Osteoporosis is a worldwide health problem that results in fractures (most often at the hip, spine and wrist). In countries such as the USA, one of every two women over the age of 50 years will have an osteoporosis-related fracture in their lifetime. It is generally agreed that adequate Ca and vitamin D are significant nutritional concerns for bone growth and maintenance and thus for the incidence of osteoporosis. Limited evidence exists that indicates other minerals, including Cu and Zn, also may be of nutritional concern for bone health. Strause et al. (Reference Strause, Saltman and Smith1) supplemented postmenopausal women (average age 66 years) daily with 1·0 g Ca, a cocktail containing 15 mg Zn, 5 mg Mn and 2·5 mg Cu, a combination of the Ca and trace element cocktail, or a placebo for 2 years. Spinal (L2–L4 vertebrae) bone density loss was a substantial 3·53 % in the eighteen subjects given the placebo, 1·25 % in the thirteen subjects given only Ca and 1·89 % in the fourteen subjects given only the trace element cocktail; these changes were not significantly different from each other. In contrast, the fourteen subjects given the Ca plus trace elements had a bone mineral density gain of 1·48 %, which was significantly different from the placebo group. In another supplementation trial, women aged 45–56 years were given daily a placebo or 2·5 mg Cu for 2 years(Reference Eaton-Evans, McIlrath and Jackson2). The Cu supplementation essentially prevented vertebral trabecular bone mineral density loss ( − 0·46 %) while the placebo group lost a significant 2·65 %. Other studies showing that Cu is needed for bone health include those showing that preterm infants provided Cu-deficient enteral nutrition exhibited osteoporotic-like bone and increased fractures(Reference Paterson and Burns3, Reference Danks4), and that plasma Cu concentration was positively correlated with lumbar spine bone mineral density(Reference Chaudhri, Kemmler and Harsch5). In rats, Cu deprivation decreased mechanical strength and changed the organic and inorganic composition of femurs(Reference Medeiros, Ilich and Ireton6, Reference Roughead and Lukaski7). In addition to Strause et al. (Reference Strause, Saltman and Smith1) including Zn as a component in their trace element cocktail supplement study, this research group reported that Zn was decreased in bones of osteoporotic patients(Reference Reginster, Strause and Saltman8). Bone growth retardation is common in different growing animal models, and animal and cell-culture experiments show that Zn is involved in or affects several metabolic pathways of bone turnover(Reference Yamaguchi9). However, the relevance of Zn in osteoporosis was questioned when it was found that long-term (3 months) marginal Zn deprivation (maintained energetic steady state) was not detrimental to the skeleton of aged (9 months) female rats(Reference Erben, Lausmann and Roschger10). Some data in this study, however, were suggestive of decreased trabecular bone mineral density and cancellous bone area in the tibia after 5 months of marginal Zn deprivation.
In the limited studies in which human subjects were supplemented with Cu and/or Zn, there was no assurance that control and treated subjects had similar and/or adequate intakes of nutrients such as Ca, vitamin D and Mg, which influence bone loss and/or maintenance. In addition, previous studies did not rigorously assess Cu and Zn intakes. Variations in all these nutrients might have affected the response of bone status indicators to Cu and/or Zn supplementation. Moreover, the number of subjects per treatment group in previous human studies was relatively small (n 15–25). Thus, we performed a supplementation trial with a relatively large number of subjects (initially n 224) with adequate Ca and vitamin D intakes to determine whether Cu and Zn intakes are significant risk factors for bone loss leading to osteoporosis.
Experimental methods
Subjects
Healthy postmenopausal women aged 51–80 years were recruited. Women eligible for the study had to not have used hormone replacement therapy for 1 year before the study, not have used medications that interfere with Ca absorption, and have normal thyroid, liver and kidney functions. The women also had to have: a BMI ≤ 32 kg/m2; bone mineral density not more than 2·5 standard deviations below that for young adults; no collapsed/compressed vertebrae determined by using dual-energy X-ray absorptiometry (DXA); history of no menses for at least 5 years; and a circulating follicle-stimulating hormone concentration < 40 IU/l.
