Introduction
The UK Overseas Territories (hereafter UKOTs) are home to more threatened bird species than the whole European continent, but this bird life, including the UKOTs’ globally important seabird populations are relatively understudied (Sanders Reference Sanders2006, Hilton and Cuthbert Reference Hilton and Cuthbert2010), and in addition environmental legislation is often weak, absent or unenforced (RSPB/FIELD 2013). This is despite the many threats facing seabirds breeding in the UKOTs such as introduced species (Hilton and Cuthbert Reference Hilton and Cuthbert2010), habitat degradation (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012), changes in fishery practice (Bertrand et al. Reference Bertrand, Rocıo, Arbulu Smet, Tremblay, Barbraud and Weimerskirch2012) and climate change (Gremillet and Boulinier Reference Gremillet and Boulinier2009), which are as great or greater than those facing their UK counterparts. Designation of terrestrial and marine Important Bird Areas (IBAs) are used to highlight priority areas for seabird conservation, and can be used to inform marine spatial planning and design of Marine Protected Areas (Arcos et al. Reference Arcos, Becares, Villero, Brotons, Rodriguez and Ruiz2012, Le Corre et al. Reference Le Corre, Jaeger, Pinet, Kappes, Weimerskirch, Catry, Ramos, Russell, Shah and Jaquemet2012).
Marine IBAs aim to capture the key at-sea areas used by seabirds when undertaking activities such as foraging, resting and rafting. Using tracking technology to record the at-sea distribution of seabirds breeding on terrestrial IBAs is one approach to collecting the data to help identify marine IBAs (Burger and Shaffer Reference Burger and Shaffer2008, Lewison et al. Reference Lewison, Oro, Godley, Underhill, Bearhop, Wilson, Ainley, Arcos, Boersma, Borboroglu, Boulinier, Frederiksen, Genovart, Gonzalez-Solis, Green, Gremillet, Hamer, Hilton, Hyrenbach, Martinez-Abrain, Montevecchi, Phillips, Ryan, Sagar, Sydeman, Wanless, Watanuki, Weimerskirch and Yorio2012). However, logistical and financial constraints preclude tracking birds from every major seabird colony. So, in the absence of site-specific data, BirdLife International (2010) advocates the use of a simple seaward extension around terrestrial IBAs with seabird colonies. The distance of the extension is based on literature reviews of the known foraging ranges of the key species present. Where spatial data do exist for a given colony, a range of more refined analytical methods can be used to identify key areas for the colony (BirdLife International 2010, O’Brien et al. Reference O’Brien, Webb, Brewer and Reid2012, Oppel et al. Reference Oppel, Meirinho, Ramirez, Gardner, O’Connell, Miller and Louzao2012, Tancell et al. Reference Tancell, Phillips, Xavier, Tarling and Sutherland2013). For example, ‘first passage time analysis’ and ‘area restricted search patterns’ identify foraging sites by using a high turning rate or the speed of travel as an indicator of foraging behaviour (Pinaud Reference Pinaud2008, Suryan et al. Reference Suryan, Sato, Balogh, Hyrenbach, Sievert and Ozaki2004) whereas the kernel density estimation approach uses the density of locations recorded to calculate probability density estimates (Worton Reference Worton1989, Calenge Reference Calenge2007). Whilst commonly used, these approaches have associated weaknesses, for example first passage time analysis is time-consuming, highly technical, and relies on the subjective assessment of plotted data rather than objective statistical inference (Barraquand and Benhamou Reference Barraquand and Benhamou2008); it also makes untested assumptions about the link between animal movement and foraging behaviour (Fauchald and Tveraa Reference Fauchald and Tveraa2003). In contrast, kernel density estimation relies on a user-defined smoothing parameter which can lead to over- or under- estimation of the extent of at-sea distributions (Row et al. 2006, Blundell et al. Reference Blundell, Maier and Debevec2001). An alternative to these is the ‘time-in-area’ approach, described by Tancell et al. (Reference Tancell, Phillips, Xavier, Tarling and Sutherland2013) as “a convenient and pragmatic approach which could be applied to large data sets and across species for the identification of a network of candidate marine protected areas in coastal and pelagic waters”. This involves drawing a grid of pre-defined sized cells around the colony and determining the proportion of the total time that tracked individuals spent in each pre-defined cell, which allows important at-sea distributions of the sample of birds to be identified (Le Corre et al. Reference Le Corre, Jaeger, Pinet, Kappes, Weimerskirch, Catry, Ramos, Russell, Shah and Jaquemet2012, Page et al. Reference Page, McKenzie, Sumner, Coyne and Goldsworthy2006, Soanes et al. Reference Soanes, Arnould, Dodd, Sumner and Green2013).
