Background
In a typical consultation, general practitioners (GPs) address three problems and make eight decisions (Ebell, Reference Ebell2010). To facilitate this process, they often use many mental shortcuts (heuristics) before coming to a diagnosis, in addition to history taking and physical examination (Phillips, Reference Phillips2010b). In this way, many accurate diagnoses are made, but occasionally mistakes lie in wait. Especially if symptoms are vague or atypical, there is a risk of taking a wrong diagnostic turn. In such cases, relying on clinical examination alone does not provide an adequate rationale for clinical decision-making.
Here, clinical decision rules (CDRs) can provide relief. These rules are developed for physicians to objectivate and guide their decision-making by facilitating the refinement process of the work-through of differentials that follows an initial suspicion (Phillips, Reference Phillips2010b). In relevant cases, they serve as evidence-based practical guidelines to make clinical practice more tangible (Green, Reference Green2013; Phillips, Reference Phillips2010a). The application of decision rules improves the clinician’s ability to treat patients consistently and to focus on the patient’s clinical context (Gaddis et al., Reference Gaddis, Greenwald and Huckson2007). Additionally, the use of CDRs can avoid diagnostic errors and prevents unnecessary tests (Phillips, Reference Phillips2010b; Reed, Reference Reed2006).
The term ‘Clinical Decision Rule’ has many definitions, but fundamentally it is a tool that quantifies and combines simple available clinical indicators in order to define a new parameter. In primary care, this parameter can be generated by combining patient signs, symptoms, and additional readily available laboratory, function, or imaging test results and can be used to estimate a score related to the probability of the presence or absence of a specific disease (diagnosis) or outcome (prognosis). Subsequently, a concise diagnostic or therapeutic ‘course of action’ or a preventive strategy should be undertaken (le Gal et al., Reference le Gal, Carrier and Rodger2012). In this way, clinical behaviour is promoted leading to a more structured and consistent decision-making, improving quality of care and patient satisfaction while reducing unnecessary costs, preferably combined with a reduction of practice variation among doctors (Reilly and Evans, Reference Reilly and Evans2006). The use of decision rules can help junior physicians to develop their clinical judgement but can also assist more experienced doctors in rapidly making decisions on complex diagnoses or potential life-threatening conditions (Carmelli et al., Reference Carmelli, Grock, Picart and Mason2018). In the end, however, the main value of a CDR lies in reducing the complexity of combining symptoms and signs to decision recommendations in terms of, typically, a ‘rule-out’ without further testing, a need for further testing, or a specific treatment (Ebell, Reference Ebell2010).
Ideally, CDRs are perfectly suited to be implemented in a primary care setting. The mere opportunity to use a CDR helps GPs find their way in the wide variety of patients presenting to them that suffer from an even wider variety of often early symptoms (le Gal et al., Reference le Gal, Carrier and Rodger2012) for which a wide range of diagnostic and treatment options is available. As a gatekeeper, one of the main strengths of GPs is avoiding unnecessary referrals for comprehensive diagnostics and specialist consultations, and CDRs are particularly suitable for this purpose (Reed, Reference Reed2006). Nonetheless, even though numerous CDRs were developed over the past few decades, their use in daily primary care is limited to only a few isolated rules.
In the present work, we focussed on providing insight into the challenges surrounding the implementation of CDRs as well as challenges in everyday use of well-established CDRs, using the CDR for deep vein thrombosis (DVT) in primary care patients as a case study. Lastly, we used these insights to formulate suggestions for a more effective application of CDRs in primary care.