Eligible applicants were invited to an information meeting that explained the purpose of the study, procedures involved and expectations of the subjects. Written informed consent was obtained from the 224 women that participated in the experimental protocol. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and by the Department of Health and Human Services and all procedures involving human subjects were approved by the Institutional Review Board of the University of North Dakota.
Experimental protocol
The experiment had a double-blind, placebo-controlled design. Following a baseline 5 d food diary and DXA determination of whole-body bone mineral content, density and T score, the women were randomly assigned to one of two treatments based on similar mean femoral neck T score and BMI. The T score is defined as the number of standard deviations by which a measured value (for example, bone mineral density) departs from the mean value of a group of young individuals matched for sex and ethnicity. The treatments were a 2-year daily supplement containing 600 mg Ca (tricalcium phosphate; Inverness Medical, Inc., Waltham, MA, USA) plus a maize starch placebo or the 600 mg Ca supplement plus a 2 mg Cu and 12 mg Zn supplement. The zinc gluconate and copper gluconate supplement and maize starch placebo were obtained from Gallipot, Inc. (St Paul, MN, USA). All women were also given daily a multivitamin supplement (Bayer Consumer Care Division, Morristown, NJ, USA) that contained 10 μg vitamin D3, 15 μg vitamin A, 60 mg vitamin C, 1·05 mg thiamin, 1·2 mg riboflavin, 13·5 mg niacin, 1·05 mg vitamin B6, 300 μg folic acid and 4·5 μg vitamin B12. Whole-body DXA measurements were made at 6-month intervals and 5 d food diaries were obtained annually during the 2-year supplementation period. Participants completing the 2-year supplementation period were invited to provide another 5 d food diary at 1 year later. Physical activity of the women also was assessed at baseline and annually by using the self-administered Physical Activity Scale for the Elderly (PASE; New England Research Institutes, Inc., Watertown, MA, USA).
Supplement distribution and compliance
A 6 months' supply of supplements was provided to the women in bubble packs in the form of 1-month packages. The first twenty-seven women received the initial supply as two 3-month sets, and then were given a full 6 months' supply at the first follow-up DXA appointment. All remaining women received 6-month sets each time. The women were required to return used bubble packs at biannual visits. Supplement compliance was determined by telephone interviews between visits, a questionnaire completed during visits, and counts of supplements remaining in bubble packs returned by the women. The study started with 224 women (112 in each group), 181 women completed year 1 and 167 women completed year 2. A power analysis based on the percentage change from baseline in bone mineral density of the spine observed in the study of Strause et al. (Reference Strause, Saltman and Smith1) indicated that eighty women in each group completing 2 years of supplementation were needed to have 90 % power to detect significant (α = 0·05) changes in bone density. All women were included in the statistical analyses of the data.
Diet and dual-energy X-ray absorptiometry determinations
The 5 d food diary obtained from the women included one weekend day. Estimated average daily Ca, Cu, Fe, Mg, Zn and vitamin D intakes were calculated by using US Department of Agriculture food composition data(11).
Whole-body bone mineral content (g) and bone area (cm2) were determined by using two dual-energy X-ray absorptiometers (QDR Delphi-W, software version 11.2.1.7; Hologic, Inc., Waltham, MA, USA). Calibration measurements were performed each day with a Hologic lumbar spine phantom before testing or if either instrument was idle for more than 2 h; calibration CV was < 0·58 % for bone mineral content and < 0·46 % for area for both instruments, over the 65 months of data collection. Although a separate between-instrument comparison yielded r>0·98 for both bone mineral content and area, a given participant was assessed on one instrument throughout their participation in the study. In addition, separately and with individuals not expected to change, a comparison of whole-body estimates over 6 months showed differences less than 1 % in bone area, mineral content and density. Three different operators performed and analysed the scans but one operator verified the quality and application of standardised analytical procedure for all scans. Participants wore uniformly lightweight clothing (‘scrubs’) and no metal during assessments.
