Despite it seeming counter-intuitive, less frequent eating has been associated with a greater risk of overweight or obesity in children and adolescents( Reference Koletzko and Toschke 1 , Reference Mesas, Munoz-Pareja and Lopez-Garcia 2 ). However, data are mixed, with numerous studies reporting no association( Reference Nicklas, Morales and Linares 3 – Reference Murakami and Livingstone 6 ) or inconsistent associations across age groups, between sexes and with other behaviours( Reference Huang, Horwath and Lin 7 – Reference Antonogeorgos, Panagiotakos and Papadimitriou 9 ). A recent meta-analysis demonstrated that more frequent eating in children and adolescents is associated with a 22 % reduction in the odds of overweight or obesity, although this association was significant only in boys when stratified by sex( Reference Kaisari, Yannakoulia and Panagiotakos 10 ). However, a major limitation inherent in much of this cross-sectional work is that reverse causation is possible, particularly in older children. That is, it is just as plausible that overweight adolescents skip meals in an effort to lose weight as it is that reduced eating frequency causes overweight.
Relatively few studies have examined eating frequency in younger children, where meal skipping for weight control would be less likely. Although six cross-sectional studies have included children as young as 3 years of age, two of these involved children up to 11 years of age( Reference Murakami and Livingstone 6 , Reference Lioret, Touvier and Lafay 11 ), an age when reverse causation is conceivably an issue. Of the remaining four studies, one reported no difference in risk( Reference He, Ding and Fong 12 ) and three reported a lower risk of overweight in children aged 3–8 years who were eating more meals per day( Reference Toschke, Kuchenhoff and Koletzko 13 – Reference Thibault, Carriere and Langevin 15 ). The risk was approximately halved in these three studies, even after adjustment for several demographic factors and behaviours that could potentially confound the association( Reference Toschke, Kuchenhoff and Koletzko 13 – Reference Thibault, Carriere and Langevin 15 ). Only one study appears to have examined eating frequency in children younger than 3 years of age, observing no association between the number of meals consumed daily and obesity risk in infants and toddlers aged 5 weeks to 2·9 years( Reference He, Ding and Fong 12 ). Such a wide age range is problematic given marked changes in the intakes of milk and complementary foods that occur from birth through the toddler years.
Although longitudinal studies would more clearly delineate the relationship between eating frequency and obesity, none appear to have been undertaken in young children. Analyses from several large cohorts of adolescent girls have shown that less frequent eating of meals( Reference Franco, Striegel-Moore and Thompson 16 ) or snacks( Reference Ritchie 17 ), or fewer total eating occasions( Reference Thompson, Ballew and Resnicow 18 ), is associated with higher BMI over time. However, inconsistency in the measurement and definition of eating frequency is also apparent in the literature( Reference Johnson and Anderson 19 ). For example, many studies restrict analyses to meals because of concerns that snacks are differentially under-reported( Reference Nicklas, Baranowski and Cullen 20 ). While this approach may be appropriate for older age groups, it seems inappropriate in very young children given the important contribution snacks make to overall energy and nutrient intakes( Reference Jahns, Siega-Ritz and Popkin 21 , Reference Skinner, Ziegler and Pac 22 ).
The aim of the current study was to determine whether eating frequency (meals and snacks) at 2 years of age is associated with past, current or subsequent BMI in children aged 1–3·5 years.
Methods
Data are from the Prevention of Overweight in Infancy (POI) study, a 2-year behavioural intervention designed to limit excessive weight gain during the first 2 years of life (Clinical Trials NCT00892983). The protocol has been presented in detail previously( Reference Taylor, Heath and Galland 23 ) and only relevant information is included here. Ethics approval was obtained from the New Zealand Lower South Regional Ethics Committee (LRS/08/12/063) and the University of Otago Human Ethics Committee (12/274) and written informed consent was obtained from all adult participants.
