The coronavirus disease 2019 (COVID-19) clinical spectrum has presented diagnostic and treatment challenges without evidence-based clinical guidelines (CG). Abnormal laboratory markers of inflammation and coagulation were obtained following prior examples of viral pneumonia evaluation and treatment (severe acute respiratory syndrome, Middle Eastern respiratory syndrome, influenza A virus subtype H1N1). Clinicians noted different laboratory and clinical patterns and began to ask about the hypercoagulable aspects of their patients’ course and publish their observations.
On February 22, 2020, Han et al.Reference Han, Yang and Liu1 submitted their study concluding, in part, that the D-dimer was found to be predictive of disease progression but did not discuss the clinical implication to recommend therapy. One week later, Lin et al.Reference Lin, Lu and Cao2 published a study concluding that the assessment of the risk of venous thromboembolism (VTE) to prevent the possibility of pulmonary thromboembolism (PE) should include the interpretation of abnormally elevated D-dimer.
The American Society of Hematology (ASH) published a COVID-19 and VTE/Anticoagulation Frequently Asked Question (FAQ)Reference Kreuziger, Lee and Garcia3 on March 27, 2020. They recommend continuing the standard pharmacologic prophylaxis and treatment irrespective of COVID-19, because the observed high rates of thrombosis in seriously ill COVID-19 patients in China occurred where routine thromboprophylaxis may not be routinely practiced.
Klok et al. studied 184 COVID-19 pneumonia patients admitted to an intensive care unit (ICU) in the Netherlands from March 7 through April 5, 2020. Despite all patients receiving at least standard VTE prophylaxis doses, 25 (13.5%) had computed tomography angiogram (CTA) proven PE, 3 (1.6%) had a VTE (1 with a proximal deep vein thrombosis [DVT] of the leg and 2 with a catheter related upper extremity thrombosis), and 3 (1.6%) had cerebrovascular thrombotic strokes. The authors proposed that physicians treating COVID-19 pneumonia patients should be vigilant for signs of thrombotic complications, despite standard VTE prophylaxis treatment.Reference Klok, Kruip and van der Meer4
Llitjos et al. studied 26 consecutive patients with all receiving anti-coagulation upon admission, in 2 French ICUs, from 19 March 2020 to 11 April 2020 with severe COVID-19: 8 received prophylactic VTE treatment, with all developing a VTE; and 18 received therapeutic anti-coagulation with 10 developing a VTE and 6 a PE. This study also noted worsening of the PaO2/FiO2 ratio as part of their surveillance while receiving anti-coagulation. The authors concluded to screen for VTE and to implement early therapeutic anticoagulation.Reference Llitjos, Leclerc and Chochois5
Most clinicians were following the CG of acute respiratory distress syndrome (ARDS) in their COVID-19 pneumonia treatment with the expectation that disseminated intravascular coagulation (DIC), bleeding, would manifest as the spectrum advanced toward multisystem organ failure (MOF). But clinicians raised questions and concluded that patients in the COVID-19 spectrum were hypercoagulable before the DIC and MOF.Reference Li, Lu and Zhang6 They began to treat patients with low molecular weight heparin (LMWH) based on the D-dimer and other readily available laboratory markers acting on what was before them with local study developing treatment plans with little, if any, outcome analysis in part due to lack of autopsy, imaging, and other definitive measures. 7-Reference Obi, Barnes and Wakefield10
The letter to the editor by Barrett et al.Reference Barrett, Moore and Yaffe11 responded to the International Society on Thrombosis and Hemostasis (ISTH) interim guidance on recognition and management of coagulopathy in COVID-19 from 22 March 2020 that recommended only prophylactic VTE treatment.Reference Thachil, Tang and Gando12 They provided a logical argument that, due to the hypercoagulable state, coagulation management needs to be pushed to the forefront.