Data analysis
Statistical analysis was performed by using the SAS procedure PROC MIXED (version 9.2; SAS Institute, Cary, NC, USA). Repeated-measures ANCOVA was used to compare treatment effects over time on bone DXA measurements. For each variable, the corresponding baseline bone DXA measure was used as the covariate in the model. The effects of each treatment on the bone measurements at baseline v. year 2 were assessed by a priori contrasts. Repeated-measures ANOVA was used to assess the association of Mg intake with bone DXA measurements. P ≤ 0·05 was considered significant.
Results
Table 1 shows the Cu, Zn and Mg intakes calculated from 5 d food diaries of 112 women in each group during different periods of the study. The mean intakes of Cu and Zn of the women were above the US RDA of 8·0 mg/d for Zn and 0·9 mg/d for Cu(12). Mg intakes were less than the RDA of 320 mg/d(13). Table 1 also shows that fifty-one women had average Cu intakes less than the RDA; fifty-nine women had average Zn intakes less than the RDA and eighty-six women had Mg intakes less than 237 mg/d (95th percentile of the estimated average requirement (EAR), estimated by using a relatively large number of balance data)(Reference Hunt and Johnson14).
Table 1 Daily copper, zinc and magnesium intakes (values without the copper and zinc supplement) calculated from 5 d food diaries obtained at baseline and once per year for 3 years
(Mean values with their standard errors)
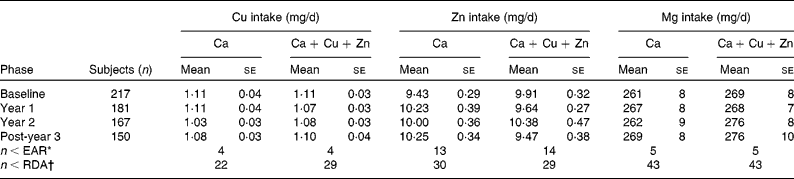
EAR, estimated average requirement.
* Number of subjects out of 112 with average intakes (mg/d) of Cu < 0·7, Zn < 6·8 or Mg (EAR based on recent balance data(Reference Hunt and Johnson14)) < 165.
† Number of subjects out of 112 with average intakes (mg/d) of Cu < 0·9, Zn < 8·0 or Mg (95th percentile of EAR based on recent balance data(Reference Hunt and Johnson14)) < 237.
Table 2 shows the Ca, Fe and vitamin D intakes calculated from 5 d food diaries of 112 women in each group during different periods of the study. The mean intakes of Fe were above the US RDA (8·0 mg/d). The mean intakes were less than both the US EAR and RDA for Ca (1000 and 1200 mg/d, respectively) and vitamin D (10 and 15 μg/d, respectively)(15). Table 2 also shows that only eleven women consumed less than the RDA for Fe, only twenty-one women consumed the RDA for Ca, and no woman consumed the RDA for vitamin D.
Table 2 Daily calcium, iron and vitamin D intakes (values without the calcium and vitamin D supplements) calculated from 5 d food diaries obtained at baseline and once per year for 3 years
(Mean values with their standard errors)
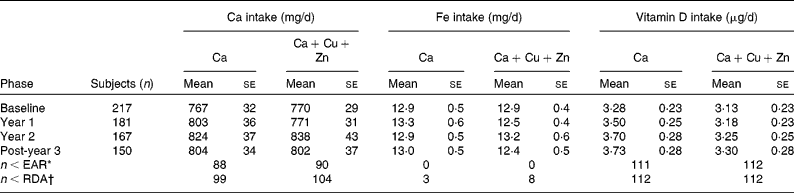
EAR, estimated average requirement.
* Number of subjects out of 112 with average intakes of Ca < 1000 mg/d, Fe < 5·0 mg/d or vitamin D < 10 μg/d.
† Number of subjects out of 112 average intakes of Ca < 1200 mg/d, Fe < 8·0 mg/d or vitamin D < 15 μg/d.
