Introduction
Tobacco smoking is much more prevalent in people with severe mental illnesses (SMI) like schizophrenia than in the general population: 50–70% of patients are regular smokers (Gurpegui et al., Reference Gurpegui, Martínez-Ortega, Aguilar, Diaz, Quintana and De Leon2005). Smoking is the largest single contributor to the poor physical health and increased mortality associated with SMI (Callaghan et al., Reference Callaghan, Veldhuizen, Jeysingh, Orlan, Graham, Kakouris, Remington and Gatley2014), and reducing smoking in this group is thus a key priority for health services (Payne, Reference Payne2016). However, health education and conventional interventions to reduce smoking may be less effective in people with SMI than in the general population (Royal College of Psychiatrists, 2013). Electronic cigarettes (e-cigarettes) can provide smokers with nicotine without the constituents of tobacco that cause cardiovascular and respiratory disease (Public Health England, 2015). They mimic tobacco cigarettes, as behavioural cues (hand-to-mouth actions) are maintained (Prochaska and Grana, Reference Prochaska and Grana2014; Hartmann-Boyce et al., Reference Hartmann-Boyce, McRobbie, Bullen, Begh, Stead and Hajek2016). They have good acceptability (Etter and Bullen, Reference Etter and Bullen2011a) and availability (Chang and Barry, Reference Chang and Barry2015), but their utility as an aid to smoking reduction or cessation is unclear (Hartmann-Boyce et al., Reference Hartmann-Boyce, Begh and Aveyard2018). One trial compared e-cigarettes (0 mg/16 mg) and nicotine patches (21 mg) in 657 general population chronic smokers (Bullen et al., Reference Bullen, Howe, Laugesen, McRobbie, Parag, Williman and Walker2013). Significantly more participants in the 16 mg e-cigarette group reduced daily smoking by at least 50% at 6 months compared with patch users (57% v. 41%, respectively), and cessation rates were 7.3% v. 5.8%, respectively (Bullen et al., Reference Bullen, Howe, Laugesen, McRobbie, Parag, Williman and Walker2013). A secondary analysis of Bullen et al. (Reference Bullen, Howe, Laugesen, McRobbie, Parag, Williman and Walker2013) studied the efficacy of e-cigarettes in SMI (O'Brien et al., Reference O'Brien, Knight-West, Walker, Parag and Bullen2015). Participants motivated to quit were stratified as having a mental illness if prescribed any of the following medications: anti-depressants, psychostimulants, antipsychotics, anxiolytics, hypnotics/sedatives and drugs for addictive disorders (O'Brien et al., Reference O'Brien, Knight-West, Walker, Parag and Bullen2015). Participants using e-cigarettes significantly reduced average cigarette consumption by 40%, significantly more than patch users (29%) (O'Brien et al., Reference O'Brien, Knight-West, Walker, Parag and Bullen2015). However, participants diagnosed with a mental illness had a significantly higher smoking relapse rate compared with those without a mental illness (79% and 67%, respectively), and reported e-cigarettes to be more acceptable (O'Brien et al., Reference O'Brien, Knight-West, Walker, Parag and Bullen2015).
A pilot study of e-cigarettes in 14 patients with schizophrenia who were not motivated to quit smoking reported a 50% reduction in tobacco cigarette use after 52 weeks, accompanied by a reduction in exhaled carbon monoxide (CO) levels (Caponnetto et al., Reference Caponnetto, Auditore, Russo, Cappello and Polosa2013). Two of the 14 patients stopped smoking completely. Another study in 19 patients with SMI reported that a provision of e-cigarettes for 4 weeks was associated with a 65% reduction in cigarette use, reduced CO levels and cessation of smoking in two patients (Pratt et al., Reference Pratt, Sargent, Daniels, Santos and Brunette2016). While these studies have provided promising results, the sample sizes were small. Moreover, if e-cigarettes are to have a sustained effect on tobacco use, their acceptability and tolerability to patients with SMI needs to be established (Prochaska, Reference Prochaska2011). This is a particular issue in smokers with psychotic disorders, as they are often heavy smokers (Evins et al., Reference Evins, Cather, Rigotti, Freudenreich, Henderson, Olm-Shipman and Goff2004), with more failed quit attempts (De Leon and Diaz, Reference De Leon and Diaz2005). The present study sought to assess the efficacy and acceptability of e-cigarettes as a harm-reduction method in patients with psychotic disorders, as well as their effects on psychotic symptoms. We predicted that e-cigarette use during an intervention period would significantly reduce tobacco cigarette consumption, without exacerbating psychotic or respiratory symptoms.
Methods
Design
Participants were referred from community mental health teams within the South London and Maudsley NHS Foundation Trust. After referral, written informed consent was obtained at screening, where detailed data on smoking behaviours, medical history and demographics were collected. Study approval was granted by the London Bromley NHS Research Ethics Committee (14/LO/0725). The study is registered on ClinicalTrials.gov, number NCT02212041, and was a pilot study with no comparison group.
