference Arup, Søchting and Frödén2013), the vast majority of which have polarilocular ascospores, that is ascospores with a cell lumen constricted into two locules situated at opposing poles of the cell and connected by a narrow isthmus. The remainder have ascospores with either no septa or locules (simple), one septum but not polarilocular (1-septate), or more than two locules (plurilocular). We differentiate locular spores from septate spores by the latter lacking any wall thickenings. The group of species with plurilocular spores was revised by Hafellner & Poelt (Reference Hafellner and Poelt1979), who recognized 17 species and one subspecies. Since then, Caloplaca lagunensis Wetmore has been described from Mexico (Wetmore Reference Wetmore1999) but we are not aware of any additional Teloschistaceae species with plurilocular ascospores.
Hafellner & Poelt (Reference Hafellner and Poelt1979) showed that immature plurilocular ascospores were initially non-septate with unthickened, three-layered walls and that the locules were formed by a gelatinous substance expanding between the inner two walls that ultimately dissipates, leaving the ascospores resembling regular septate spores. In C. tswaluensis, however, at least one septum develops before the formation of locules because immature spores lacking wall thickening are clearly 1-septate. In addition, although overmature spores are 3-septate in water, the same spores become plurilocular in 10% KOH.
Hafellner & Poelt (Reference Hafellner and Poelt1979) were of the opinion that the species with plurilocular ascospores did not form a monophyletic group and that the character had evolved in the Teloschistaceae on at least three separate occasions. Previously, molecular data were available for only three Teloschistaceae species with plurilocular ascospores. Molecular data for Xanthocarpia ochracea (Schaer.) A. Massal. & De Not. was first provided by Søchting et al. (Reference Søchting, Huneck and Etayo2007; as Caloplaca ochracea (Schaer.) Flagey), with additional sequences provided by Arup et al. (Reference Arup, Søchting and Frödén2013) and Gaya et al. (Reference Gaya, Högnabba, Holguin, Molnar, Fernandez-Brime, Stenroos, Arup, Søchting, van den Boom and Lucking2012, Reference Gaya, Fernandez-Brime, Vargas, Lachlan, Gueidan, Ramirez-Mejia and Lutzoni2015). Arup et al. (Reference Arup, Søchting and Frödén2013) showed that X. ochracea belonged in the subfamily Xanthorioideae and that several other species with regular polarilocular ascospores were congeneric, indicating that plurilocular ascospores were not a genus-level character. Subsequently, Wilk et al. (Reference Wilk, Pabijan, Saługa, Gaya and Lücking2021) provided molecular data for Caloplaca brebissonii (Fée) Zahlbr. and C. quadrilocularis (Nyl.) Zahlbr., with both species included in the subfamily Caloplacoideae, and with C. brebissonii occupying an isolated position. Caloplaca tswaluensis, newly described here, occupies a position in the subfamily Teloschistoideae, distant from both C. brebissonii and C. quadrilocularis in the Caloplacoideae as well as X. ochracea in the Xanthorioideae. This reaffirms Hafellner & Poelt's opinion that species with plurilocular ascospores do not form a monophyletic group and have evolved in the Teloschistaceae on at least three separate occasions.
Of the species treated by Hafellner & Poelt (Reference Hafellner and Poelt1979), most have only three locules, unlike the current species, which has four. Of the quadrilocular species treated by Hafellner & Poelt (loc. cit.), Caloplaca tetramera (Müll. Arg.) Imshaug ex Hafellner & Poelt and Xanthocarpia ochracea are both saxicolous and occur only in Costa Rica and the Europe/Mediterranean region respectively; Caloplaca oahuensis Hafellner, C. quadrilocularis and C. spadicea (Tuck.) Zahlbr. are corticolous but all have brown apothecia and are known only from the Americas (including Hawai'i). In addition, C. quadrilocularis and C. spadicea have a cream-coloured to grey thallus and C. spadicea is also isidiate. Caloplaca lagunensis from Mexico (Wetmore Reference Wetmore1999) was described as having ascospores with only three locules but otherwise resembles C. spadicea in having a grey isidiate thallus.