Women were recruited in late pregnancy from the only maternity hospital serving the city of Dunedin, New Zealand (population approximately 120 000). After exclusion criteria (not living locally, booked into the maternity centre after 34 weeks’ gestation, prematurity or congenital abnormality likely to affect feeding or growth), a participation rate of 59 % yielded a final sample size of 802 participants. Participants were randomised to control, a sleep intervention, a food and activity intervention, or a combination of both interventions (within strata for parity and family socio-economic status)( Reference Taylor, Heath and Galland 23 ). The sleep group received a brief intervention antenatally and at 3 weeks of age designed to encourage appropriate sleep patterns from birth. The food and activity intervention (from late pregnancy to 18 months of age) provided a lactation consultant and used anticipatory guidance and resources to promote breast-feeding, healthy family eating and use of appropriate parental feeding strategies. No guidance or information on eating frequency was provided as part of the intervention( Reference Fangupo, Heath and Williams 24 ). The current analysis concerns data collected when the children were 1, 2 and 3·5 years of age.
All outcome assessments were collected by researchers blinded to group allocation. Information on birth weight was obtained from hospital records. Infant and child weight (Tanita WB-100 MA/WB 110 MA), infant length (Rollameter 100c length board; Harlow Healthcare, UK) and child height (Harpenden Portable Stadiometer; Holtain Ltd, UK) were measured in duplicate following WHO protocols( Reference de Onis, Onyango and Van den Broeck 25 ) at 12 (weight, length), 24 and 42 (weight, height) months of age. BMI (weight in kilograms divided by the square of height in metres) was calculated at each time point and WHO reference data used to calculate BMI Z-scores( Reference de Onis, Onyango and Borghi 26 ). Demographic information was obtained from mothers at baseline (late pregnancy) including date of birth, education, household income, parity, household deprivation( Reference White, Gunston and Salmon 27 ), self-reported pre-pregnancy height and weight, maternal smoking status during pregnancy (yes/no) and maternal ethnicity( 28 ). Infant birth weight was determined from hospital records; infant birth date and sex were reported by the mother. Weeks of exclusive breast-feeding (defined as the number of continuous weeks from birth when the infant was given only breast milk) was determined using questionnaire data collected monthly to 27 weeks and at 12 months of age.
Eating frequency at 2 years of age was calculated from a feeding diary (Fig. 1) completed over the course of two full days. Parents recorded when their child ate a meal or snack (the distinction between these was determined by the parent) in five-minute blocks. Frequency information on drinks was also collected, but information on whether the drinks were hypoenergetic (water or artificially sweetened) or not (milk, fruit juice, etc.) was not collected. Meal and snack codes for consecutive five-minute blocks were entered into a spreadsheet with formulas to calculate timing and frequencies of both meals and snacks. To qualify as a separate eating occasion, the start of the next meal or snack had to be more than 15 min after the end of the previous meal or snack (i.e. separated by at least four five-minute blocks)( Reference Berteus Forslund, Torgerson and Sjostrom 29 ). Any snacks that were eaten within 15 min of a meal were coded as that same meal and not as a separate snack. The mean total number of eating occasions per day was calculated as the average from meals and snacks combined across the two days.