Participants
Between September 2014 and November 2016, 248 referrals were screened after meeting the following criteria: (1) aged 18–70 years; (2) daily smoker (unwilling to quit soon); (3) exhaled CO level of more than five parts per million; (4) an established clinical diagnosis of schizophreniform, schizophrenia, schizoaffective disorder or bipolar disorder, or attending an early detection service in a high-risk state.
Exclusion criteria included: (1) the use of e-cigarettes on more than two occasions in the past 30 days; (2) intention to quit smoking in the next 30 days; (3) medication use that may reduce smoking (including, bupropion, nicotine replacement therapies, acamprosate, varenicline, baclofen, clonidine, naltrexone, buprenorphine, nortriptyline, disulfiram and anti-seizure medications); (4) hospitalisation/change in dose of psychotropic medication(s) in the last 30 days; (5) unstable physical health in the past 3 months; (6) a previous serious stomach ulcer and/or phaeochromocytoma; (7) severe heartburn, stroke, unstable kidney/liver disease, an uncontrolled overactive thyroid gland in the past 3 months; (8) individuals who meet the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for illicit/alcohol drug dependency; (9) medical contraindications to nicotine; (10) asthma; (11) suicidal ideation/suicide attempt in the past month; and (12) pregnancy. This visit was followed by a session in which baseline measures were obtained.
Intervention
Participants were provided with free tobacco flavoured e-cigarettes in an amount equivalent to 150% of their daily tobacco use (as recommended by the manufacturer) for 6 weeks. The NJOY traditional bold (NJOY Inc., Scottsdale, AZ, USA) disposable e-cigarette was used, which contains 4.5% nicotine. This model of e-cigarette was chosen because: they were widely available; were popular; they mimic the flavour, length, diameter and mouth-feel of cigarettes; NJOY was not owned by a tobacco company; and do not require charging or re-filling. Participants were instructed in the use of the e-cigarette after the baseline assessment. They were not required to stop smoking tobacco, but were encouraged to replace it with e-cigarettes as much as possible. No behavioural (stop-smoking) support was provided during the intervention period. This was followed by a 4-week post-intervention follow-up, in which participants were encouraged to continue e-cigarette use. Participants were informed about e-cigarette types, and where these could be purchased. A final follow-up assessment was conducted at week 24.
Measures
Primary outcome
Tobacco cigarette use measured weekly using the Time Line Follow Back (TLFB) (Brown et al., Reference Brown, Burgess, Sales, Whiteley, Evans and Miller1998) and reported at the subsequent session. Participants were required to report the number of tobacco cigarettes consumed a week prior to the assessment. The mean number of cigarettes per day (cigarettes/day) was calculated by dividing the total number of cigarettes consumed between assessments by the number of days between assessments.
Secondary outcomes
E-cigarette use was measured weekly using the TLFB and reported at the subsequent session. To facilitate recall and recycling, participants returned used and un-used e-cigarettes at each assessment. The mean number of e-cigarettes consumed per day (e-cigarettes/day) was calculated by dividing the total number of e-cigarettes consumed between assessments by the number of days between assessments. The number of e-cigarettes/day was multiplied by 18 to approximate the tobacco equivalent per day, on the basis of data from the manufacturer that each NJOY e-cigarette is equivalent to 15–20 tobacco cigarettes.
Tobacco cigarette use was also indexed weekly by measuring exhaled CO levels with a Smokerlyzer ED50 CO meter (Bedfont Instruments, UK). E-cigarette acceptability was measured using a visual analogue scale (VAS) at baseline, and at 2, 6, 10 and 24 weeks (Blank et al., Reference Blank, Sams, Weaver and Eissenberg2008). Participants were asked to rate the occurrence of side effects associated with e-cigarette use on a weekly basis (see online Supplementary material).
Respiratory symptoms were assessed at baseline, and at 2, 6, 10 and 24 weeks using an abbreviated and adapted version of the American Thoracic Society Questionnaire (ATS) (Comstock et al., Reference Comstock, Tockman, Helsing and Hennesy1979). A Wright's Mini Peak-flow Meter (Clement Clarke International Ltd., UK) was used to assess lung capacity at baseline, and weeks 6, 10 and 24. Peak flow was obtained three times at each assessment, so a representative mean could be calculated.
Urinary cotinine was measured at baseline, 2, 6 and 10 weeks. Cotinine level was determined using high-performance liquid chromatography coupled to tandem mass spectrometry with multiple reaction monitoring (LC-MS/MS) after liquid–liquid extraction with ethyl acetate using cotinine-d₃ as the internal standard. The original assay developed was performed by gas chromatography with nitrogen phosphorous detection. In a subsample of participants (N = 8), 3-hydroxypropylmercapturic acid (3-HPMA, a measure of the toxicant acrolein) and formic acid were measured at baseline and week 6. These participants were chosen as their tobacco intake had decreased by more than 50% in this period. The measurement of 3-HPMA and formic acid was also performed by validated LC-MS/MS assays. Analysis was conducted by Advanced Bioanalytical Service Laboratories Ltd., Welwyn Garden City, UK.