From derivation to implementation of a viable CDR
Over 20 years of research on the topic of CDRs has led to the formulation of a set of preconditions that must be met by every decision rule. Such a rule should have a clear purpose and be relevant, accurate, concise, and reproducible. Additionally, CDRs should demonstrate content validity; they should be composed of well-recognised, clinically sensible, and independent predictors. Typically, three or more predictor variables should be used, which can be obtained from the patient’s medical history, physical examination, or straightforward diagnostic tests (Graham et al., Reference Graham, Stiell, Laupacis, McAuley, Howell, Clancy, Durieux, Simon, Emparanza, Aginaga, O’connor and Wells2001). These predictors should be incorporated in a rule that is easy to apply and may involve a diagnostic, therapeutic, or preventive course of action, and preferably limits practice variation (le Gal et al., Reference le Gal, Carrier and Rodger2012; Rodger et al., Reference Rodger, Le Gal, Wells, Baglin, Aujesky, Righini, Palareti, Huisman and Meyer2014). This list of preconditions implies that different stages must be successfully passed when developing a valid and useful CDR. Indeed, at least three stages of CDR development can be distinguished. These three stages are presented in Figure 1, which was initially conceptualised by McGinn et al (Reference McGinn, Guyatt, Wyer, Naylor, Stiell and Richardson2000).

Figure 1. The development process of a clinical decision rule, as conceptualised by McGinn et al (McGinn et al., Reference McGinn, Guyatt, Wyer, Naylor, Stiell and Richardson2000)
After the primary idea that a simple decision rule may improve decision-making in response to a specific clinical question, the first stage can be initiated. This stage involves the development, or more specifically, the derivation of the CDR and its variables, which is subject to strict guidelines (Laupacis et al., Reference Laupacis, Sekar and Stiell1997; le Gal et al., Reference le Gal, Carrier and Rodger2012; McGinn et al., Reference McGinn, Guyatt, Wyer, Naylor, Stiell and Richardson2000; Rodger et al., Reference Rodger, Le Gal, Wells, Baglin, Aujesky, Righini, Palareti, Huisman and Meyer2014; Stiell and Wells, Reference Stiell and Wells1999). Typically, this derivation process is supported by a set of specific methodologic standards relating to characteristics such as outcome events, predictors, and study subjects based on original data collection and multivariate statistical analysis. The next step is to confirm the need for the actual newly generated rule after all its details are elaborated, its clinical relevance and ease to use in real life, as well as the expected savings in terms of time and resources (McGinn et al., Reference McGinn, Guyatt, Wyer, Naylor, Stiell and Richardson2000; Stiell and Wells, Reference Stiell and Wells1999). The results of this step may require the rule or the generated score to be refined. An informative illustration of the importance of incorporating this ‘clarification’ step is presented in a publication on the STONE score, a rule whose developers seem to have failed to ask these questions (Green and Schriger, Reference Green and Schriger2016). The authors of the publication question both the need and clinical relevance of the decision rule, stating that it is not clear what its role is in actual clinical decision-making, and that the rule does not outperform clinical judgement.
Once the derivation stage has been completed, a prospective external validation should take place in an independent cohort of patients (le Gal et al., Reference le Gal, Carrier and Rodger2012). The rule should be first applied in a narrow setting and in a patient population that is similar to the derivation group in order to validate if the rule’s behaviour is actually the same as in the derivation cohort. Later on, validation in broader, more general, clinical settings is required with varying prevalence and outcomes of disease (McGinn et al., Reference McGinn, Guyatt, Wyer, Naylor, Stiell and Richardson2000) in order to discover if the rule is suitable for application in these situations. This action should only be done after adequate training of the study physicians, because otherwise, results will most probably be biased by differences in the application of the – hitherto unknown – CDR (Stiell and Wells, Reference Stiell and Wells1999). In the end, the total spectrum of patients in which the CDR is to be validated must fit the entire patient – and physician – population in which the CDR is intended to be applied. It is important to ensure that the application of the rule will remain strictly limited to the validated settings and patients, especially after implementation in routine clinical practice (Carmelli et al., Reference Carmelli, Grock, Picart and Mason2018) and to confirm that generalisability issues are adequately addressed (Toll et al., Reference Toll, Janssen, Vergouwe and Moons2008).