Fig. 1 shows the whole-body bone mineral density changes during the study; T score and bone mineral determinations demonstrated the same type of changes. Repeated-measures ANCOVA found that neither Ca plus placebo nor Ca plus Cu and Zn supplementation prevented whole-body bone mineral content, density or T score from decreasing from baseline during the supplementation period. However, when each treatment was examined individually by using a priori contrasts between baseline and year 2, the decreases in bone mineral density and T score were not significant in the subjects supplemented with Ca plus placebo, whereas they were highly significant (P < 0·001) in women supplemented with Ca plus Cu and Zn. Change in physical activity assessed by the Physical Activity Scale for the Elderly (PASE) and ANCOVA analyses using both baseline values and weight as covariates indicated that the observed changes were not affected by changes in physical activity or weight.

Fig. 1 Effect of a daily supplement of 600 mg Ca or 600 mg Ca plus 2·0 mg Cu and 12 mg Zn for 2 years on whole-body bone mineral density in intent-to-treat postmenopausal women aged 51–80 years. (![]() ), Baseline; (
), Baseline; (![]() ), year 1; (
), year 1; (![]() ), year 2. Values are means, with standard errors represented by vertical bars. The density significantly decreased (P = 0·0001; repeated-measures ANCOVA) from baseline during the supplementation period. Significant changes determined by a priori contrasts between year 2 and baseline for Ca and Ca + Zn + Cu supplementation at different calculated dietary intakes of Cu and Zn are indicated: * P < 0·05, *** P < 0·0001.
), year 2. Values are means, with standard errors represented by vertical bars. The density significantly decreased (P = 0·0001; repeated-measures ANCOVA) from baseline during the supplementation period. Significant changes determined by a priori contrasts between year 2 and baseline for Ca and Ca + Zn + Cu supplementation at different calculated dietary intakes of Cu and Zn are indicated: * P < 0·05, *** P < 0·0001.
About 26 % of the women completed food diaries indicating Zn intakes less than the RDA of 8·0 mg/d (Table 1). These women and those with food diaries indicating Zn intakes ≥ 8·0 mg/d were analysed separately by repeated-measures ANCOVA to determine whether the significance of the decrease in bone status indicators differed according to Zn intake between the two treatments groups. A priori contrasts found that the decrease between baseline and year 2 in whole-body bone mineral density was significant in the women supplemented with Ca only, but not in the women supplemented with Ca plus Cu and Zn (Fig. 1); T scores showed the same difference. The decreases in bone mineral density and T score essentially occurred between years 1 and 2 in the women supplemented with Ca only. Based on the findings with all subjects and those with intakes less than the RDA, the analysis of women with Zn intakes ≥ RDA gave expected results (Fig. 1). A priori contrasts found that whole-body bone mineral densities and T scores were not significantly decreased between baseline and year 2 in Ca plus placebo-supplemented women with calculated Zn intakes ≥ 8·0 mg/d. Women supplemented with Ca plus Cu and Zn exhibited highly significant decreases in bone mineral densities and T scores between baseline and year 2 that appeared to occur throughout supplementation.
Fig. 1 also shows that whole-body bone mineral density was significantly inferior in women with food diaries indicating Cu intakes < 0·9 mg/d than in women with food diaries indicating Cu intakes ≥ 0·9 mg/d. Whole-body mineral contents and T scores also were inferior. Bone mineral contents, densities and T scores were neither improved in women consuming < 0·9 mg/d Cu, nor exacerbated in women consuming ≥ 0·9 mg/d Cu, by the Zn plus Cu supplement. However, Cu intake might have had some effect on the response of the bone status indicators to the Ca and vitamin D supplementation. A priori analysis suggested that the indicators were significantly decreased in the Ca plus placebo group when Cu intakes were ≥ 0·9 mg/d but not with intakes < 0·9 mg/d.
A high (53 mg/d) v. deficient (3 mg/d) Zn intake has been reported to decrease Mg balance in postmenopausal women(Reference Nielsen and Milne16), and Mg deficiency may be a risk factor for bone loss in postmenopausal women(Reference Rude, Singer and Gruber17). Thus, an analysis was made of whether the negative response to Zn supplementation was greater with Mg intakes < 237 mg/d through exacerbating the negative effect of Mg deprivation on bone health. The results of this analysis (Table 3) indicated that Mg intake did not significantly influence the responses to the treatments. Regardless of Mg intake, women in each treatment group exhibited significantly decreased whole-body bone mineral contents, densities and T scores. Of interest, however, was the finding that bone mineral contents, densities and T scores were significantly better in women with calculated Mg intakes ≥ 237 mg/d than in women with intakes < 237 mg/d.