Available VTE/PE prophylaxis guidelines 7-Reference Obi, Barnes and Wakefield10 have been collected with various dates of inception based on their own institutional or network data as well as early publications regarding the hypercoagulable state to assist this study. How to build CG (the plane) based on the findings of a scoping review while practicing (flying) in the early phase of the COVID-19 pandemic uses an understanding of CG development as the science becomes refined while carefully examining measurable outcomes of the incidence of VTE and PE when available. All-cause mortalityReference Zhang, Yan and Fan13 without autopsy or confirming imaging of PE, complications from the LMWH treatment, and control for adherence to the adopted local CG with set D-dimer parameters based on prophylaxis and treatment LMWH dosages can be followed by the institutions that adapt, adopt, and create their own CG. Reference Minze, Kwee and Hall14,Reference Hull, Garcia and Burnett15 The aim of this scoping review is to determine the options for anti-coagulation therapy during the SARS-nCoV-2 clinical spectrum from available peer-reviewed literature from clinical researchers’ observations, some published on-line ahead of print during the study. Specifically of interest is to learn the change in the D-dimer over a period of time that would indicate when to escalate the LMWH dose from standard VTE/PE prophylaxis to VTE/PE treatment.
METHODOLOGY
A scoping review is preferred over a systematic review in a pandemic, as it is able to quickly identify the evidence available to identify knowledge gaps, scope a body of literature, and clarify concepts.Reference Munn, Peters and Stern16 During the SARS-n-CoV-2 pandemic, an increase in scientific production has occurred, with on-line publication ahead of print, providing a massive amount of data in a span of weeks. As many different studies, with different sizes and search questions, were hastily published to spread knowledge on the COVID infection, scoping reviews are specially fitted to collect the essence from different studies and highlight emerging evidence in current peer- reviewed literature when it is still unclear what other, more specific, questions can be posed and valuably addressed by a more precise systematic literature review.Reference Armstrong, Hall and Doyle17
METHODS
Search Questions
Which D-dimer levels require clinical actions in COVID-19 patients?
What COVID-19 hypercoagulation evaluation and treatment data are available in the midst of the pandemic?
How common are thrombotic and hemorrhagic events in the COVID-19 spectrum?
Information Sources
PubMed electronic records were searched for publications regarding, coronavirus and anticoagulation, using the key words “coronavirus” or “COVID” or “SARS-nCov-2” AND the search terms listed in Table 1.
TABLE 1 Search Keywords

Search keywords used with the results for each query. The strings reported in the table were alternatively put in in the parenthesis of the search query: “coronavirus” or “COVID” or “SARS-nCoV-2” AND (*).
Eligibility Criteria and Selection Process
Papers that met inclusion criteria were exported from PubMed as XML files and entered on a Google spreadsheet, which included the following fields: Subject title, Authors, Name of the journal or book, Abstract, Date of publication.
Inclusion: Studies published from 1 January to 23 April 2020, English studies, Chinese studies with an English abstract.
Exclusion: Studies that did not answer the search questions, Single case reports, Papers that reported data from other studies.
The first screening was a review of the title, by 2 independent reviewers (A.C., E.W.) each reviewer would note, “yes” or “no” on the spreadsheet. If there was “yes” consensus, the article was included in the second screening review. If there was “no” consensus, the article was discarded. If consensus was not reached, the 2 reviewers discussed the subject, and then, if consensus was not reached, then the third reviewer (D.S.) was the arbiter.
The second screening was a review of the abstract, again noting, “yes” or “no” on the spreadsheet. If there was “yes” consensus, the article was included in the scoping review. If there was “no” consensus, the article was discarded. If consensus was not reached, the 2 reviewers discussed the subject, and then, if consensus was not reached, then the third reviewer (D.S.) was the arbiter.