A CDR should also do what it promises to be valuable in practice. An estimate of the potential impact of use is vital to verify if this is the case (McGinn et al., Reference McGinn, Guyatt, Wyer, Naylor, Stiell and Richardson2000; Reilly and Evans, Reference Reilly and Evans2006; Toll et al., Reference Toll, Janssen, Vergouwe and Moons2008). In such an impact analysis, evidence is collected to demonstrate whether the rule actually changed physicians’ behaviour, improved clinically relevant process parameters or patient outcomes, or reduced costs (le Gal et al., Reference le Gal, Carrier and Rodger2012; McGinn et al., Reference McGinn, Guyatt, Wyer, Naylor, Stiell and Richardson2000). Ideally, an impact study is able to demonstrate a so-called positive resource utilisation impact in an index group, which was exposed to the use of the decision rule, versus a control group, which was subjected to care or clinical judgement as usual. This positive resource utilisation impact amounts to a decrease in the use of the resource in question, for example, diagnostic test ordering, without a corresponding increase in adverse outcomes such as an increase in morbidity/mortality or a decline in quality of life.
Many barriers have been reported between awareness of and adherence to a new approach (Gaddis et al., Reference Gaddis, Greenwald and Huckson2007). In other words, even if a CDR passed an impact analysis successfully and results have been widely communicated to the professionals involved, this still does not mean that the CDR will actually be implemented in routine daily practice, let alone that it continues to be used in the long run (Laupacis et al., Reference Laupacis, Sekar and Stiell1997; Reilly and Evans, Reference Reilly and Evans2006; Toll et al., Reference Toll, Janssen, Vergouwe and Moons2008; van Doorn and Geersing, Reference van Doorn and Geersing2018).
As a rule of thumb, a CDR will only be embraced by physicians if its use is evidently clinically useful, if the CDR is incorporated into guidelines, if it is, for example, used by both opinion leaders and immediate colleagues, if it results in saving money or time, and if it improves both patients’ and doctors’ lives (Phillips, Reference Phillips2010b). Often, however, not all of these criteria are met. In fact, many factors are known to pose barriers to the use of a CDR. An overview of these factors was selected from the literatureFootnote 1 and is presented in Table 1. The Appendix provides an extended version of this table, including some additional context and a few examples.
Table 1. Overview of factors that pose barriers for the use of a clinical decision rule in routine clinical practice. NB: the examples that are applicable to a specific CDR differ widely from country to country, as has been demonstrated by the application of the Ottawa Ankle Rules (Graham et al., Reference Graham, Stiell, Laupacis, McAuley, Howell, Clancy, Durieux, Simon, Emparanza, Aginaga, O’connor and Wells2001). The Addendum provides an extended version of the table with references, some additional context and a few examples

1) this might explain why 50% of physicians do not use CDRs at all (Le Marechal et al., Reference Le Marechal, Martinot, Duhamel, Pruvost and Dubos2013).
CDR: clinical decision rule.
Due to a combination of these factors, decision rules are poorly implemented in clinical practice; this even applies to the mother of all decision rules, the Ottawa Ankle Rules (Brehaut et al., Reference Brehaut, Stiell, Visentin and Graham2005; Gaddis et al., Reference Gaddis, Greenwald and Huckson2007). Obviously, some factors can be dealt with, for instance, a CDR that is too complicated should be redesigned, while it is to be accepted that others will persist – for instance, the fear of litigation. Most important is to identify which barriers prevent the CDR from being used, and then formulate a targeted strategic approach to overcome these barriers. Many approaches have been suggested (Reilly and Evans, Reference Reilly and Evans2006), but most of them have not been proven to be effective. Exceptions thus far include targeted mailing and local implementation strategies equipped with known effective elements, such as audit and feedback, delivered by respected local clinicians (Graham et al., Reference Graham, Stiell, Laupacis, McAuley, Howell, Clancy, Durieux, Simon, Emparanza, Aginaga, O’connor and Wells2001; Stiell and Wells, Reference Stiell and Wells1999).
With so many obstacles, it is not surprising that in the end almost all CDRs do not make it to the endFootnote 2 . A small subset of CDRs has undergone an appropriate prospective external validation, while only a few have undergone a formal impact analysis (Ban et al., Reference Ban, Chan, Muthee, Paez, Stevens and Perera2021; Ebell et al., Reference Ebell, Rahmatullah, Cai, Bentivegna, Hulme, Thompson and Lutz2021; Laupacis et al., Reference Laupacis, Sekar and Stiell1997; Reilly and Evans, Reference Reilly and Evans2006; Toll et al., Reference Toll, Janssen, Vergouwe and Moons2008)Footnote 3 . However, once a CDR has passed the entire process, clinicians have an additional tool at their disposal that can be very helpful for them and their patients.