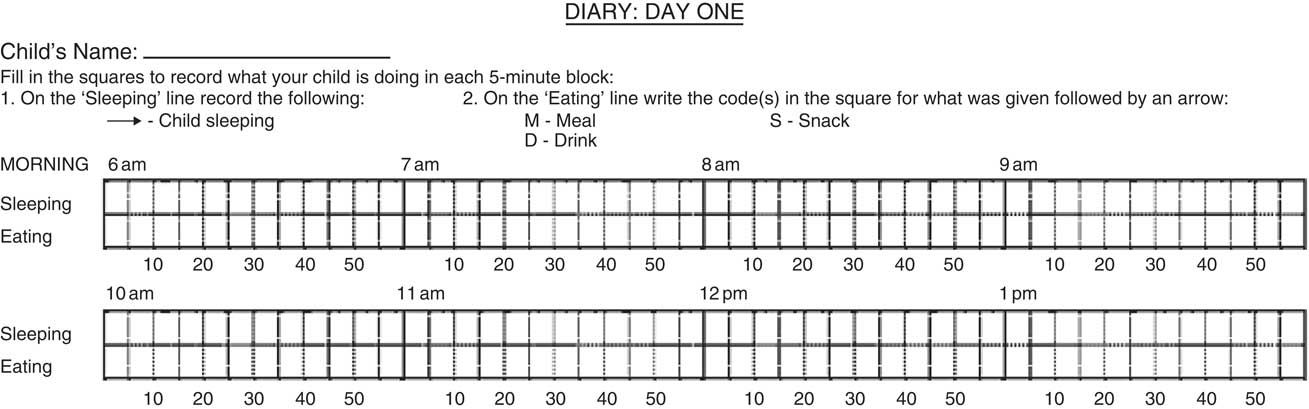
Fig. 1 Example of the sleep and feeding diary
Statistical analyses
Data were used from both control and intervention groups. The χ 2 test and the t test were used for categorical and continuous variables, respectively, to investigate potential discrepancies between participants who provided a diary at 2 years and those who did not. Associations between eating frequency at 2 years of age and BMI Z-score at 2 years (‘current’ model) and at 3·5 years (‘subsequent’ model) of age were assessed using unadjusted linear regression analyses and then adjusted for potential confounders (note: the unadjusted ‘subsequent’ model also included BMI at 2 years as change in BMI was the outcome). These models investigated the extent to which eating frequency predicted current BMI and subsequent changes in BMI in order to determine whether the finding in older children that eating frequency is associated with BMI( Reference Kaisari, Yannakoulia and Panagiotakos 10 ) also applies in very young children. Unadjusted and adjusted linear regression models were then used to model the association between BMI at 1 year and eating frequency at 2 years (‘past’ model). This model investigated the extent to which eating frequency was in fact predicted by past BMI, a proposed mechanism for the association between eating frequency and BMI seen in some cross-sectional studies. All three models were adjusted for POI intervention group and stratification variables (three levels of household deprivation (NZDep 1–3, 4–7 and 8–10) and maternal parity (dichotomised into primaparous and multiparous)). Adjustments were also made for infant sex, birth weight, maternal education, maternal pre-pregnancy self-reported BMI, household income, smoking during pregnancy and weeks of exclusive breast-feeding as potential confounders or otherwise explanatory variables. The residuals of each linear regression model were assessed for evidence of heteroscedasticity and non-normal distribution. Variance inflation factors were also investigated for the presence of substantial collinearity. The degree of agreement in the total number of eating occasions per day across the two days was assessed using the intra-class correlation coefficient. The statistical software package Stata version 13.1 was used for all statistical analyses and the two-sided significance level α=0·05 was considered to indicate statistical significance.
Results
Table 1 presents the demographics of the participants according to whether or not the feeding diary was completed and returned at 2 years of age. At this time, 695 (87 %) participants remained in the study, although only 371 (46 %) provided a completed diary. Mothers who completed diaries were significantly older by 2·1 years (P<0·001) and had more years of education (P<0·001) than mothers who did not complete diaries. Similarly, a smaller proportion of these mothers had smoked in pregnancy (12·1 % v. 21·1 %, P<0·001) and fewer were Māori or Asian (P=0·018). No significant differences were noted in any other demographic variables including maternal BMI, level of household deprivation, infant sex or birth weight.