This time-in area approach provides an objective representation of actual areas of use. Grid cell size can also be set to reflect the aim of the study. For example, small grid cells (e.g. 1 x 1 km) may be most appropriate for relating bird movements to environmental characteristics, to match the scale of most bathymetric and satellite-derived environmental data (e.g. SST and chlorophyll a abundance), whilst a larger scale may be preferable for marine spatial planning relating to activities such as fisheries, renewable energy developments and mineral extraction (Kidd et al. Reference Kidd, Plater and Frid2011).
We have previously used the time-in-area approach to assess if the at-sea distribution identified from given samples of tracked birds was representative of the area used by the whole colony (Soanes et al. Reference Soanes, Arnould, Dodd, Sumner and Green2013). However, we did not consider the effect of grid size on our predictions. Here, we develop this approach using data from one of the first tracking studies conducted on breeding seabirds in the Caribbean, specifically the UKOT of Anguilla, where we used GPS data loggers to track individuals from the globally important Brown Booby Sula leucogaster colony breeding on the Dog Island terrestrial IBA (Birdlife International 2012; Figure 1).
Figure 1. Location of Anguilla, Lesser Antilles in relation to other Caribbean islands (Source: ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute).
The data collected provide important preliminary seabird spatial distribution information for the Dog Island IBA which will be used to inform future tracking studies in the region. As such, we assess how representative the results from our sample are likely to be of the at-sea distribution of the whole colony and compare these with at-sea distributions predicted from the BirdLife International seaward extensions approach, using data from previous tracking studies. We also highlight the need to consider how increasing grid cell size can decrease the number of birds that need to be tracked from the colony in order to identify important at-sea distributions of the colony as a whole.
Methods
Fieldwork was conducted on Dog Island, Anguilla (18o16’N, 63o15’W) between 27 March and 4 April 2012. Twenty Brown Boobies with chicks aged 4–5 weeks were captured at their nests using a crooked pole. Females could be easily distinguished from males by the pink rather than blue tinge of their bills (Weimerskirch et al. Reference Weimerskirch, Shaffer, Tremblay, Costa, Gadenne, Kato, Ropert-Coudert, Sato and Aurioles2009). GPS data loggers (IgotU G120, Mobile Action, Taiwan) were waterproofed with heat-shrink PVC tubing and attached to the bird’s central 2–3 tail feathers using Tesa ® Extra Power tape (Wilson et al. Reference Wilson, Pütz, Peters, Culik, Scolaro, Charrassin and Ropert-Coudert1997). Data loggers weighed c.17g which on average constituted 1.2% of the mean body mass of female birds and 1.6% of males. Loggers were scheduled to record GPS locations every two minutes, and were retrieved 5–7 days after deployment.
Data were interpolated to 10 second intervals using the R package “trip” (Sumner, Reference Sumner2011) and plotted in Arcmap (ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute). Foraging trips were identified and the total trip duration (hrs), distance (km), and maximum distance travelled from the breeding colony (km) calculated for each one.
The trip package was used to create a grid of time spent in pre-defined cells from the interpolated data. This package allows the user to set the size of grid cells before calculating the time birds spend in each cell. At-sea distributions are commonly described in terms of “utilisation distribution” (UD) (Ford Reference Ford1979, Kappes et al. Reference Kappes, Weimerskirch, Pinaud and Le Corre2011, Copello et al. Reference Copello, Pon and Favero2013). We define the 95% UD as the grid cells where 95% of all time was spent, when cells were ranked in order by seconds spent in them, and the 50% UD as the grid cells where 50% of all time was spent (Worton Reference Worton1989, Soanes et al. Reference Soanes, Arnould, Dodd, Sumner and Green2013). A further intermediate 75% UD was also defined. We simulated BirdLife International’s seaward extension approach based on maximum foraging distances using the maximum foraging range recorded from the present study and a previous study of Brown Boobies (Weimerksirch et al. Reference Weimerskirch, Shaffer, Tremblay, Costa, Gadenne, Kato, Ropert-Coudert, Sato and Aurioles2009).