The Motivation to Stop Scale (MTSS) (Kotz et al., Reference Kotz, Brown and West2013) and Smoking Consequences Questionnaire-Adult (SCQ-A) (Copeland et al., Reference Copeland, Brandon and Quinn1995; Rash & Copeland, Reference Rash and Copeland2008) were used at baseline, weeks 2, 6, 10 and 24 to assess perceived consequences of smoking, symptoms of withdrawal and motivation to quit. Psychotic and mood symptoms were measured weekly using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., Reference Kay, Fiszbein and Opler1987) and the Calgary Depression Scale for Schizophrenia (CDSS) (Addington et al., Reference Addington, Addington and Maticka-Tyndale1993). Heart rate, blood pressure and weight were measured weekly to assess physical changes. Finally, the occurrence of (serious) adverse events was assessed on a weekly basis by asking participants whether any event had occurred. When this was not possible, electronic medical notes were checked for events. For a table of measures and time points, see Table 1.
Table 1. Study measures and assessment timepointsa

a BL, baseline; FU, follow-up; ← indicates retrospective reporting from week 10 to 24.
Statistical methods
A sample size of at least 30 was sought as per Browne's (Reference Browne1995) suggestions for a pilot study. We anticipated a drop-out rate of approximately 30%, so a sample size of 50 was the target with the aim to complete data collection in around 35 participants. In order to compare screening characteristics, categorical variables were summarised as n (%) and continuous variables as means and standard deviations. Prior to the intervention period, three measures of cigarettes/day were available. In order to obtain a more accurate measure of cigarettes/day, a mean was calculated from these time points (later referred to as the pre-intervention baseline). To evaluate changes in the PANSS, CDSS, cigarettes/day, e-cigarettes/day, CO and peak flow between baseline and subsequent assessments, one-way repeated-measures analyses of variance (ANOVA) were conducted. The significance level was set a priori at p < 0.05 and all statistical methods used were two-tailed. In order to carry out the statistical analysis, missing data were treated using the last-observation-brought-forward method. Data were analysed using SPSS 22 (IBM Corp., Armonk, NY, USA).
Results