Following the screening process, data were entered on to the spreadsheet for the following elements: D-dimer levels of COVID patients, thrombotic and hemorrhagic events of COVID patients, protocols and therapeutic approaches for the use of anticoagulants in COVID patients, open questions and unanswered research questions on coagulation and related complications, D-dimer values that trigger the start of anticoagulation, first anticoagulation drug used and relative dosing, D-dimer values that trigger the escalation of anticoagulation, surveillance subjective (symptoms) or objective (labs, imaging) data to monitor complications of the hypercoagulable state or treatment, escalation of anticoagulant therapy, discharge with anticoagulant therapy.
RESULTS
The search retrieved 130 publications, once 35 duplicate results were removed, 95 papers were screened then 35 were excluded. Reference Han, Yang and Liu1,Reference Lin, Lu and Cao2,Reference Klok, Kruip and van der Meer4,Reference Llitjos, Leclerc and Chochois5,Reference Casini, Alberio and Angelillo-Scherrer9,Reference Obi, Barnes and Wakefield10,Reference Zhang, Yan and Fan13,Reference Li, Du and Wang18-Reference Lodigiani, Iapichino and Carenzo47 Based on title and abstract after the second screening, 60 papers were selected for further review. There were 5 Chinese papers with an English abstract and Chinese-only full text that made it to the second phase, and it was agreed by both reviewers to evaluate these based on the abstract (Figure 1).

FIGURE 1 Scoping Review
The expanded Google spreadsheet served as a data extraction tool for the 39 papers that were selected to have met the search questions (Table 2).
TABLE 2 Search Results

Papers selected after the two steps screening process. This table presents the content of the 39 studies.
D-Dimer Values
D-dimer values (n = 25) (Table 3) were increased in all the studies, compared with general control population, in all COVID patients. D-dimer on admission greater than 4 times the upper limit of normal (ULN) could effectively predict in-hospital mortality in patients with COVID-19, which indicated D-dimer could be an early and helpful marker to improve management of COVID-19 patients.Reference Zhang, Yan and Fan13 A mean increase in concentration is appraised in more severe patients, with higher concentrations suggesting a higher mortality rate. Reference Zhang, Yan and Fan13,Reference Zhou, Yu and Du24,Reference Tang, Li and Wang31 D-dimer levels higher than twice ULN are associated with worse outcome by 2 studies. Reference Zhang, Zhang and Wang19,Reference Zhou, Yu and Du24 The recommendation to hospitalize patients with values higher than 3-4 times the ULN was questioned by Akima et al.Reference Akima, McLintock and Hunt44 Patients with D- dimer higher than 6 times ULN seem to benefit the most from the use of heparin therapy. Reference Tang, Bai and Chen48,Reference Yin, Huang and Li49
TABLE 3 D-Dimer and Complications

D-dimers data and hypercoagulation/hemorrhagic complications in the selected studies.
Thrombotic and Hemorrhagic Complications
Manifestations of hypercoagulation (n = 6) (Table 3) are widespread, with the most common reported in reduced numbers: DVT, PE, ST segment myocardial infarction (STEMI), and cerebrovascular accident (CVA).Reference Lodigiani, Iapichino and Carenzo47 No hemorrhagic complication was reported by any of the studies. Close monitoring is always suggested due to the increased risk of bleeding with anticoagulation following standard monitoring of symptoms, signs, and blood tests.
Therapeutic Evidence
Except for Lin et al.,Reference Lin, Lu and Cao2 no study suggests a D-dimer cutoff to start anticoagulation therapy (n = 16) (Table 4). The criteria for beginning anticoagulation treatment with COVID-19 patients is debated, with some studies starting prophylactic anticoagulation on every hospitalized patient, Reference Obi, Barnes and Wakefield10,Reference Ranucci, Ballotta and Di Dedda51 others are based on the stratified risk of VTE,Reference Bikdeli, Madhavan and Jimenez52 while some others suggest the inclusion of ambulatory patients with risk factors.Reference Lodigiani, Iapichino and Carenzo47
TABLE 4 Therapeutic Approaches

Papers with data regarding the therapeutic approach.