Table 1 Characteristics of the participants at birth according to completion of a child feeding diary at age 2 years, Prevention of Overweight in Infancy (POI) study, Dunedin, New Zealand
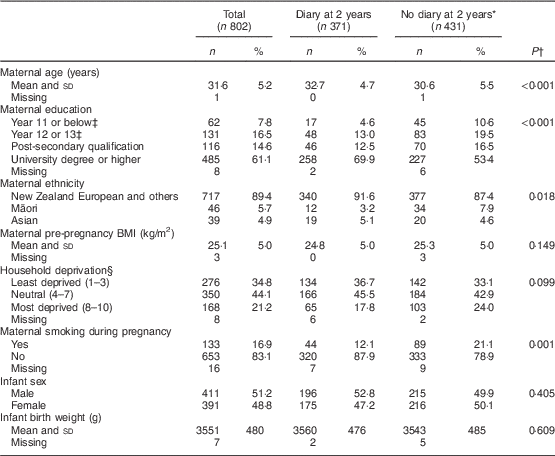
Data are presented as n and % unless otherwise indicated·
* Of the 431 who did not complete and return a diary at 2 years of age, 324 were active participants, twenty were lost to follow-up, seventy-two had withdrawn from the project, and fifteen had moved out of Dunedin so were only asked to provide data for the main outcomes of the POI study.
† P value compares those who did and did not complete a diary.
‡ Year 11 or below is about age 15 years; Year 12 or 13 is about age 16 and 17 years.
§ NZ Deprivation Index 2006( Reference Salmond, Crampton and Atkinson 36 ) is an index of socio-economic deprivation developed by Statistics New Zealand.
Table 2 reports the number of eating occasions for each of the two days, demonstrating that the majority of children were eating 4–7 times daily (88·1 % on day 1 and 88·9 % on day 2). Over the two days, children ate on average 5·5 (sd 1·2) times each day. Some variation in the total number of eating occasions was apparent between days: the variance within children was comparable to the variance between children, resulting in an intra-class correlation coefficient of only 0·52 between days 1 and 2. By using the mean of two days, we increased the reliability of this measure to 0·68. The mean difference in the number of eating occasions between the two days was 0·1 (sd 1·3), providing 95 % CI of −2·6, +2·8. Thus 95 % of individual children ate up to 2·6 times less often to as much as 2·8 times more often between days 1 and 2.
Table 2 Eating frequency of children at age 2 years (n 371), Prevention of Overweight in Infancy (POI) study, Dunedin, New Zealand
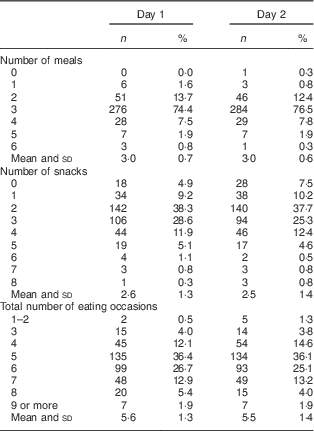
Data are presented as n and % unless otherwise indicated.
Analyses for the ‘current’ model show that eating frequency at 2 years of age was not related to BMI Z-score at 2 years of age either before (difference in BMI Z-score per additional eating occasion; 95 % CI: −0·04; −0·12, 0·04) or after (−0·02; −0·10, 0·05) adjustment for intervention group, household deprivation, parity, infant sex, birth weight, maternal education, maternal pre-pregnancy self-reported BMI, household income, smoking during pregnancy and weeks of exclusive breast-feeding. Similarly, in the ‘subsequent’ model, eating frequency at 2 years of age did not predict change in BMI Z-score when the children were 3·5 years of age before (0·02; −0·03, 0·07) or after (0·02; −0·03, 0·06) adjustment for these potential confounding variables.
The ‘past’ model determined whether children with higher BMI Z-score at 1 year of age displayed a different eating frequency at 2 years. We found no significant relationship between BMI Z-score at 1 year of age and eating frequency at 2 years either before (difference in eating frequency per additional BMI Z-score unit; 95 % CI: −0·06; −0·20, 0·07) or after (−0·03; −0·19, 0·13) adjustment for potential confounding variables.
Figure 2 provides a graphical representation of the three adjusted regression models (past, current, subsequent) described above, illustrating the statistically significant changes in BMI Z-score (or number of eating occasions) per unit increase in each independent variable.