A total of 50 participants were recruited from 248 referrals (Fig. 1). Two participants were lost to follow-up (due to loss of contact and disengagement), and seven withdrew consent (two due to the time commitment, two for unknown reasons, one did not like the e-cigarettes, one wanted to quit immediately and one could not comply with the protocol). Seventy-six per cent of participants were male, and the majority of participants identified as white/white British (46%) (Table 2). The most common diagnosis in this sample was schizophrenia (54%). The mean PANSS positive, negative and general scores were 10.47 (s.d. = 3.6), 9.04 (s.d. = 2S4) and 20.94 (s.d. = 5.0), respectively, suggesting a low and stable level of symptom severity.
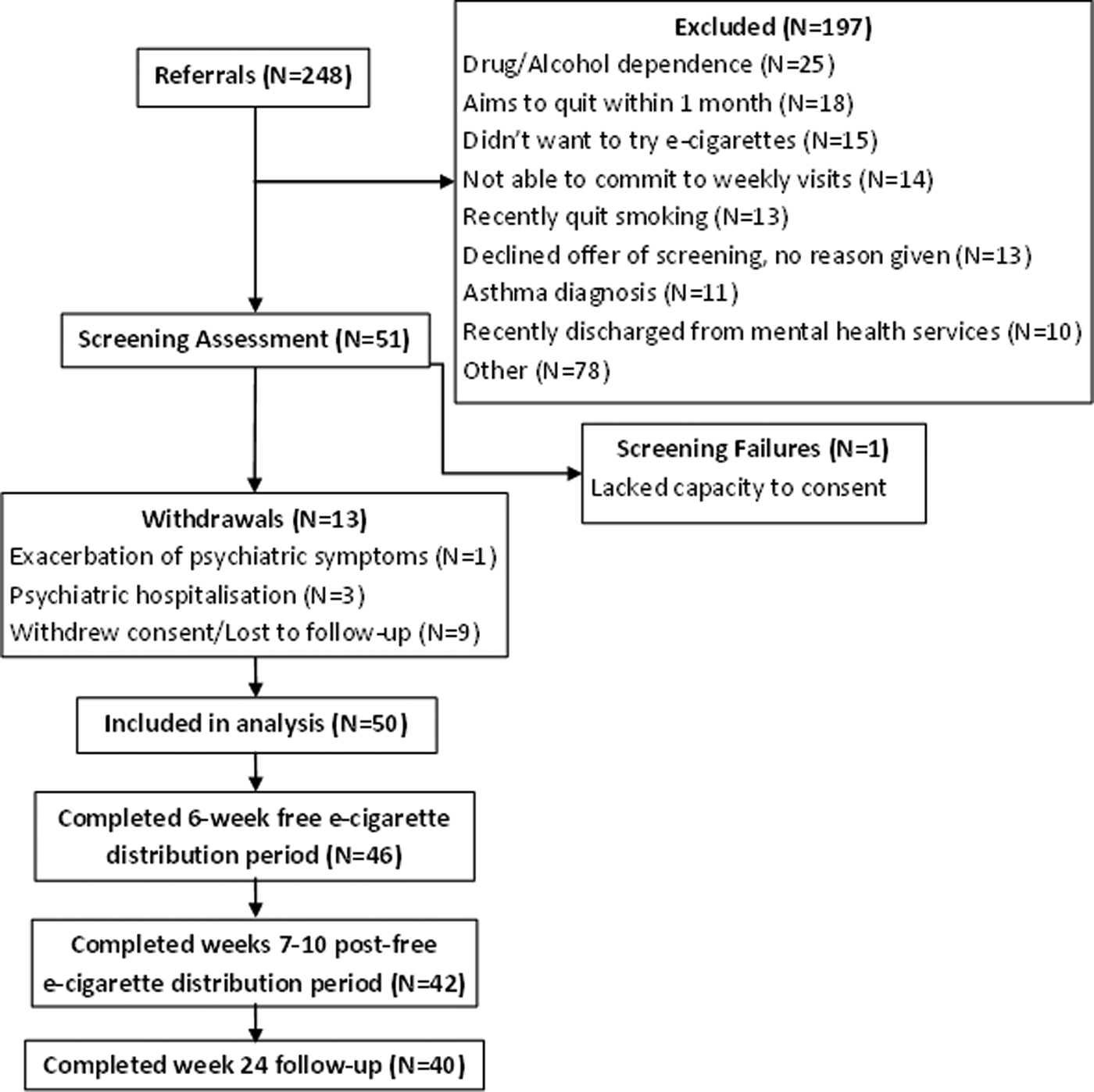
Fig. 1. Participant flow chart.
Table 2. Characteristics of the sample at baselinea, N = 50

a PANSS, Positive and Negative Syndrome Scale; GCSE/CSE, General Certificate of Secondary Education/Certificate of Secondary Education.
The mean age at screening was 39.0 years (s.d. = 10.7). At baseline, the mean number of cigarettes/day consumed was 17.9 (s.d. = 11.9). Of those who had previously tried to quit, only 22% reported that they had managed to abstain from smoking for more than 3 months. At baseline, 26.5% of participants reported that they thought they should stop smoking, but did not really want to, and 46% of participants reported smoking their first cigarette within 5 min of waking.
Cigarette/e-cigarette use
During 6 weeks free e-cigarette distribution
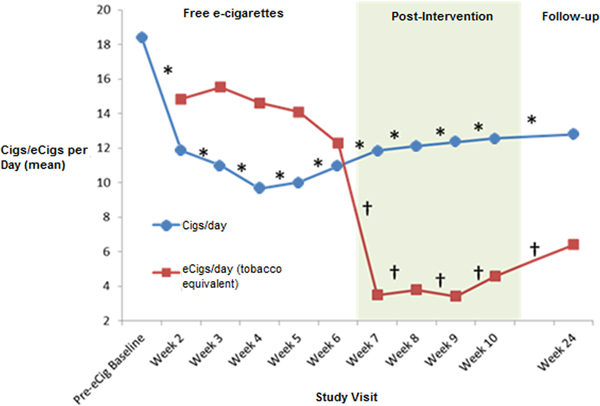
A one-way repeated-measure ANOVA showed a significant reduction in cigarettes consumed per day between the pre-intervention baseline and week 6 [F (2.596,116.800) = 25.878, p < 0.001] (Fig. 2). E-cigarette use remained stable over the free e-cigarette distribution period [F (2.932,46.504) = 2.023, p = 0.115]. From the pre-intervention baseline to week 6, there was a significant reduction in exhaled CO level [F (3.335,126.633) = 5.063, p = 0.002] (Fig. 3), and there was a trend for reduction in urinary cotinine concentration [F (2,46) = 2.608, p = 0.085]. By the end of the free e-cigarette period, 37% of participants had reduced the number of cigarettes per day by ⩾50% (this subgroup will now be referred to as reducers), while 7% reported having stopped smoking entirely.