Most studies suggest as a starting therapy LMWH, with dosing adjusted for weight and creatinine clearance (CCr) to be in prophylactic range or, alternatively, unfractionated heparin (UFH), depending on the CCr. The dosages would follow each institution’s standard dosage algorithms and are not discussed in this study after a careful standard assessment of bleeding risk and other contraindications. Just a single study suggests as first-line therapy the use of therapeutic doses of LMWH or UFH.Reference Bikdeli, Madhavan and Jimenez52 Additionally, a study suggests using D-dimer OR thromboelastography (TEG) for monitoring therapeutic efficacy.Reference Song, Wang and Zhang41
While authors recognize the role of ultrasound (US) in the surveillance of DVT complications, Reference Llitjos, Leclerc and Chochois5,Reference Cui, Chen and Li28,Reference Marietta, Ageno and Artoni42 no study suggests routine sonographic screening. The use of CTA is preferred by some authors in case of high bleeding risk,Reference Obi, Barnes and Wakefield10 while clinical monitoring followed by US and CTA is still the most common approach, Reference Obi, Barnes and Wakefield10,Reference Ranucci, Ballotta and Di Dedda51,Reference Bikdeli, Madhavan and Jimenez52 D-dimerReference Oudkerk, Büller and Kuijpers50 is suggested only once in this findings as a tool to escalate therapy.
Escalation therapy is unclear in many studies. The studies that mention the increase of dosing, refer to the use of LMWH or UFH to full therapeutic dosing Reference Marietta, Ageno and Artoni42,Reference Oudkerk, Büller and Kuijpers50,Reference Bikdeli, Madhavan and Jimenez52 with just a single study suggesting the use of novel oral anticoagulant (NOAC).Reference Obi, Barnes and Wakefield10
Few studies refer to the use of anticoagulant therapy after discharge, with direct oral anticoagulant (DOAC) or LMWH as reference drugs. Reference Marietta, Ageno and Artoni42,Reference Lodigiani, Iapichino and Carenzo47,Reference Bikdeli, Madhavan and Jimenez52 Therapy duration is referred from 7 to 14 dReference Marietta, Ageno and Artoni42 up to 2 mo suggested by Obi et al.Reference Obi, Barnes and Wakefield10
LIMITATIONS
The highest level of evidence is a systematic literature review of randomized controlled studies with meta-analysis of data. When this study commenced at that phase of the COVID-19 clinical spectrum there were no such studies. Tricco et alReference Tricco, Antony and Zarin53 raised the concern of poor quality of reporting of numerous rapid reviews and recommends a prospective study comparing the results of systematic literature reviews and scoping reviews as a measure of quality analysis of available data.
Unfortunately, at the time of this study, the incidence of thrombotic complications and outcomes of anticoagulation therapy are unknown due to lack of standardized reporting, lack of autopsy studies, and other data gaps. Most studies are retrospective and present small sample sizes. Additionally, the majority are focused on Chinese patients, which could lead to biases in population selection and therapeutic management.Reference Thachil, Tang and Gando12 Studies are highly heterogeneous in reporting anticoagulant management and therapeutic suggestions, and not many papers have considered the possibility of escalating therapy to reduce the potential of thrombotic complications. Additionally, little evidence is available of the use of antiplatelet aggregation therapy, with a single study reporting its use,Reference Liu, Li and Liu43 while most of the therapeutic approaches are based on anticoagulant use alone. This was not 1 of the search questions, but it is an open point for further clinical research.
Time zero has proven difficult to define when the initial D-dimer test was obtained in the time course of the COVID-19 spectrum in available studies. Each patient presents at a different point in time in their disease course, comparing patient to patient or an aggregate analysis of patients’ D-dimer levels would require a careful analysis.Reference Howick, Chalmers and Glasziou54 Perhaps in due time this can and likely will be defined, but in the midst of the evolving science of the COVID-19 spectrum, our only alternative is to appreciate the global phenomena of the hypercoagulable state and extrapolate what we do know in the absence of double-blind random control studies or a registry of studies that use the same treatment guideline with clinical outcome measurements, including autopsy, imaging, and other determinants of VTE and PE.