Previously described South African species
We considered the possibility that the species described here had been included among the numerous taxa described from South Africa by previous lichenologists (e.g. Stizenberger Reference Stizenberger1890, Reference Stizenberger1891; Vainio Reference Vainio1926; Zahlbruckner Reference Zahlbruckner1926, Reference Zahlbruckner1932, Reference Zahlbruckner1936). Many of these are known only from their type species and a short Latin description but the plurilocular ascospores of C. tswaluensis are a distinctive and uncommon character that make searching protologues for similar species much easier. We therefore searched the protologues of all the species assigned to Caloplaca and its segregate genus Blastenia (sensu A. Massal. non Arup et al.) listed by Fryday (Reference Fryday2015) but failed to find any that mentioned this character. It is reasonable to conclude, therefore, that C. tswaluensis has not been described previously.
Systematic position of Caloplaca tswaluensis
The Teloschistaceae is a large, cosmopolitan family of lichenized fungi that is frequent in most habitats worldwide. Until recently, the family was divided into three main genera based on growth form: Teloschistes (fruticose), Xanthoria (foliose) and Caloplaca (crustose). The crustose species, with more than 1000 described species, are particularly frequent and widespread. However, investigation of the genetics of the family using molecular methods has shown this simplistic arrangement to be untenable (Arup et. al. Reference Arup, Søchting and Frödén2013). Although the three main groups were largely supported, and recognized as the subfamilies Teloschistoideae, Xanthorioideae and Caloplacoideae, there was a considerable variation of thallus type within each subfamily and, consequently, the family was divided into 39 separate genera, either resurrected from synonymy or described as new (Arup et al. Reference Arup, Søchting and Frödén2013). Since then, the process of separating new genera has continued by further dividing existing genera and recognizing newly revealed lineages as new genera (e.g. Søchting et al. Reference Søchting, Garrido-Benavent, Seppelt, Castello, Pérez-Ortega, de los, Murillo, Frödén and Arup2014; Frolov et al. Reference Frolov, Vondrák, Košnar and Arup2021) with the result that over 100 genera have now been erected (Wikispecies 2023a, b, c, d). Although some of these have been reduced to synonymy and many more are not widely accepted (Wilk et al. Reference Wilk, Pabijan, Saługa, Gaya and Lücking2021; Consortium for Classification of Fungi and Fungus-like Taxa 2023), over 50 genera are now recognized in the Teloschistaceae by most lichenologists.
Unfortunately, most molecular work on the Teloschistaceae has been carried out on Northern Hemisphere species, with work on Southern Hemisphere species being largely restricted to Antarctic, Australian and South American taxa. Sixty seven species of crustose Teloschistaceae have been reported from South Africa (Fryday Reference Fryday2015), 54 in Caloplaca and 13 in the segregate genus Blastenia. However, to the best of our knowledge molecular sequence data have not been obtained from any South African collection. It is no surprise, therefore, that the only sequenced collection that was at all close to C. tswaluensis was from Mexico and that even less closely related collections were also all from Central and South America (Bolivia, Peru, Mexico).
The current arrangement of the Teloschistaceae, coupled with the relative genetic isolation of our new species, meant we had two alternatives: erect a new genus for the species or describe it in a widely circumscribed Caloplaca s. lat. It is probable that a molecular analysis of other southern African crustose Teloschistaceae would show that at least some are congeneric with C. tswaluensis, which would reduce its isolation in the phylogeny of the family and justify erecting a new genus to accommodate them. However, because the systematic position of the new species is unclear, and monotypic genera serve little practical purpose, and also because the last thing lichen systematics needs right now is another new genus of crustose Teloschistaceae, we choose to describe the species in a widely circumscribed Caloplaca. This is despite it clearly not being congeneric with the type species of that genus (C. cerina (Hedw.) Th. Fr.).