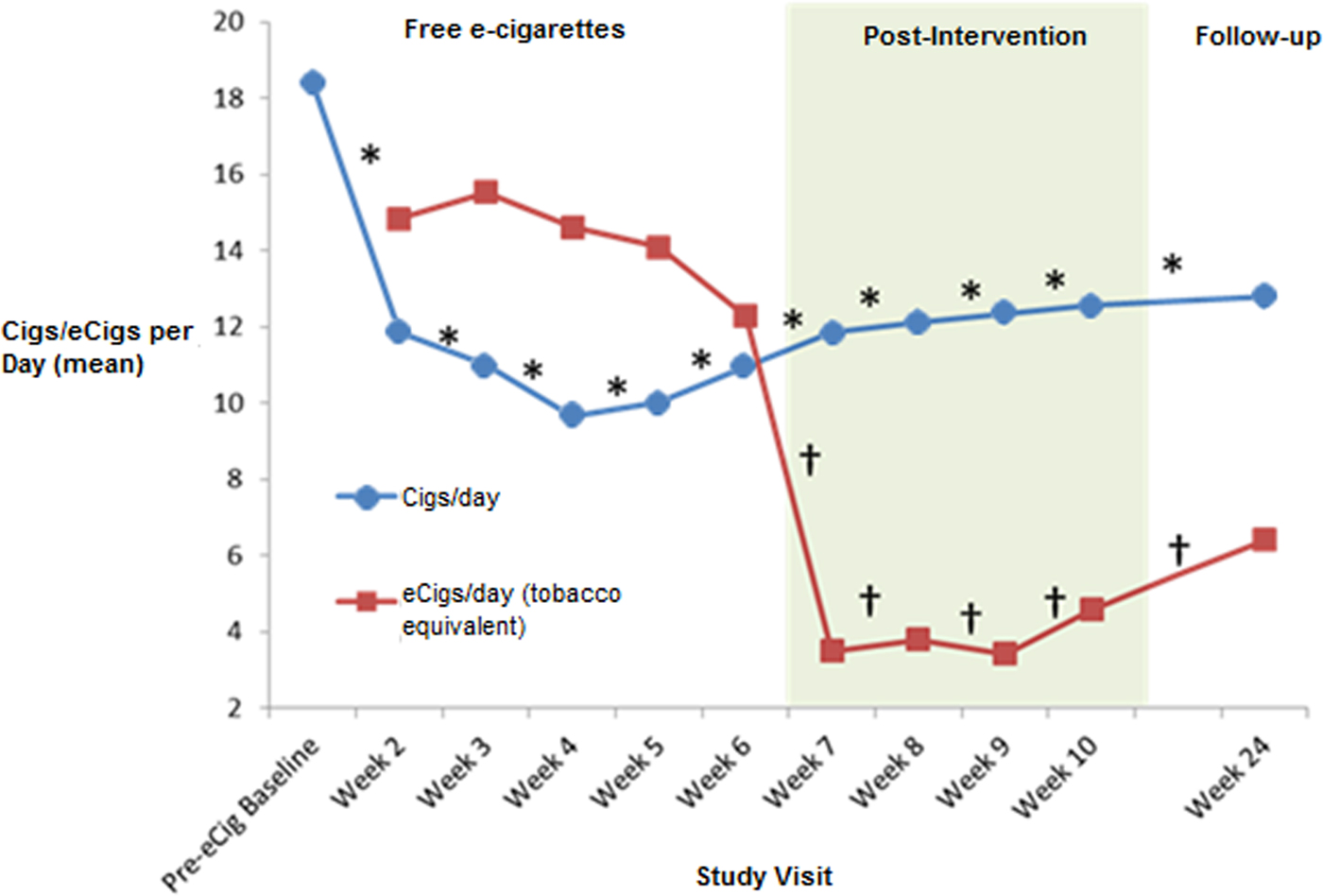
Fig. 2. Tobacco cigarette and e-cigarette use during and post-intervention. aCigs/day, cigarettes per day; eCigsday, e-cigarettes per day. *Repeated-measures ANOVA F value significant p < 0.05 – cigarettes from baseline to week 24. †Repeated-measures ANOVA F value significant p < 0.05 – e-cigarettes from week 2 to 24.