Decision rules within primary care
Traditionally, CDRs have been implemented primarily in the emergency room, cardiology and paediatrics. Within these clinical settings, in the majority of cases, they serve as a screening tool for safely ruling out a disease outcome prior to imaging (Carmelli et al., Reference Carmelli, Grock, Picart and Mason2018; Ebell, Reference Ebell2010; Le Marechal et al., Reference Le Marechal, Martinot, Duhamel, Pruvost and Dubos2013; Lim, Reference Lim2018; Monahan et al., Reference Monahan, Barton, Taylor, Roalfe, Richard Hobbs, Cowie, Davis, Deeks, Mant, McCahon, McDonagh, Sutton and Tait2017; Phillips, Reference Phillips2010a; Pugh, Reference Pugh2016; Reed, Reference Reed2006), next to other clinical tasks of CDRs: diagnosis, prognosis, treatment and prevention (Ebell, Reference Ebell2010).
As previously described, only a few CDRs are regularly used in primary care. An explanation for this phenomenon may be found in the limited number of impact studies that have been performed on the topic of primary care CDRs (Keogh et al., Reference Keogh, Wallace, O’Brien, Galvin, Smith, Lewis, Cummins, Cousins, Dimitrov and Fahey2014). Decision rules that are known to be routinely applied in primary care in some countries are the Ottawa Ankle Rules and a few cardiovascular rules. Examples of these cardiovascular rules are i) a rule to screen patients with a suspicion of cardiac failure for the need of a cardiac ultrasound (Monahan et al., Reference Monahan, Barton, Taylor, Roalfe, Richard Hobbs, Cowie, Davis, Deeks, Mant, McCahon, McDonagh, Sutton and Tait2017; Van Riet et al., Reference Van Riet, Hoes, Limburg, Landman, Kemperman and Rutten2016), ii) the CHA2DS2-VASc score for determining whether or not treatment is required with anticoagulation therapy or antiplatelet therapy – although this score is mainly used in the hospital (Lucassen, Reference Lucassen2018), and iii) the primary care DVT rule, also called the Oudega rule (Oudega et al., Reference Oudega, Moons and Hoes2005b). This rule is widely used in a primary care setting and hence will be discussed in more detail below.
The Oudega rule was developed for primary care patients and appeared to be better suited for this population than the Wells rule, which was based upon data from hospital outpatients (Oudega et al., Reference Oudega, Moons and Hoes2005b, Reference Oudega, Hoes and Moons2005a; Wells et al., Reference Wells, Anderson, Bormanis, Guy, Mitchell, Gray, Clement, Robinson and Lewandowski1997). The rule was developed to rule out DVT, using a clinical score (see Table 2) combined with a D-dimer test (a blood test) to reduce the need for imaging throughout the exclusion process (see Figure 2). In other words, applying the rule ensures that an ultrasound exam is only performed if strictly necessary. Several factors contribute to making this primary care rule a textbook example of a decision rule as intended. These factors will be further explained in the next paragraph.