Table 3 Whole-body bone mineral content, density and T scores of subjects consuming less than or at least 237 mg magnesium per d through the three periods of the study
(Mean values with their standard errors)

* Significant effects: Mg intake, P = 0·007; year, P = 0·0001; Mg × supplement × year, P = 0·05.
† Significant effects: Mg intake, P = 0·006; year, P = 0·0003.
‡ Significant effects: Mg intake, P = 0·005; year, P = 0·0007.
Discussion
The findings that bone mineral density and T score did not decrease between years 1 and 2 of supplementation and that a priori contrasts indicated no significant loss in these bone status indicators between baseline and 2 years in women supplemented with Ca plus placebo suggest that long-term Ca plus multivitamin supplementation containing vitamin D may slow bone loss in ageing postmenopausal women. Cu and Zn supplementation did not provide further benefit to bone compositional characteristics, and a priori contrasts indicate that the Cu and Zn supplement was detrimental by year 2 to any beneficial effect of the Ca and vitamin D supplementation. This finding was just the opposite of that of Strause et al. (Reference Strause, Saltman and Smith1) who found that a cocktail of Cu, Zn and Mn enhanced the effect of Ca supplementation on bone mineral density, and was not consistent with the finding that Cu supplementation prevented vertebral trabecular bone density loss(Reference Eaton-Evans, McIlrath and Jackson2). Thus, a possible explanation for the discordant findings was sought.
In the present study, a 2 mg/d Cu supplement over a 2-year period did not significantly improve bone mineral densities, contents and T scores in women with calculated intakes less than the RDA for Cu, although these bone status indicators were inferior to those in women with calculated intakes ≥ the RDA. The inferior bone status is consistent with animal studies and premature infant supplementations showing that bone formation is impaired by a Cu-deficient state(Reference Paterson and Burns3, Reference Danks4, Reference Medeiros, Ilich and Ireton6, Reference Roughead and Lukaski7). It is unclear why a positive response to the 2 mg/d Cu supplement was not obtained in women with Cu intakes < the RDA, which would be inconsistent with the findings of Strause et al. (Reference Strause, Saltman and Smith1) and Eaton-Evans et al. (Reference Eaton-Evans, McIlrath and Jackson2) that Cu supplementation at about three times the RDA was beneficial to bone mineral density. Perhaps another environmental or nutritional factor promoting bone loss or poor Ca utilisation inhibited the response to Cu supplementation. We found no report indicating that Cu supplementation to about three times the requirement is beneficial or detrimental to bone health in Cu-adequate animals. In the present study, we did not find that Cu supplementation was detrimental to bone maintenance in postmenopausal women. Thus, Cu apparently was not the cause of the negative effect of the Cu plus Zn supplement on bone mineral density and T score in women supplemented with Ca and vitamin D.