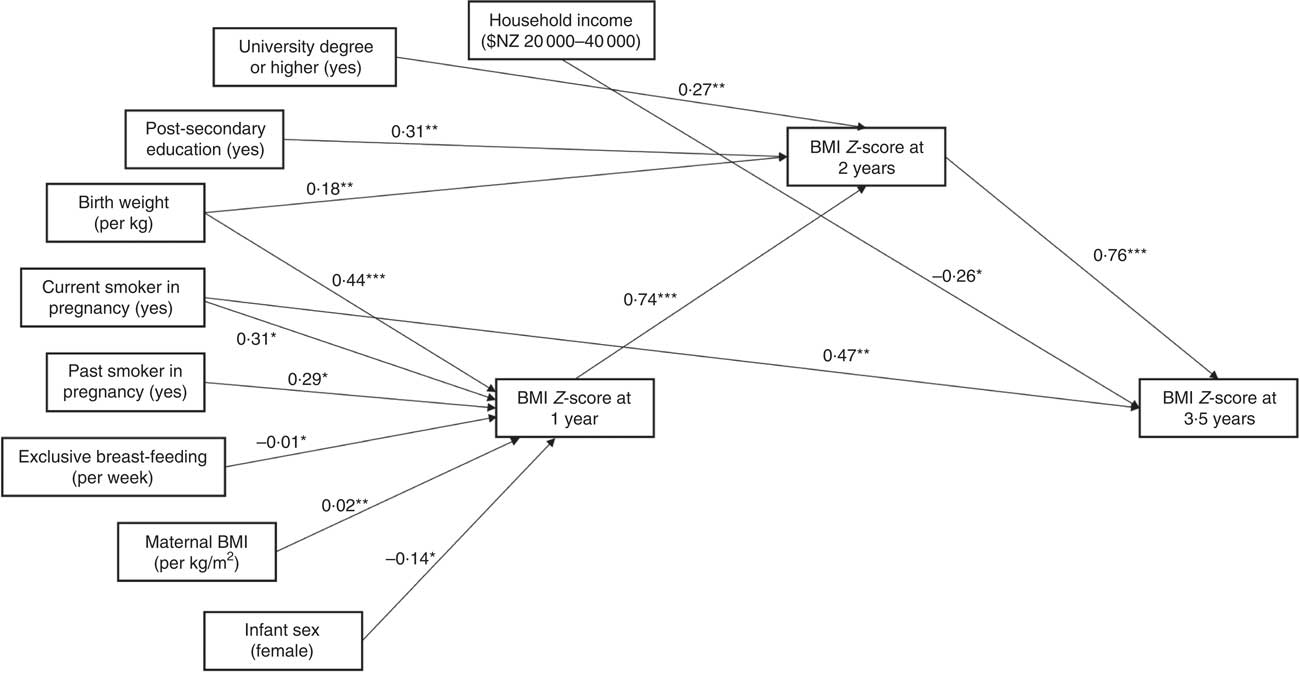
Fig. 2 Relationships between eating frequency, BMI Z-score and demographic variables of interest among children (n 371) aged 1–3·5 years, Prevention of Overweight in Infancy (POI) study, Dunedin, New Zealand. Figure indicates the statistically significant changes in BMI Z-score (or number of eating occasions) per unit increase in each independent variable, or for the stated category compared with the reference level, from the regression analyses: *P<0·05, **P<0·01, ***P<0·001. Units/categories for each variable are indicated in parentheses
Discussion
Eating frequency does not appear to be related to BMI in very young children, either cross-sectionally or longitudinally. Eating less often at 2 years of age was not associated with higher BMI at the same age or 18 months later. Similarly, BMI does not appear to be related to later eating frequency: children who had a higher BMI at 1 year of age did not have a different eating frequency when they were 2 years of age. Our sample size was large and the confidence intervals for the differences in BMI Z-score per additional eating occasion were very narrow, suggesting that either no association exists or any association that does exist is not of practical significance.