Table 2. Items (risk factors) of the primary-care-adapted Wells rule (Oudega rule) for deep vein thrombosis (Oudega et al., Reference Oudega, Moons and Hoes2005b) with their corresponding weight (1 or 2). A total score can be calculated for each patient in order to discriminate low (CDR score < 4) from high (CDR ≥ 4) risk patients. Only a high-risk patient is instantly subjected to ultrasonography. CDR: clinical decision rule
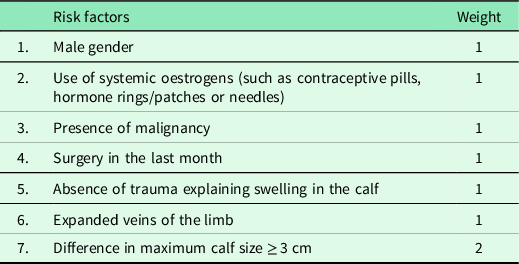

Figure 2. Simplified diagnostic flowchart presenting the clinical application of the primary-care-adapted Wells rule (Oudega rule) for deep vein thrombosis (Oudega et al., Reference Oudega, Moons and Hoes2005b). Only a high-risk patient (CDR ≥ 4) is instantly subjected to ultrasonography. In low-risk patients (CDR score < 4), a D-dimer blood test is performed before performing an ultrasound exam. In case of a non-elevated D-dimer test, no ultrasound is needed to safely exclude a DVT. This figure is a simplification of the original flowchart (NHG-werkgroep 2017). DVT: deep vein thrombosis. CDR: clinical decision rule. US: ultrasonography
For one, the Oudega rule successfully passed all stages involved in CDR introduction, from derivation to extensive external validation in various settings (Buller et al., Reference Buller, Ten Cate-Hoek, Hoes, Joore, Moons, Oudega, Prins, Stoffers, Toll, van der Velde and van Weert2009; Geersing et al., Reference Geersing, Erkens, Lucassen, Büller, Ten Cate, Hoes, Moons, Prins, Oudega, van Weert and Stoffers2012; Green, Reference Green2013; Hendriksen et al., Reference Hendriksen, Geersing, Van Voorthuizen, Oudega, Ten Cate-Hoek, Joore, Moons and Koffijberg2015; Oudega et al., Reference Oudega, Moons and Hoes2005b; Toll et al., Reference Toll, Oudega, Bulten, Hoes and Moons2006), as well as several impact analyses (Hendriksen et al. Reference Hendriksen, Lucassen, Erkens, Stoffers, van Weert, Büller, Hoes, Moons and Geersing2016; Ten Cate-Hoek et al., Reference Ten Cate-Hoek, Toll, Büller, Hoes, Moons, Oudega, Stoffers, van der Velde, van Weert, Prins and Joore2009; van Maanen et al., Reference van Maanen, Kingma, Oudega, Rutten, Moons and Geersing2020). Also, the rule possesses several ‘bonus properties’, making it simpler, more sensible, and more suitable for incorporation in daily practice. These characteristics are explained below.
-
i. The first feature is the fact that the Oudega rule is both a dichotomised and a two-way rule. The dichotomisation involves a classification into only two groups: a low-risk and a high-risk group (Reilly and Evans, Reference Reilly and Evans2006). In a two-way rule, a recommendation is made for each of the patient groups, whereas in a one-way rule, a patient either meets the criteria and the CDR recommends a specific outcome or the patient does not meet the criteria, and no recommendation is made. This can be confusing, leading to inability to act and can, paradoxically, result in an increase in testing (Carmelli et al., Reference Carmelli, Grock, Picart and Mason2018; Green, Reference Green2013). In the Oudega rule, a recommendation describing a clear course of action – ordering a D-dimer test versus a radiologic exam – is made for both patient groups (low-risk and high-risk), which makes it a two-way rule.
-
ii. The second advantage is the fact that the rule was designed for exclusion (rule-out) rather than for inclusion (detection). Clinicians are generally much more comfortable with a rule that is primarily able to correctly rule out an illness (high sensitivityFootnote 4 ) than with a rule that is designed to efficiently detect an illness (high specificityFootnote 5 ) (Ebell, Reference Ebell2010; Laupacis et al., Reference Laupacis, Sekar and Stiell1997; Reilly and Evans, Reference Reilly and Evans2006). This preference can be explicated by the fact that clinicians are more eager not to miss a diagnosis than about making the diagnostic process more efficient. Subsequently, the benefit of using the rule itself lies in a specificity that measurably outperforms clinical judgement with a comparable high sensitivity (Carmelli et al., Reference Carmelli, Grock, Picart and Mason2018; Green and Schriger, Reference Green and Schriger2016), as is indeed the case of the Oudega rule (Geersing et al., Reference Geersing, Janssen, Oudega, van Weert, Stoffers, Hoes and Moons2010; van Maanen et al., Reference van Maanen, Kingma, Oudega, Rutten, Moons and Geersing2020).