We estimated the areas of the 95%, 75% and 50% UDs following the procedure outlined by Soanes et al. (Reference Soanes, Arnould, Dodd, Sumner and Green2013), by assuming an asymptotic relationship between the number of birds tracked and the percentage of the area of the whole colony UD. We used a bootstrapping procedure (BirdLife International 2010), implemented in the R package Boot (Canty and Ripley 2007), to randomly resample the UD of 1–16 individuals and then plotted the number of individuals included in the sample against the size of the predicted sample UD. The most appropriate asymptotic model to fit our data was identified as the Michaelis-Menten model, based on AIC values and ability of the model to extrapolate the data (Equation 1). Multiple trips were included from each individual as this gives more information on the size of the whole colony’s UD (Soanes et al. Reference Soanes, Arnould, Dodd, Sumner and Green2013). However as not all tracked individuals made the same number of foraging trips, we restricted analysis to the first three trips per individual, and to the 16 individuals that made at least three trips, as a compromise between including the most birds and the most individual foraging trips in the sample to avoid pseudo-replication. Data were re-sampled 10,000 times and 95% confidence limits estimated as the 2.5th and 97.5th percentiles. We assessed the effect of using four different grid cell sizes (2 x 2 km, 5 x 5 km, 10 x 10 km and 20 x 20 km) on predictions of the UDs of our sample of birds by repeating this procedure for each grid cell size.
Equation 1: Michaelis-Menten model:
Where a = the asymptotic value of the y axis, and b = the value of x at which half of the maximum response is attained
We then extrapolated each of the nonlinear model functions to estimate the area of the Dog Island colony’s 50%, 75% and 95% UDs, based on colony size. We used these nonlinear functions to calculate UD sizes, using varying numbers of birds and sizes of grid cell, and expressed this as a percentage of the predicted UDs for our sample of birds. Plotting these percentages as a three dimensional surface allowed rapid visual evaluation of the size of the at-sea areas that would be estimated using different sampling and grid cell size protocols. Finally, we used our models to calculate how many birds would need to be tracked to estimate 95% of the colony’s 50%, 75% and 95% utilisation distributions. We selected 95% here due to the asymptotic nature of the relationships. See Soanes et al. (Reference Soanes, Arnould, Dodd, Sumner and Green2013) for a more detailed description of this methodology.
Results
Data loggers were retrieved from 19 of the 20 Brown Boobies, with the remaining bird evading recapture. Individuals made between two and seven foraging trips during the 5–7 day tracking period (individual tracks are shown in Figure 2a). Table 1 shows the mean trip length, duration and maximum distance from the colony travelled by Brown Boobies breeding on Dog Island, compared to those breeding at colonies in Mexico and the Johnston Atoll, Pacific Ocean (Lewis et al. Reference Lewis, Schreiber, Daunt, Schenk, Orr, Adams, Wanless and Hamer2005, Weimerskirch et al. Reference Weimerskirch, Shaffer, Tremblay, Costa, Gadenne, Kato, Ropert-Coudert, Sato and Aurioles2009). However, it should be noted that these differences could also be due to the three studies being undertaken at different stages of the breeding cycle. Figure 2 shows the foraging trips made by Brown Boobies tracked on Dog Island along with two seaward extensions to the breeding colony (1) the maximum distance from the colony recorded during this study and (2) the maximum distance from the colony recorded at a Mexican breeding colony (Weimerskirch et al. Reference Weimerskirch, Shaffer, Tremblay, Costa, Gadenne, Kato, Ropert-Coudert, Sato and Aurioles2009). Based on this foraging radii approach (BirdLife International 2010), the maximum distance travelled from the Mexican colony predicts an estimated total at-sea area of 11,198 km2 whereas the maximum distance travelled in this study of 100 km, predicts a foraging area of 34,247 km2. Despite the large differences in predicted foraging areas the seaward extension using the maximum distance recorded at the Mexican colony actually encompasses at least 99–100% of the 50% UD, 98–100% of the 75% UD and 86–94% of the 95% UD of the individual foraging trips made by birds tracked on Dog Island in 2013 depending on the grid cell size.