DISCUSSION
Pathophysiologic Effects of COVID-19
The binding of the SARS-CoV-2 virion to an angiotensin-converting enzyme 2 (ACE2) receptor, largely found in alveolar epithelial cells, activates cytokine release. In some individuals, the number of proinflammatory cytokines released sharply increases due to loss of negative feedback to the immune system, and positive feedback from the inflammatory cytokines leading to further production of inflammatory cytokines. This results in a phenomenon known as cytokine release syndrome (CRS), or cytokine storm, a dynamic process that may not be clinically apparent.
CRS Lab Findings
Cytokine measurement is theoretically appealing, but most markers are not easily assessed in the peripheral blood and others have not yet proven to be predictive of poor outcomes.Reference Henderson, Canna and Schulert55 In 2002, Shorr et al.Reference Shorr, Thomas and Alkins56 showed that D-dimer levels in critically ill patients correlate with activation of the proinflammatory cytokine cascade. Chen et alReference Chen, Wu and Guo20 in a small sample size observation study of COVID-19 patients demonstrated similar with CRS markers and the D-dimer markedly (5 × ULN) elevated in the severe group (n = 11) than the moderate group (n = 10) with normal D-dimer levels. Neutrophil extracellular traps (NETs) are extracellular webs of chromatin, microbicidal proteins, and oxidant enzymes that are released by neutrophils to contain infections. However, when not properly regulated, NETs have potential to propagate inflammation and microvascular thrombosis. Zuo et al.Reference Zuo, Yalavarthi and Shi57 found that patients with COVID-19 (n = 50 patients; n = 84 samples) have elevated levels of markers of NETs that strongly correlate with elevated D-dimer levels.
Importantly, these NET markers were higher in hospitalized patients receiving mechanical ventilation as compared with hospitalized patients breathing room air. Experience suggests that trends in laboratory studies rather than threshold values will be most informative.Reference Mehta, McAuley and Brown58 The D-dimer is readily available in most hospital laboratories and most providers are familiar with the association between VTE and elevated D-dimer levels. In a COVID-19 patient with CRS and worsening coagulopathy as evidenced by a rising D-dimer, it may make sense to administer escalated doses of anti-coagulation before thrombotic events occur. Reference Dolhnikoff, Duarte-Neto and de Almeida Monteiro39,Reference Mehta, McAuley and Brown58
TEG
TEG is a noninvasive test using blood obtained by means of venipuncture that can measure the ability of whole blood to form a clot. Over the years, TEG has been highly studied and used in various capacities, including assisting in liver transplant patients, cardiac surgery, and in traumatic blood loss. It can detect and quantify changes in the viscoelastic properties of a blood sample during clotting. Normal range for coagulation index (CI) is -3.0 to +3.0, where a CI > +3.0 would indicate a hypercoagulable state, and CI < -3.0 indicates a coagulopathy.Reference Whiting and Dinardo59 A study conducted by Panigada et al.Reference Panigada, Bottino and Tagliabue38 used TEG testing on 24 COVID-19 patients admitted to the ICU in Milan, Italy, to show evidence of hypercoagulability with a severe inflammatory state, but not acute DIC. Elevated TEG results were shown to correlate with elevated D-dimer results in COVID-19 patients in severe respiratory failure, supporting a hypercoagulable state.Reference Spiezia, Rossetto and Campello60 Chinese expert consensus supported the use of TEG in severe COVID- 19 patients in part because TEG was available and an actionable test, interpretable to assist management.Reference Song, Wang and Zhang41
D-Dimer in CGs