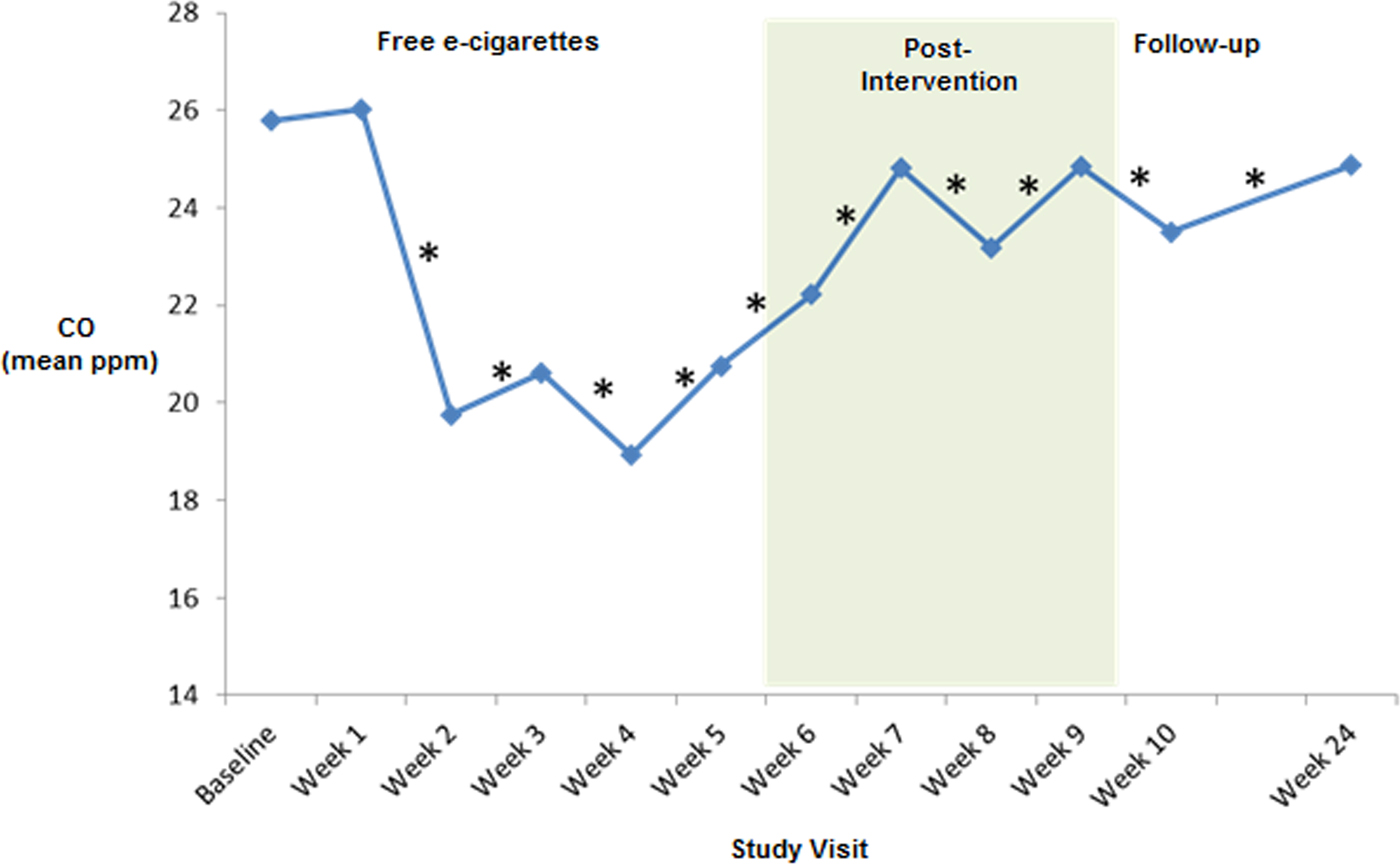
Fig. 3. Mean carbon monoxide (CO) levels during post-intervention. *Repeated-measures ANOVA F value significant p < 0.05 from baseline to week 24.
Four weeks post free e-cigarette distribution
Although e-cigarette use significantly reduced after they were no longer provided for free [F (2.392,107.659) = 25.738, p < 0.001], tobacco cigarette use at week 10 was still significantly lower than at baseline [F (3.779,170.076) = 14.718, p < 0.001]. Exhaled CO levels were also lower at week 10 than at baseline [F (4.672,154.188) = 2.987, p = 0.015]. There was a trend in urine cotinine-level reduction between baseline and week 10 [F (3,66) = 2.714, p = 0.052]. At week 10, 26% of participants had reduced the number of cigarettes/day by ⩾50% compared with baseline, with 5% reporting being non-daily smokers.
Twenty-four weeks after baseline
The reduction in cigarettes used per day from baseline remained significant at week 10 [19.3(±12.4)] and week 24 [12.8(±9.9)] [F (3.944,153.806) = 11.545, p < 0.001], although e-cigarette use was also significantly reduced between weeks 2 and 24 [F (2.886,112.562) = 18.335, p < 0.001]. A significant reduction in CO levels was still evident at week 24 [F (4.921,191.930) = 2.794, p = 0.019]. At this stage, 25% (10/40) of participants had reduced the number of tobacco cigarettes consumed by ⩾50%, one participant had become a non-daily smoker, and another had quit completely.
Acceptability of e-cigarettes
The most common reasons for quitting e-cigarettes after the free distribution period ended included financial reasons (14/46), and not getting around to (8/46), or not feeling like purchasing one (5/46). Others reported they had not used e-cigarettes because shops were out of stock (4/46), they were researching a future purchase (4/46) or preferred tobacco cigarettes (4/46). A minority cited wanting to quit e-cigarettes as well as cigarettes (2/46), not receiving the same nicotine hit (2/46), disliking the taste/smell (2/46), experiencing adverse effects (2/46), being too busy to make a purchase (2/46) or purchasing e-cigarettes that ran out very quickly (2/46). Individual participants reported stopping e-cigarettes due to stigma (1/46), a current cough (1/46), finding tobacco cigarettes better for stress relief (1/46), not wanting to become addicted to vaping (1/46) and waiting for an ordered e-cigarette to arrive (1/46).