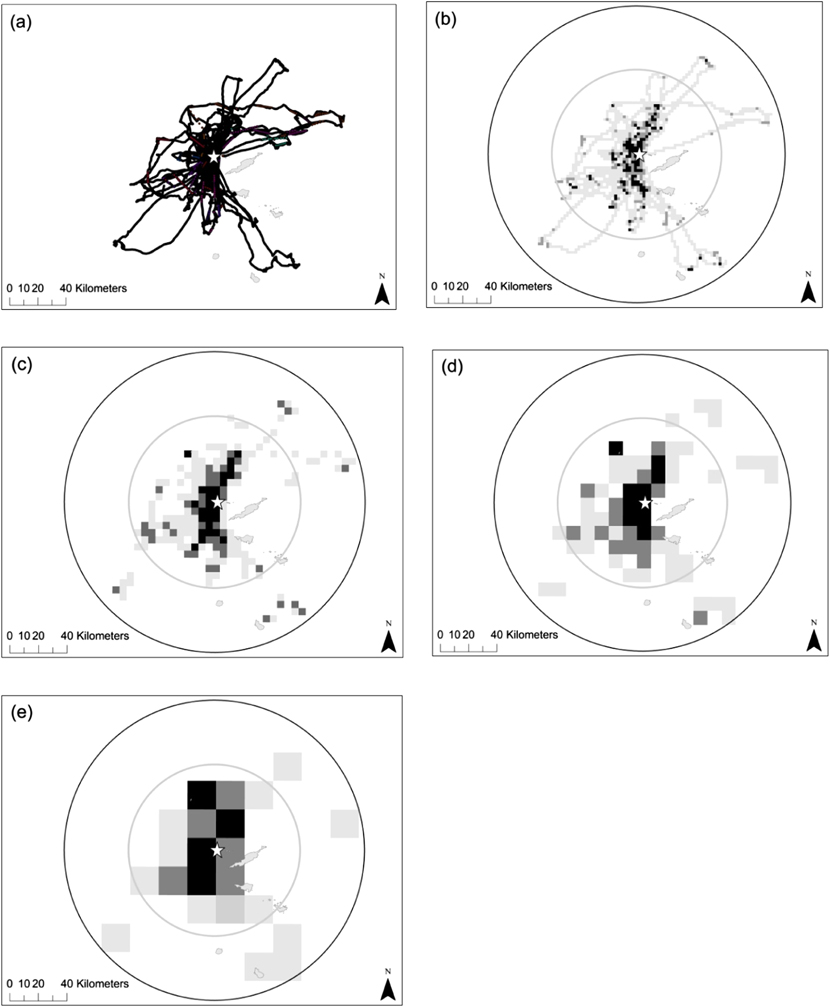
Figure 2. (a) First three foraging tracks of 16 Brown Boobies, and areas of foraging activity identified for each trip when using time spent in cells of a grid with resolution of (b) 2 x 2 km (c) 5 x 5 km (d) 10 x 10 km and (e) 20 x 20 km. Light grey cells = where the sample of birds spent 95% of time, dark grey = 75% of time and black = 50% of time. All panels also show the predicted foraging radii of the colony based on the furthest point from the colony recorded from this study (black circle), and a previous tracking study of Brown Boobies (inner grey circle) (Weimerskirch et al., Reference Weimerskirch, Shaffer, Tremblay, Costa, Gadenne, Kato, Ropert-Coudert, Sato and Aurioles2009).
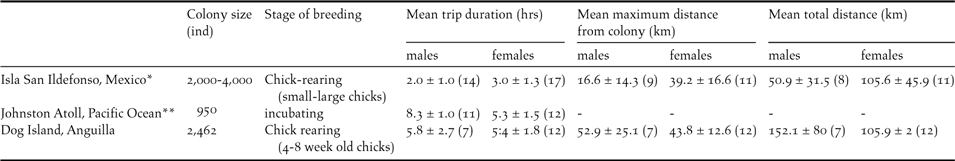
Table 1. Mean foraging trip duration, maximum distance from colony and total distance travelled by Brown Boobies on Dog Island, Anguilla compared to two other colonies (± SD, sample sizes in brackets). *Weimerskirch et al. (Reference Weimerskirch, Shaffer, Tremblay, Costa, Gadenne, Kato, Ropert-Coudert, Sato and Aurioles2009), ** Lewis et al. (Reference Lewis, Schreiber, Daunt, Schenk, Orr, Adams, Wanless and Hamer2005).