D-dimer is a major fibrin degradation product released upon cleavage of cross-linked fibrin by plasmin. Although other fibrin degradation products are generated with this cleavage of fibrin, D-dimer is the most studied and validated for clinical assessment. Normal levels of D-dimer vary by laboratory, depending on their equipment and process. For the purpose of how to create a CG while interpreting observational studies from across the world, the D-dimer level will be expressed as “times ULN” or “× ULN”. Although not studied with COVID-19 patients using an assessment of pretest clinical probability with age-adjusted D-dimer, there was a low likelihood of clinical venous thromboembolism.Reference Righini, Van Es and Den Exter61 The recommended (and most used) adjustment is by the formula for patients over 50 y of age is (patient’s age × 10) µg/L.Reference Nybo and Hvas62
The development of CGs to diagnose, treat, and monitor the hypercoagulable state with COVID-19 patients requires an appreciation of small sample observation studies. Although Lippi et al. established a weighted mean difference among 4 studies (Huang,Reference Huang, Wang and Li63 Tang,Reference Tang, Li and Wang31, Wang,Reference Wang, Hu and Hu64 and ZhouReference Zhou, Yu and Du24) of a D-dimer level of 6 × ULN, acknowledging the heterogeneity across the studies, the main point raised is that the evolution of a worsening clinical course can be determined by serial D-dimer levels.
Use of Direct Oral Anticoagulants
For over 50 y, warfarin was the only oral anticoagulant available. In the past 10 y, we have seen the development of NOACs. These drugs are now commonly classified as DOACs. The 5 DOACs currently available in the United States are dabigatran (Pradaxa®), rivaroxaban (Xarelto®), apixaban (Eliquis®), edoxaban (Savaysa®), and betrixaban (Bevyxxa®). DOACs are termed “direct” because they block a single blood-clotting factor to treat or prevent thrombus. These drugs have different and often overlapping FDA indications, but all indications are for the treatment or prevention of thromboembolic disease. Given the increased risk and prevalence of thrombotic complications in COVID-19, the question has been raised if DOACs would play a role in the treatment of or reduction of risk of VTE in this subset of patients.
Current consensus supports the universal administration of thromboprophylaxis for all patients admitted for COVID-19, who do not have an increased risk of bleeding or contraindication to anticoagulation. Reference Kreuziger, Lee and Garcia3,Reference Thachil, Tang and Gando12,Reference Zhai, Li and Chen46,65 Many support a varied dosage strategy based on VTE risk (typically using D-dimer), weight, and renal function. Most experts recommend LMWH or fondaparinux over UFH, as it minimizes patient interaction with managing drips and eliminates difficulties maintaining therapeutic levels. It would seem likely that DOACs would offer these same advantages. However, most experts do not recommend DOACs for thromboprophylaxis or treatment of VTE for COVID-19 inpatients. The principal reason for this is due to interactions between investigational drugs for COVID-19 and DOACs. Multiple medications under investigation for COVID-19 have drug-drug interaction with DOACs due to their effect on the cytochrome-P450 enzyme activity, the enzyme-substrate by which most DOACs are metabolized. The University of Liverpool has collated a list of drug interactions at http://COVID19-druginteractions.org/ .66 Given this, it is even recommended that patients on DOACs (and warfarin) should be switched to LMWH on admission for COVID-19, to dosages corresponding to DOAC or warfarin dosing.Reference Hunt and McClintock67
Extended Thromboprophylaxis