Only limited comparisons can be made with the literature given the scarcity of published results at this age. The single study that appears to have examined eating frequency in toddlers also observed no association between the daily number of meals consumed and risk of obesity( Reference He, Ding and Fong 12 ). That case–control study in 359 young children used a retrospective questionnaire to simply categorise children as consuming more than three meals per day or three or fewer meals( Reference He, Ding and Fong 12 ). In contrast, we used a more comprehensive 48 h diary collected in ‘real time’. Although findings are mixed in older children (3–11 years), several studies do report significantly lower odds of being overweight or obese( Reference Lioret, Touvier and Lafay 11 , Reference Eloranta, Lindi and Schwab 14 , Reference Thibault, Carriere and Langevin 15 ) or a lower prevalence of obesity( Reference Toschke, Kuchenhoff and Koletzko 13 ) in children consuming more daily meals. Measurement of eating frequency in these studies varied, ranging from single questions( Reference Toschke, Kuchenhoff and Koletzko 13 , Reference Thibault, Carriere and Langevin 15 ) to more detailed 4–7 d diet records( Reference Lioret, Touvier and Lafay 11 , Reference Eloranta, Lindi and Schwab 14 ), although the majority of studies have used questionnaires( Reference Kaisari, Yannakoulia and Panagiotakos 10 ). A recent meta-analysis reported that the odds of being overweight or obese are lower by 22 % in children who eat more frequently than their peers( Reference Kaisari, Yannakoulia and Panagiotakos 10 ). However, this association was significant only in boys and both publication bias and significant heterogeneity in findings were apparent, illustrating that further, well-conducted research is warranted( Reference Kaisari, Yannakoulia and Panagiotakos 10 ).
Why eating frequency might be unrelated to BMI in very young children, when a relationship potentially exists in older children, is not clear. However, it may be a result of the extrinsic and intrinsic influences on eating frequency and energy intake that coexist in young children. Current nutrition guidelines advise parents to feed young children small meals and snacks often, because their smaller stomach capacity limits the size of meals at individual sittings( 30 ). This advice certainly appears to be being followed as only 5 % of the children in our study were eating fewer than four times daily. At the same time that the frequency with which meals are available is determined externally, by parents, young children are also likely to have good internal energy self-regulation. Young children are considered to be more receptive to hunger and satiety cues than older children and thus to have improved energy self-regulation( Reference Rolls, Engell and Birch 31 , Reference Fox, Devaney and Reidy 32 ). It is possible, therefore, that in the usual situation when meals are made available with high frequency, the young child eats only what s/he needs – a relatively straightforward process when the next meal is never far away. It is also possible that if meals are offered less frequently, children of this age are less likely to overcompensate by overeating both because they have improved appetite control( Reference Ventura and Birch 33 ) and because of their smaller stomach capacity.
Strengths of our study include a large sample size, allowing for reasonably precise estimates as indicated by the narrow confidence intervals; and multiple measures of body size over time, allowing investigation of longitudinal associations between eating behaviour and BMI. We also used 48 h diaries to estimate eating frequency, which should theoretically provide a more definitive measure than one obtained from questionnaires asking parents to indicate how many times per day their child ‘typically’ eats( Reference Kaisari, Yannakoulia and Panagiotakos 10 , Reference Johnson and Anderson 19 ). The importance of collecting multiple measurements for eating frequency is illustrated by the relatively low intra-class correlation coefficient observed using the number of eating occasions over the two days in our study even with diaries used. However, both techniques (questionnaire and diary) are limited in that self-report or parental proxy must be used and validation studies determining whether parents can accurately estimate eating frequency in children do not appear to have been undertaken. Although overall study retention was high (87 %) at 2 years of age, less than half of POI parents provided a useable two-day diary for these analyses. While the diary itself was not particularly time consuming to complete, it was one of several questionnaires at the 24-month time point, which may have influenced our low collection rate. Participants who completed diaries did differ for some, but certainly not all, demographic variables compared with participants who did not complete them, but these differences may limit wider generalisation. This is particularly important in terms of maternal education, which was quite different across the two groups. A further limitation is that we did not adjust for physical