According to the VAS scale at week 6, 25.5% of participants believed that e-cigarettes did not taste like tobacco smoking ‘at all’, while 19.6% reported that the e-cigarettes felt like a tobacco cigarettes (rated 5, where 7 is ‘extremely’). At week 6, 41.3% of participants said they would like to use e-cigarettes more and cigarettes less, and 82.6% perceived e-cigarettes to be less harmful than tobacco cigarettes. The χ2 tests showed some significant differences in the perception of tobacco cigarettes compared with e-cigarettes at week 6 (see online Supplementary material).
Predictors of e-cigarette use
There was a significant association between smoking reduction status at 10 weeks and agreement with the following statement at baseline ‘the more I smoke, the more I risk my health’ [χ2(1) = 5.027, p = 0.025]. There were no significant differences between reducers and non-reducers in age, cigarettes used per day (at baseline), years smoking, years since first contact with mental health services, gender, employment status, qualification level, motivation to quit, PANSS total and subscale scores at baseline (all p > 0.05).
Adverse effects
During the intervention period, the most commonly reported adverse effects (endorsed in response to named adverse effects) were throat irritation (13/46), dry cough (9/46) and dry mouth (7/46). There were no significant changes in the reporting of adverse effects between baseline and week 6 (all p > 0.05). When asked about adverse effects in an open question, one participant reported a dry cough.
Changes in respiratory symptoms
There was no significant increase or decrease in cough, phlegm production, breathlessness, tightness in the chest or wheezing from baseline to week 6 (all p > 0.05). There was no significant change in the peak-flow rate between baseline and week 6 [F (1,25) = 0.986, p = 0.330], between weeks 6 and 10 [F (1,44) = 0.013, p = 0.860] or between weeks 10 and 24 [F (3,114) = 0.691, p = 0.559]. There were no significant differences in respiratory symptoms or peak-flow rate between reducers and non-reducers (all p > 0.05).
Psychiatric symptoms
There were no significant changes in the PANSS positive, negative, general symptoms or total score (Kay et al., Reference Kay, Fiszbein and Opler1987) at any time point (all p > 0.05). There were also no significant changes in CDSS (Addington et al., Reference Addington, Addington and Maticka-Tyndale1993) score between baseline and any time point (all p > 0.05). There were also no differences in scores on the PANSS or CDSS between reducers and non-reducers (all p > 0.05).
Serious adverse events
Five serious adverse events occurred during the study. All were psychiatric hospitalisations, four due to a worsening of psychotic symptoms and one a worsening of depressive symptoms. All were considered to be unrelated to the study intervention.
Toxicants in urine
There were no significant changes in 3-HPMA or formic acid concentrations from baseline to the end of the free e-cigarette intervention [F (1,7) = 3.808, p = 0.092] and [F (1,7) = 0.403, p = 0.546], respectively.
Discussion
The findings from this pilot study suggest that the provision of e-cigarettes can significantly reduce tobacco consumption and CO level, with no significant change in respiratory and psychiatric symptoms in people with SMI. This is consistent with similar findings in the general population (Bullen et al., Reference Bullen, Howe, Laugesen, McRobbie, Parag, Williman and Walker2013), which indicate that people using e-cigarettes significantly reduce their tobacco intake. Few studies to date have investigated e-cigarettes as a harm reduction method on people diagnosed with a mental illness (Caponnetto et al., Reference Caponnetto, Auditore, Russo, Cappello and Polosa2013; O'Brien et al., Reference O'Brien, Knight-West, Walker, Parag and Bullen2015; Pratt et al., Reference Pratt, Sargent, Daniels, Santos and Brunette2016). As in these studies, we found that although e-cigarette use dropped after free distribution ended, a reduction in cigarette use was still maintained (Caponnetto et al., Reference Caponnetto, Auditore, Russo, Cappello and Polosa2013). Our results also suggest that smokers with SMI, like smokers in the general population (Hartmann-Boyce et al., Reference Hartmann-Boyce, McRobbie, Bullen, Begh, Stead and Hajek2016), find e-cigarettes acceptable. We also found that e-cigarettes were perceived to be healthier and more socially acceptable than tobacco cigarettes, supporting previous findings in smokers with SMI (Pratt et al., Reference Pratt, Sargent, Daniels, Santos and Brunette2016) and in the general population (Etter and Bullen, Reference Etter and Bullen2011b; Bullen et al., Reference Bullen, Howe, Laugesen, McRobbie, Parag, Williman and Walker2013).