Because Cu apparently was not responsible, Zn was closely examined as the factor responsible for the negative effects of the Cu and Zn supplement on bone mineral density and T score in the Ca and vitamin D-supplemented women. A plot of bone mineral density or T score v. calculated Zn intake did not reveal a threshold value at which differences in Zn intakes might affect the response to the Zn plus Cu supplement. Thus, the RDA of 8·0 mg/d was chosen as the criterion for dividing the Zn intakes (excluding the supplement) into low and high groups. As a result, intakes of Zn ≥ 20 mg/d (including the supplement) were associated with the negative effects of the Cu and Zn supplement. Thus, a Zn intake more than two times the RDA may impair the beneficial effects of Ca and vitamin D on bone maintenance in postmenopausal women. However, the findings with women with food diaries indicating Zn intakes less than the RDA suggest that the Zn supplementation may have been beneficial for bone mineral maintenance when Zn intakes are deficient. This suggestion is consistent with the demonstration that Zn has a stimulatory effect on osteoblastic bone formation and mineralisation(Reference Yamaguchi9). The present findings also suggest that a low Zn intake may limit or prevent the bone health benefits of Ca plus vitamin D or that Ca supplementation exacerbates the detrimental effects of Zn deficiency on bone maintenance. Consistent with this latter suggestion is the report that high Ca intakes reduce Zn absorption and balance in humans(Reference Wood and Zheng18). Thus, long-term inadequate Zn intake may be detrimental for bone health in postmenopausal women; a suggestion supported by the finding of decreased bone status indicators with Zn intakes less than the RDA in the present study. The suggestion that Zn supplementation can have both positive (in Zn deficiency) and negative (in Zn excess) effect is not unprecedented. There are reports indicating that feeding excessive Zn is similar to Zn deficiency in affecting immune function(Reference Ibs and Rink19, Reference Raqib, Hossain and Kelleher20) and mineralisation of bone nodule osteoblast-like cells in culture(Reference Cerovic, Miletic and Sobajic21). The excessive Zn in these reports generally was much higher than the Zn supplementation in the present study (for example, 100 mg/d for humans)(Reference Ibs and Rink19). However, Zn supplementation at only 22 mg/d was found to lower measures of Fe status in young women with low Fe reserves(Reference Conangelo, Woodhouse and King22) and in physically active adolescents(Reference Oliveira, Donangelo and Oliveira23).
Possible causes for the negative effect of Zn supplementation in women consuming ≥ the RDA for Zn was focused on a possible change in the status of a mineral involved in bone maintenance. In a controlled feeding study with postmenopausal women, high Zn intake (about 53 mg/d) v. a low Zn intake (about 3 mg/d) increased urinary N-telopeptide and decreased serum calcitonin concentrations(Reference Nielsen and Milne16), which suggests that a high Zn intake may affect bone breakdown or Ca metabolism. Thus, Zn supplementation in excess of requirement may have prevented a beneficial action of Ca through interfering with Ca metabolism. This is speculation only because the negative effect of Zn was unexpected, and, thus, evaluation of indicators that could suggest a possible effect of high Zn on Ca metabolism was not made in the present study.
Mg is another mineral that may be affected by low or high Zn intakes. In an experiment performed on postmenopausal women, the high Zn intake (about 53 mg/d) compared with low Zn intake (about 3 mg/d) significantly increased the percentage of Mg intake found in both faeces and urine, which resulted in significantly decreased Mg balance or retention(Reference Nielsen and Milne16). In rats, marginal Zn deficiency was found to increase Mg retention and impair Ca utilisation(Reference Nielsen24). In a prospective study of 4035 men, a combined low Zn and high Mg intake was associated with decreased all-cause mortality risk(Reference Leone, Courbon and Ducimetiere25). In a supplementation trial, a relatively high Zn intake (142 mg/d) was found to inhibit Mg balance and absorption in men(Reference Spencer, Norris and Williams26). Thus, it is unclear whether the change in Mg metabolism in the postmenopausal women in the metabolic unit study(Reference Nielsen and Milne16) was the result of the low Zn increasing Mg retention, the high Zn intake decreasing Mg balance, or both Zn deficiency and excess playing a role in changing the Mg balance. Nonetheless, because Zn intake apparently affects Mg metabolism, and Mg deficiency is associated with osteoporotic-type bone changes in humans and induces such changes in rats(Reference Rude and Gruber27), exacerbating a low Mg status was explored as a possible basis for the negative effect of Zn supplementation in the present study. A recent report indicated that the US EAR of 265 mg/d may be too high(Reference Hunt and Johnson14). This report indicated that neutral Mg balance in healthy individuals occurred at about 165 mg/d and that 265 mg/d would meet the requirements of most individuals. Thus, an Mg intake of 237 mg/d, an intake that was the 95th percentile of the EAR estimated in the report(Reference Hunt and Johnson14), was used as the breakpoint between a deficient and adequate intake of Mg. The results in Table 3 show that the changes induced in whole-body bone mineral content, density and T score by the Zn and Cu supplementation were not markedly different between women with Mg intakes less or more than 237 mg/d. However, the analysis did reveal, regardless of Ca, Cu and Zn supplementation, that whole-body bone mineral content, density and T score was markedly lower in the 38 % of the women with Mg intakes < 237 mg/d than in women with intakes ≥ 237 mg/d. This finding suggests that a low Mg intake is associated with decreased bone health that could eventually result in osteoporosis. Confirmation of this suggestion would be significant because National Health and Nutrition Examination Survey (NHANES) data indicate that over 25 % of US women in the age group in the present study have Mg intakes < 237 mg/d(Reference Moshfegh, Goldman and Ahuja28).