Distribution of species with plurilocular ascospores
The crustose Teloschistaceae with plurilocular spores appear to have a mainly tropical/subtropical distribution with only Xanthocarpia ochracea, the distribution of which extends into NW Europe, and Caloplaca homologa (Nyl.) Hellb. from New Zealand laying outside this range. Six species have previously been reported from Africa, all corticolous, and only one from South Africa. The African species are Caloplaca auratopruinosa Sambo (Ethiopia), C. crocea (Kremp.) Hafellner & Poelt subsp. crocea (South Africa and Madagascar), C. erythroleuca (Nyl.) Zahlbr. (Eritrea and Guinea), C. erythroleucoides (Nyl.) Zahlbr. (Tanzania), C. vainioi Hafellner & Poelt (Angola) and C. zavattarii Sambo (East Africa). The South African record of C. crocea was collected from Howick in KwaZulu-Natal by van der Bijl [Byl] in 1920 (Hafellner & Poelt Reference Hafellner and Poelt1979). In addition, Stizenberger (Reference Stizenberger1890) described the new taxon Lecanora ochracea var. parvula Stizenb., with ascospores reported as 8–10 × 5–6 μm, from a collection on basaltic rocks near Mashishing (Lydenburg), Mpumalanga by Friedrich Wilms. However, the typical variety of Xanthocarpia ochracea is known only from the Mediterranean region and Europe so it is unlikely that this Mashishing collection is related to that species. Its true identity can be determined only by examination of the type collection, which is probably in a European herbarium but could not be found in either Herbarium Berolinense, Berlin (B) where Wilms was employed on his return from South Africa, or Herbarium der Eidgenössische Technische Hochschule Zürich (ZT), which houses Stizenberger's herbarium.
Conclusion
The new species, described here, along with the new genera described by the first author and co-workers (Fryday et al. Reference Fryday, Medeiros, Siebert, Pope and Rajakaruna2020; A. Fryday et al., unpublished data) and the nine new genera described by Brusse between 1985 to 1994 (Brusse Reference Brusse1985a, Reference Brusseb, Reference Brusse1987a, Reference Brusseb, Reference Brussec, Reference Brusse1988a, Reference Brusseb, Reference Brusse1994; Brusse & Kärnefelt Reference Brusse and Kärnefelt1991) are symptomatic of the poorly understood nature of the lichenized fungi of southern Africa. Add to this the numerous new species described by Brusse (cf. Fryday Reference Fryday2015) and the putative undescribed taxa collected during the fieldwork that resulted in the discovery of the new species described here, and the case for a significant undiscovered biodiversity of lichenized fungi in southern Africa is irrefutable. This is in need of serious and concerted study that can be achieved only by a well-funded, joint field- and laboratory-based research programme.
Acknowledgements
We thank the Explorers Club for an Exploration Fund Grant to DAW that permitted DAW and AF to travel to South Africa, and the Tswalu Foundation, Oppenheimer Generations Research and Conservation ‘OGRC’ and the Tswalu Kalahari Reserve for funding the fieldwork and providing logistical support. AF and DAW are particularly grateful to Dylan Smith, Executive Dedeben Research Centre at Tswalu Kalahari Reserve for initiating our visit and for sharing his time and knowledge of, and enthusiasm for, the reserve and all its biodiversity. We also thank Dr Nishanta Rajakaruna for organizing the project and assisting with fieldwork, and the curators and staff of the herbaria of Herbarium Berolinense, Berlin (B), Duke University, Durham, North Carolina, USA (DUKE) and Eidgenössische Technische Hochschule Zürich (ZT) for searching their collections for relevant material. Molecular work was supported by a long-term research development grant RVO (grant number 67985939) to JV and SS.
Author ORCIDs
Alan Fryday, 0000-0002-5310-9232; Stanislav Svoboda, 0000-0001-9797-4984; Jan Vondrák, 0000-0001-7568-6711; Danielle A. Ward, 0009-0000-0595-643X; Madeleen Struwig, 0000-0001-9626-4796.
Competing Interests
The authors declare no competing interests.
Supplementary Material
The Supplementary Material for this article can be found at https://doi.org/10.1017/S0024282924000185.