As might be expected, the extent of each of the whole colony’s UD predicted by our sample increased with increasing grid cell size, but the number of birds required to predict 95% of the colony’s UDs decreased (Table 2). Thus, whilst increasing the grid cell size decreased the precision of the boundary of the at-sea area used, it increased the coverage of total area used by the Dog Island colony (Figure 2). Figure 3 shows how the percentage of the area of the sample UDs predicted increases when more individuals are included in a sample, or when a larger grid cell is defined. Our sample of 16 birds would have predicted between 48 and 83% (depending on grid cell defined) of the whole colony’s 50% UD, 62 and 78% of the 75% UD and between 43 and 55% of the 95% UD. The 50% UD is clearly of great importance to the birds since, irrespective of grid cell size chosen, the birds spent 50% of their time in an area less than 10% of the size of the 95% UD (Table 2) and even our limited sample was accurate in identifying the majority of the colony’s predicted at-sea distribution at the coarsest scale used.
Table 2. Number of individuals required to predict 95% of the 50%, 75% and 95% whole colony utilisation distributions of Brown Boobies breeding on Dog Island, Anguilla, based on a colony size of 1,231 pairs (Bright et al. 2013) and using the model parameters derived from equation 1 (confidence intervals are shown in brackets). Predicted area (km2) of each UD are shown for different grid cell sizes.
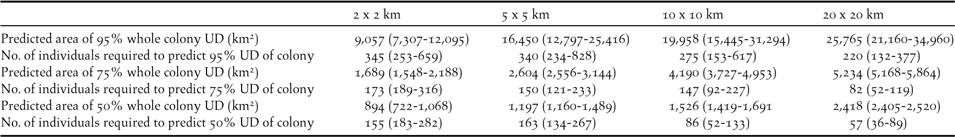
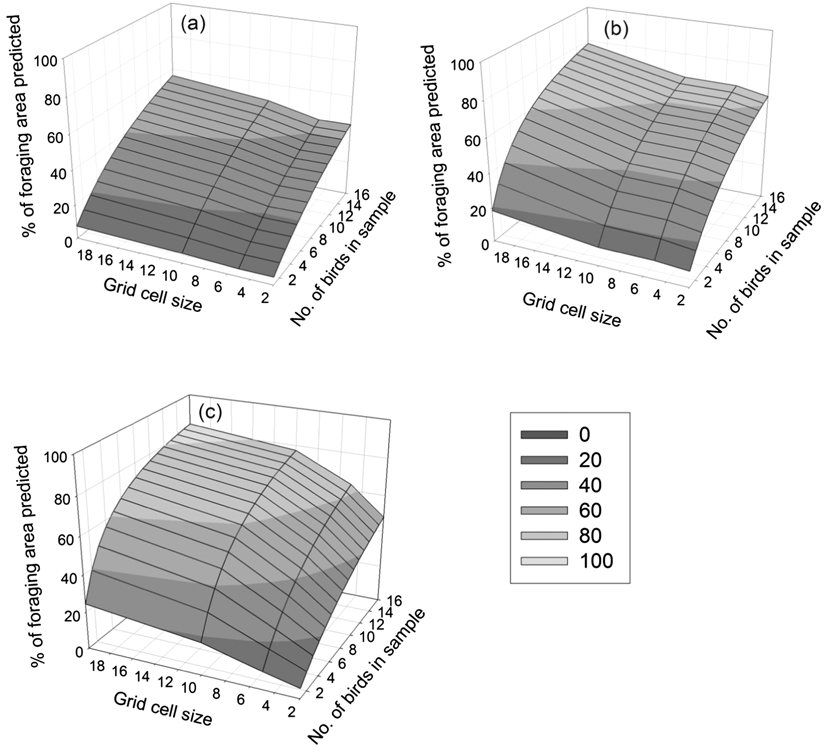
Figure 3. Percentage of the predicted utilisation distribution of the whole colony as a function of number of individuals tracked and size of grid cells used to make predictions (2 x 2 km, 5 x 5 km, 10 x 10 km and 20 x 20 km) for (a) 95% utilisation distribution (b) 75% utilisation distribution and (c) 50% utilisation distribution.