The greatest use for DOACs to prevent VTE in the COVID-19 patient appears to be at discharge and in the outpatient treatment space. After hospital discharge from acute medical illness, extended prophylaxis with LMWH or DOAC’s can reduce the risk of VTE. Reference Amin, Varker and Princic68,Reference Schünemann, Cushman and Burnett69 While there are no current data specific to thromboprophylaxis in COVID-19, it stands to reason that, due to the increased risk of thrombotic events identified beyond what is typically seen in the acutely medically ill patient population, upon discharge COVID-19 inpatients could benefit from extended thromboprophylaxis. Many experts and academic institution guidelines support risk stratification for the use of DOACs for extended thromboprophylaxis postdischarge in patients without increased bleeding risk. Reference Zhai, Li and Chen46,Reference Zhang, Sun and Feng70 Rivaroxaban, betrixaban, and apixaban have all demonstrated extended thromboprophylaxis in the acutely medically ill patients, in which patients with infectious diseases were part of their inclusion criteria Reference Cohen, Spiro and Büller71-Reference Spyropoulos, Ageno and Albers75 (Supplementary Table 1, which is available online). Reference Gibson, Halaby and Korjian76,Reference Raskob, Spyropoulos and Zrubek77
These studies all compared the DOAC to enoxaparin, placebo, or enoxaparin plus placebo. The studies varied in patient population characteristics, inclusion criteria, and primary efficacy outcomes. Their results varied in superiority and noninferiority compared with the respective control group. Rivaroxaban and betrixaban did achieve FDA approval for the indication of extended thromboprophylaxis in the treatment of the acutely medically ill. There are no studies that have reviewed extended thromboprophylaxis in the treatment of COVID-19 patients. However, many experts, as well as the authors of this study, suggest constructing guidelines that strongly encourage the use of DOACs or LMWH for extended thromboprophylaxis postdischarge for all admitted COVID-19 patients without contraindications or increased risk of bleeding. Reference Kreuziger, Lee and Garcia3,Reference Zhai, Li and Chen46,Reference Bikdeli, Madhavan and Jimenez52,65
With no studies in the COVID-19 thromboprophylaxis space, it is challenging to determine dosing at discharge for DOACs. Most experts prescribe dosages for DOACs that are typically in line with dosages studied in the thromboprophylaxis studies for treatment of the medically ill.
COVID-19 patients have an increased risk of thrombotic events, with many patients demonstrating markedly elevated D-dimer or have suspected or confirmed VTE and are treated as inpatients with enoxaparin at treatment doses. For those patients with confirmed VTE, the DOAC at discharge should be one that has an FDA approved indication for VTE treatment, and the dose should be the treatment regimen. For those patients who have markedly elevated D-dimer and treated with therapeutic doses of LMWH, confirming or ruling out VTE would be prudent before discharge. If there is no confirmatory VTE, one would expect that a COVID-19 patient who is well enough to be considered for discharge would have a D-dimer level that is at least below 3 times ULN and could be discharged on a DOAC or LMWH at prophylactic dosages.
As far as the duration of prophylactic treatment, expert opinion has ranged from 14 to 45 d. Reference Kreuziger, Lee and Garcia3,Reference Lodigiani, Iapichino and Carenzo47,Reference Bikdeli, Madhavan and Jimenez52,65 Again, given that clinicians are out of the realm of the randomized control trials in thromboprophylaxis of COVID-19 patients to create CGs, determining duration of treatment for thromboprophylaxis is challenging. How long inflammation and thrombotic derangements last after recovering from the COVID-19 acute lung injury spectrum remains unclear, because the incidence of post-COVID-19 acute lung injury spectrum VTE/PE and other thrombotic complications is unknown. Cohoon et al.Reference Cohoon, De Sanctis and Haskell73 as a subgroup analysis of the MAGELLAN study concluded that rivaroxaban for thromboprophylaxis among patients recently hospitalized for acute infectious diseases after 35 d reduced the occurrence of VTE. The risk/benefit ratio remains a clinical decision based on the in-patient team’s rationale for thromboprophylaxis to continue postdischarge. The authors of this study recommend consideration of up to a 35-d DOAC course with a careful assessment of continued risk, bleeding precautions, with close follow-up.