There were trends for reduction in cotinine levels during both intervention and the follow-up periods. A lack of significant changes despite reduced cigarette consumption may have resulted from the use of e-cigarettes which also contain nicotine (Public Health England, 2018). Furthermore, it could be because less nicotine is absorbed while using e-cigarettes compared with tobacco cigarettes. A previous study demonstrated that the e-cigarettes performance of nicotine delivery into serum is more similar to delivery in nicotine replacement therapies than tobacco cigarettes (Bullen et al., Reference Bullen, McRobbie, Thornley, Glover, Lin and Laugesen2010), therefore we might not see as dramatic a fall in cotinine levels initially. The reduction in tobacco use was not accompanied by a proportional reduction in CO levels. It is possible that CO levels may still have reflected tobacco use prior to assessment. Another potential factor is that participants may have had difficulty performing the technique correctly. It is likely that participants were engaging in compensatory smoking, a previously documented phenomenon (Strasser et al., Reference Strasser, Lerman, Sanborn, Pickworth and Feldman2007). In addition, although we gave participants 150% of their usual tobacco use in e-cigarettes, not everyone used all of this allowance, or replaced as many cigarettes, so may not have had such a dramatic decrease in CO level. Future studies could limit the amount of tobacco smoked immediately prior to an assessment. In addition, because of the non-significant rise in CO from week 6 to 24, more support may need to be offered to reducers after intervention in order to maintain initial gains.
Although urinary 3-HMPA and formic acid levels did not significantly change after 6 weeks of e-cigarette use, research has suggested that dual use of tobacco and e-cigarettes is not very helpful when attempting to reduce levels of carcinogens/toxicants (Shahab et al., Reference Shahab, Goniewicz, Blount, Brown, McNeill, Udeni Alwis, Feng, Wang and West2017). However, it is not suggested that dual use is more harmful, as e-cigarettes are shown to have low levels of carcinogens, therefore the goal should be tobacco cessation (Shahab et al., Reference Shahab, Goniewicz, Blount, Brown, McNeill, Udeni Alwis, Feng, Wang and West2017).
One limitation of the present study was the use of self-report for various measures: further biochemical verification of toxins such as aldehydes or tobacco-specific N-nitrosamines could address this issue. As the e-cigarette used in this intervention was a first-generation model, the results may not be generalisable to other e-cigarettes. Previous research has shown that when compared with newer e-cigarette models, first-generation e-cigarettes are less effective in terms of nicotine delivery (Farsalinos et al., Reference Farsalinos, Spyrou, Tsimopoulou, Stefopoulos, Romagna and Voudris2015), and a survey revealed that e-cigarette type and frequency of use are important factors to encourage cessation in UK smokers (Hitchman et al., Reference Hitchman, Brose, Brown, Robson and McNeill2015). Therefore, it would be sensible to conduct future trials using contemporary types of e-cigarettes. Although the present study involved a modestly sized sample, the promising findings indicate that further research in larger sample sizes is merited, alongside a comparison group. A larger study may also be merited, given the possibility of the current study being underpowered. Finally, it can be seen that a majority of the participants in this study were male. However, this is in keeping with the ratio of male-to-female psychosis patients in mental health services in South London.
Despite these limitations, this pilot study employed a representative sample of participants diagnosed with SMI, and with a low rate of loss to follow-up in a difficult to engage population. The study also administered a number of standardised measures, thereby increasing validity. This study also employed a long period of follow-up. Despite recruiting a population including heavy smokers, who were not motivated to quit, with a history of failed cessation attempts, the results of this pilot study suggest that e-cigarettes may be a useful intervention as a means of harm reduction in smokers diagnosed with a psychotic illness. This was achieved without any exacerbation of psychotic symptoms or change in respiratory symptoms.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718001782.
Acknowledgements
The authors thank all participants in the study, and all of the SLaM Psychosis CAG teams. Especially thanks to the COAST team in Croydon, the North East Promoting Recovery Service in Lambeth and the North East Psychosis Community Service in Southwark. The protocol was developed in collaboration with Dr J. Tidey (Brown University) and Dr A.E. Evins (Harvard Medical School).
Conflict of interest
R.P-I. has received honoraria and speaker support from Lundbeck. L.D. has provided consultancy for the pharmaceutical industry (Johnson & Johnson 2015, 2017) and acted as an expert witness for an e-cigarette patent infringement case (Porzio, Bromberg & Newman Attorneys at Law, 2015). Between 2011 and 2013, she conducted research for several independent electronic cigarette companies (Totally Wicked, SKYCIGS and E-Lites) for which the University of East London received funds. The e-cigarette companies involved had no input into the design, conduct or write up of these projects and she has not received any funds from e-cigarette companies in the last 4 years. She has no links with, and has not received any funds from, the tobacco industry, although two e-cigarette companies that she worked with in 2013 were subsequently acquired by the tobacco industry (SKYCIGs and E-Lites). L.H., T.R., K-V.S., J.M., A.M. and P.M. have no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Funding
This work was funded by the Maudsley Charity (grant number 715); and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London.