Discussion
We present results from one of the first seabird tracking studies undertaken in the Caribbean, for the globally important colony of Brown Booby breeding on Dog Island, Anguilla, one of the most important sites for seabirds in the region (Lowrie et al. Reference Lowrie, Lowrie and Collier2012). The mean foraging trip length, and maximum distance travelled of the tracked birds were longer than those of Brown Boobies in Mexico (Weimerskirch et al. Reference Weimerskirch, Shaffer, Tremblay, Costa, Gadenne, Kato, Ropert-Coudert, Sato and Aurioles2009), but mean trip duration was comparable to that of the Johnston Atoll colony in the Pacific Ocean (Lewis et al. Reference Lewis, Schreiber, Daunt, Schenk, Orr, Adams, Wanless and Hamer2005) (Table 1). Foraging trips made by Brown Boobies on Dog Island extended beyond Anguilla’s Exclusive Economic Zone and into those of neighbouring Sint Eustatius and Saba (territories of the Netherlands), Saint Barthelemy (territory of France) and Saint Maarten/Sint Martin (territory of France and the Netherlands). Ensuring the multinational protection of important habitats for such wide ranging species presents a challenge to governments and environmental organisations, but is essential to achieving successful conservation. The results from this and future studies will be relevant to multinational initiatives such as the Caribbean Challenge (Nature Conservancy 2013) and in informing how seabird tracking data can be used to define Marine Protected Areas.
Identification of important foraging areas is essential in order to show the areas where protection of breeding seabird populations is most needed and can help inform the design, designation and management of protected areas. In the absence of tracking data, BirdLife International (2010) suggests using a seaward extension approach based on known foraging radii. Our study shows that this approach has potential in the identification of areas that are likely to encompass the most heavily used areas (50 and 75% UDs) but still risks under-representing all key at-sea areas for the Dog Island Brown Booby colony. Ideally, several datasets from different colonies over different years should be used to inform the seaward extension approach. The size of the colony is also a key factor in the foraging behaviour of seabirds (Wakefield et al. Reference Wakefield, Bodey, Bearhop, Blackburn, Colhoun, Davies, Dwyer, Green, Gremillet, Jackson, Jessopp, Kane, Langston, Lescroel, Murray, Le Nuz, Patrick, Peron, Soanes, Wanless, Votier and Hamer2013) and would be a useful refinement that should be incorporated into future predictive models in the absence of colony-specific tracking data.
The logistics of seabird tracking studies, including time and budgetary constraints, mean that sample sizes are often low. To begin to make population-level conservation decisions using tracking data it is vital to assess the representativeness of such small samples in predicting important at-sea areas for the wider population. Our analysis indicates that our sample of 16 birds only identified 43–55% of the Dog Island colony’s 95% UD and 48–83% of the colony’s 50% UD. Thus, many more individuals from this colony would need to be tracked to fully identify the 95%, 75% and 50% UDs for this colony (Table 2, Figure 2), though this number decreases with increasing grid cell size. For example, if using a 2 x 2 km grid cell to estimate the 50% UD then 155 (CI 183–282) individuals would be required, compared to just 57 (CI 36–89) when a coarser spatial scale of 20 x 20 km is defined. Increasing grid cell size should therefore increase the coverage of the whole colony’s at-sea distribution, since there is a greater chance of including key areas for a given sample size. However precision will be decreased as the coarse scale is likely to include areas not actually used by birds. The exact grid cell size to be selected will be a question for marine planners and policy makers and depend on the scale at which other marine spatial planning decisions are made (Kidd et al. Reference Kidd, Plater and Frid2011). An additional way of increasing the representativeness of the sample would be to include a greater number of foraging trips for each individual (see Soanes et al. Reference Soanes, Arnould, Dodd, Sumner and Green2013), but this is made difficult by the limited battery life of commonly used low-cost GPS data loggers.
Understanding seabird foraging behaviour and identifying important at-sea areas are key to minimising the impact of the increasing global pressures on our marine ecosystems, through activities such as fisheries and marine renewable energy development. Accurate and representative species distribution information is essential to assessing how area use varies over time and to aid species protection via the designation of Marine Protected Areas. Seabird populations in the UKOTs are currently understudied, despite their global importance, and studies such as this investigating seabird foraging behaviour and identifying important at-sea areas for seabirds are crucial to redress this.
Acknowledgements
Thanks to Mr Karim Hodge of the Department of Environment and to the Government of Anguilla for granting research permissions for this work. Thanks to the Anguilla National Trust staff and volunteers for logistical support and the Dog Island rat eradication team for making us welcome and supporting our research, in particular Elizabeth ‘Biz’ Bell (Wildlife Management International Ltd.). Thanks also to Steve Holliday, Colin Wilkinson and Steffen Oppel for advice on planning the fieldwork. We are also grateful to the anonymous reviewers who provided constructive comments on previous drafts of this work.