-
iii. Lastly, there is a substantial difference between the risk of disease of patients classified as low risk versus those classified as high risk. Such a difference makes it worthwhile to actually apply the rule (Ebell et al., Reference Ebell, Rahmatullah, Cai, Bentivegna, Hulme, Thompson and Lutz2021). The probability of disease in the low-risk-DVT group followed by a negative D-dimer test, ≈1.5%Footnote 6 , is low enough to require only a D-dimer test for these primary care patients and taking the (small) risk of a false-negative result for granted (Kingma et al., Reference Kingma, van Stel, Oudega, Moons and Geersing2017). Simultaneously, in the high-risk group, performing an instant ultrasound exam, which is more accurate but also more expensive and time-consuming than a D-dimer test, can be justified by an actual risk, ≈33%, of suffering from a clinically relevant thrombo-embolic event.
Once more, it appeared that applying a CDR in daily practice has limitations that are not addressed in study settings. In practice, it is found that the Oudega rule is often used incorrectly (Kingma et al., Reference Kingma, van Stel, Oudega, Moons and Geersing2017; van Maanen et al., Reference van Maanen, Kingma, Oudega, Rutten, Moons and Geersing2020), or it is used in only a limited number of eligible patients (Kingma et al., Reference Kingma, van Stel, Oudega, Moons and Geersing2017). These factors contribute to a lower efficiency and higher failure rate of the diagnostic work-up (Geersing et al., Reference Geersing, Janssen, Oudega, van Weert, Stoffers, Hoes and Moons2010; van Maanen et al., Reference van Maanen, Kingma, Oudega, Rutten, Moons and Geersing2020) than in the case of optimal use of the CDR.
This limited adoption of the rule may occur due to several causes. The most obvious cause for the suboptimal compliance with this primary care rule seems to be the time pressure in the primary care setting. During busy office hours in a crowded practice, GPs are tempted to base estimates on experience and clinical judgments, or only to use those clinical parameters that are readily available (Seaberg, Reference Seaberg2001; Toll et al., Reference Toll, Janssen, Vergouwe and Moons2008; Xu et al., Reference Xu, de Wit, Geersing, Takada, Schutgens, Elf, Kearon and Parpia2021). Moreover, this effect is enhanced by a low exposure rate of DVT suspicions, combined with a rule that incorporates a relatively high number of items (Green, Reference Green2013), seven of which at least one is considered non-trivial by many GPsFootnote 7 (le Gal et al., Reference le Gal, Carrier and Rodger2012). Based on these items (Table 2), in case of a low clinical score, the diagnostic procedure requires a D-dimer test prior to further diagnostic testing, and the need to refer to a laboratory can be an obstacle when an urgent decision has to be made.
On closer inspection, however, these issues do not seem insurmountable.
First, both the trigger of the application of the CDR and the memorisation of its content can be taken over by computer software or personnel other than the primary care physician. Such can be realised by the introduction of a DVT care pathway (DCP), in which an elevated D-dimer test, carried out at the phlebotomy service, is directly followed by an ultrasound exam without the intervention of the GP (Heerink et al., Reference Heerink, Péquériaux, Oudega, de Jong, Koffijberg and Kusters2022). In the DCP electronic request form, a window pops-up containing the CDR and its items, which need not necessarily be filled out by the physician that orders this protocol. Besides, it has been demonstrated that the mere application of a CDR in patients with suspected DVT, in the context of a DVT care pathway, acts as a ‘selection filter’, potentially preventing many unnecessary D-dimer tests from being performed (Heerink et al., Reference Heerink, Péquériaux, Oudega, de Jong, Koffijberg and Kusters2022).