Because supplemental Zn was found to lower the measure of Fe status in young women(Reference Conangelo, Woodhouse and King22) and adolescents(Reference Oliveira, Donangelo and Oliveira23), and dietary Fe has been positively associated with bone mineral density in healthy postmenopausal women(Reference Harris, Houtkooper and Stanford29), Fe intakes were determined. The data in Table 2 indicated that only eleven women had usual intakes less than the RDA for Fe. Thus, exacerbation of an Fe deficiency was not considered as a possible reason for the negative effect of Zn supplementation on bone maintenance in the present study.
In summary, Zn and Cu supplementation did not enhance any beneficial effect of a daily Ca supplement of 600 mg/d on DXA determinations of whole-body bone status indicators in vitamin D-adequate postmenopausal women. Instead, Ca-supplemented women given a daily supplement of 2 mg Cu and 12 mg Zn had significant decreases in whole-body bone mineral densities and T scores between the start and end of 2 years of supplementation while those supplemented with Ca only did not exhibit a significant decrease, especially between years 1 and 2. Based on Cu intakes indicated by 5 d food diaries, the Cu supplementation apparently did not have an impact on whole-body bone contents, densities or T scores. Findings were obtained indicating that Zn and Cu supplementation may be helpful and Ca supplementation alone may not be beneficial for bone maintenance in vitamin D-adequate postmenopausal women with usual Zn intakes less than the RDA, but just the opposite occurs when Zn intakes are ≥ the RDA. The reason for the negative effect of Zn intakes in excess of requirements remains to be determined, but it apparently was not caused by exacerbating a low Mg (indicated by intakes >237 mg/d) or low Fe (indicated by intakes < 8 mg/d) status. However, Mg intakes < 237 mg/d (determined by 5 d food diaries), as well as Cu intakes < 0·9 mg/d and Zn intakes < 8·0 mg/d, were associated with decreased DXA bone status measurements, which suggests that long-term low intakes of these elements may increase the risk of osteoporosis.
Acknowledgements
The research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The study was funded by Congressional appropriations allocated to the US Department of Agriculture, Agricultural Research Service, Grand Forks Human Nutrition Research Center, which was the research location.
Mention of a trademark or proprietary product does not constitute a guarantee or warranty by the US Department of Agriculture and does not imply its approval to the exclusion of other products that also might be suitable. The US Department of Agriculture, Agricultural Research Service, Northern Plains Area, is an equal opportunity/affirmative action employer, and all agency services are available without discrimination.
The authors thank the members of the Grand Forks Human Nutrition Research Center human studies staff that made the present study possible: Wesley Canfield (medical affairs), Sandra Gallagher (clinical chemistry), Bonnie Hoverson (supplements), Brenda Ling (recruiting), Emily Nielsen (study coordination), Angela Scheett (food diaries), and William Siders and Clint Hall (DXA measurements). The authors also thank Inverness Medical (Waltham, MA, USA) for donating the Ca supplements and Bayer Consumer Care Division (Morristown, NJ, USA) for donating the multivitamins.
F. H. N. participated in the planning of the experiment and analysis of the data and wrote the manuscript. H. C. L. was a co-principal investigator in the design and conduct of the experiment and participated in the analysis of the data. Z. K. R. was a co-principal investigator in the design and conduct of the experiment. L. K. J. helped in designing the experiment, performed the statistical analyses and helped with the interpretation of the data.
The authors report no conflict of interests.