activity; although POI collected accelerometer measures of activity at 24 months of age, sufficient data were available for only 303 participants in total. We decided not to include activity as a potential confounder as it would further reduce the available sample size. Although parental definition of whether each eating occasion was a meal or a snack has been considered a limitation( Reference Nicklas, Baranowski and Cullen 20 ), our use of the total number of eating occasions (meals and snacks combined) is relevant at this age, given the important contribution that snacks make to energy and nutrient intakes in young children( Reference Jahns, Siega-Ritz and Popkin 21 , Reference Skinner, Ziegler and Pac 22 ). While our study was longitudinal with repeated measures of body size, we only collected diary data to assess eating behaviour at 2 years of age. Additional diary measurements would have allowed us to examine whether changes in eating frequency were associated with changes in relative body weight. Ideally more than two days of real-time, eating frequency data would have been collected. With only two days, the mean may not be particularly reliable and could be an inaccurate reflection of any particular child’s true consumption due to within-person variability. This could result in decreased power to detect meaningful effects. However, reliability problems can be overcome with a larger sample and our study did include more than 300 individuals; a mean based on 2·2 d would be sufficient to achieve a reliability of 0·7 which is often considered acceptable. The confidence intervals from the regression models ranged from only −0·10 to 0·05 for differences in BMI per one unit increase in eating frequency in the ‘current’ model and from −0·03 to 0·06 for the ‘subsequent’ model. These upper and lower limits are much lower effects than what would be considered of practical or clinical importance. We had no data on parental meal frequency or other family-related factors that may influence children’s food behaviours and body weight( Reference Vaughn, Ward and Fisher 34 ). We also had to exclude drinks from the analyses; milk is an important source of nutrients and energy in the diets of 2-year-old children and might generate additional ‘eating occasions’ not measured by our study if it was consumed alone (i.e. not in a meal or snack with solid foods). Finally, we collected no data on the foods consumed, energy or diet quality over the two days of diary recording. Recent analyses of the US National Health and Nutrition Examination Survey demonstrated that higher meal (but not snack) frequency in children aged 6–19 years is associated with greater diet quality( Reference Murakami and Livingstone 35 ), although no corresponding analyses appear to have been conducted in younger children.
Conclusion
Eating frequency at 2 years of age was not associated with past, current or subsequent BMI in very young children. Associations that are apparent in some studies in older children may be a function of changes in energy self-regulation with increasing age or of eating behaviours in response to weight.
Acknowledgements
Financial support: The POI study was funded by the Health Research Council of New Zealand (grant numbers 08/374, 12/281 and 12/310). R.W.T. is supported by the KPS Fellowship in Early Childhood Obesity. S.L.C. is supported by the University of Otago, Health Sciences Postdoctoral Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflict of interest: None. Authorship: R.W.T. was the co-principal investigator of the POI study, conceived the idea for this paper, and wrote the first and subsequent drafts of the manuscript. E.I. designed and completed all statistical analyses, was involved in writing the paper and had final approval of the submitted and published versions. A.-L.M.H. contributed to study design, was involved in writing the paper and had final approval of the submitted and published versions. A.R.G. contributed to study design, was involved in writing the paper and had final approval of the submitted and published versions. B.J.T. was the co-principal investigator of the POI study, contributed to study design, was involved in writing the paper and had final approval of the submitted and published versions. J.A.L. collected the data, was involved in writing the paper and had final approval of the submitted and published versions. M.H. collected the data, was involved in writing the paper and had final approval of the submitted and published versions. S.L.C. collected the data, was involved in writing the paper and had final approval of the submitted and published versions. R.S. collected the data, was involved in writing the paper and had final approval of the submitted and published versions. B.G. contributed to study design, had responsibility for the integrity of the diary data, was involved in writing the paper and had final approval of the submitted and published versions. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the New Zealand Lower South Regional Ethics Committee and the University of Otago Human Ethics Committee. Written informed consent was obtained from all adult participants.