Also, applying the latest medical insights to the Oudega criteria may lead to a modification (update) of this CDR, which is essentially untouched for about 25 years while it is known that CDRs in general can become ‘out of date’ rapidly due to new insights or new tests (le Gal et al., Reference le Gal, Carrier and Rodger2012; Toll et al., Reference Toll, Janssen, Vergouwe and Moons2008). Indeed, a recent study proposed a simplification of the current rule consisting of a D-dimer test and two simple items that can be applied even more rapidly in primary care (Xu et al., Reference Xu, de Wit, Geersing, Takada, Schutgens, Elf, Kearon and Parpia2021), similar to the YEARS score for hospital outpatients with a suspicion of pulmonary embolism (van der Hulle et al., Reference van der Hulle, Cheung, Kooij, Beenen, van Bemmel, van Es, Faber, Hazelaar, Heringhaus, Hofstee, Hovens, Kaasjager, van Klink, Kruip, Loeffen, Mairuhu, Middeldorp, Nijkeuter, van der Pol, Schol-Gelok, Ten Wolde, Klok and Huisman2017; Van Es et al., Reference Van Es, Beenen, Douma, den Exter, Mos, Kaasjager, Huisman, Kamphuisen, Middeldorp and Bossuyt2015). Even the application of D-dimer as a stand-alone test –without a CDR– for all patients with suspected DVT seems to be safe and efficient, as recent data have been demonstrated (Rinde et al., Reference Rinde, Fronas, Ghanima, Vik, Hansen and Brækkan2020) Footnote 8 .
Lastly, promising new diagnostic opportunities are emerging, such as easy-to-use point-of-care (POC) D-dimer tests, which enable performing a D-dimer test, preceded or not by a CDR, in a one-stop visit at the GP’s office (Ellis et al., Reference Ellis, Johnston, Craig, Scribner, Simon and Kirstein2021; Heerink et al., Reference Heerink, Gemen, Oudega, Hopstaken, Geersing and Kusters2020; Price et al., Reference Price, Fay and Hopstaken2021). In this way, the time delay that comes with referring the patient to the laboratory for a D-dimer test can be eliminated, potentially lowering the threshold for using a CDR.
Taking all these trends into consideration, it is difficult to say which arguments will ultimately determine whether or not a rule like in this case the Oudega rule will continue to exist in its present form.
Generally speaking, one can postulate that the opposite is also true: changing conditions may lead to new opportunities for introducing sensible CDRs, or for making current CDRs more useful. For example, due to the SARS-CoV-2 pandemic, visits to the GP’s office have been replaced increasingly by time-consuming home visits. The time spent on these home visits may be decreased by using CDRs that can be used remotely and, if necessary, be combined with a POC test on the spot. Accordingly, the added value of such CDRs will be immediately visible, since patients could be discharged instantaneously in case of a favourable CDR score. As a result, such a practical benefit will most probably increase compliance among physicians, which is in turn a key success factor in a fruitful implementation process of a CDR. Hence, by taking advance of such new trends in the development of future decision rules, primary care CDRs could still be incentivised to maximally exploit the simple nature of CDRs.
Conclusion
In conclusion, the potential benefits of using CDRs in primary care are numerous but only come into their own if tailor-made CDRs are being developed and used that take the specific context of primary care into consideration, and if they anticipate the latest developments such as the use of POC tests. Current relevancy of the few CDRs in use should be evaluated periodically. Accordingly, new powerful well-validated primary care CDRs can make a meaningful contribution to improving the quality of primary health care and patient satisfaction while reducing unnecessary costs.
Authors’ Contribution
JH wrote the first draft of the manuscript. All authors have reviewed and edited the manuscript and approved the final version.
Funding
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Appendix

Factors that pose barriers for the use of a clinical decision rule in routine clinical practice. NB: the examples that are applicable to a specific CDR differ widely from country to country, as has been demonstrated by the application of the Ottawa Ankle Rules (Graham et al., Reference Graham, Stiell, Laupacis, McAuley, Howell, Clancy, Durieux, Simon, Emparanza, Aginaga, O’connor and Wells2001).
1) this might explain why 50% of physicians do not use CDRs at all (Le Marechal et al., Reference Le Marechal, Martinot, Duhamel, Pruvost and Dubos2013).
CDR: clinical decision rule.