DOAC’s at Emergency Department Discharge
Autopsy reports of COVID-19 patients have demonstrated PE, cerebrovascular (stroke) and coronary artery (myocardial infarction [MI]) emboli. Reference Giannis, Ziogas and Gianni78-Reference Julien, Julien and Morgan80 It could be extrapolated that patients with milder pulmonary symptoms of COVID-19 could also benefit from thromboprophylaxis as outpatients with DOACs or LMWH. Some experts recommend that patients with mild and moderate pulmonary COVID-19 who have a high or moderate risk of VTE (using IMPROVEDD,Reference Gibson, Spyropoulos and Cohen81 among other validated scoring systems), began pharmacological thrombotic prevention,Reference Zhai, Li and Chen46 others believe pharmacological prophylaxis should be reserved for inpatients.Reference Phend82 This is a controversial topic currently without evidence-based recommendations. However, with the increase in thrombotic events, some consideration for thromboprophylaxis should be given to the COVID-19 patient at high risk for VTE with no other indications for admission.
Risk stratification using the D-dimer seems to be the best approach, because it has been shown to predict severity of illness and thromboembolic risk.83 ISTH guidance suggests any COVID-19 patient with a D-dimer great than 3 times ULN be admitted regardless of the presence of any other admission criteria. Reference Thachil, Tang and Gando12,Reference Giannis, Ziogas and Gianni78 However, that still leaves the at-risk patient with a D-dimer less than 3 times ULN without guidance. Patients with VTE risk and D-dimer levels from 1 to 3 times ULN with no other criteria for admission still have significant risk, and perhaps guidance for prescribing DOACs for those without significant bleeding risk, at the same dosage and duration as those discharged inpatients, would be a life-saving maneuver.
Aspirin Use
The hypercoagulable thrombotic potential for COVID-19 patients that do not require admission based on oxygen requirements or other supportive care does raise the question of aspirin therapy without standard contraindications. Guzik et al.Reference Guzik, Mohiddin and Dimarco84 summarize that COVID-19 may lead to plaque instability and myocardial infarction, which is a common cause of death in COVID-19 patients. The most common cardiac complications include dysrhythmia (atrial fibrillation, ventricular tachydysrhythmias, and ventricular fibrillation). A study from the Zhongnan Hospital of Wuhan University shows that 16 of the 138 COVID-19 patients required cardiologic intensive care after developing arrhythmias.Reference Wang, Hu and Hu64 In the Lombardy region of Italy during the study period in 2020, a total of 9806 cases of COVID-19 were reported in the study territory. During this period, 362 cases of out-of-hospital cardiac arrest (OHCA) were identified, compared with 229 cases identified during the same period in 2019 (a 58% increase). COVID-19 was diagnosed or suspected in 103 of the 362 OHCA patients.Reference Baldi, Sechi and Mare85 Studies presented earlier have demonstrated the occurrence of MIs and CVAs. For these reasons, the recommendation without standard contraindication is for 325 mg86 of aspirin daily for the extended thromboprophylaxis period stated earlier in the DOAC discussion of up to 35 d.
COVID-19 VTE/PE Prophylaxis CG
The CG developed (Figure 2 with accompanying Table 5) addressed the 4 main presentations of a COVID-19 patient: (1) not requiring further evaluation after confirmation based on the clinical exam, there is no recommendation to obtain a D-dimer at this time; (2) requiring further evaluation based on the clinical exam but not requiring hospitalization with a D-dimer that is normal or < 3 times ULN; (3) requiring hospitalization without risk or clinical indication for VTE or PE with a D-dimer that is normal or <3 times ULN; (4) requiring hospitalization with risk or clinical indication for VTE or PE or with a D-dimer > 3 times ULN. A key aspect of this CG is to educate the patient upon discharge from the initial presentation or after acute in-patient care to be aware of symptoms and signs of VTE: DVT, PE, chest pain or angina equivalents, CVA/transient ischemic attack (TIA), mesenteric ischemia, and limb ischemia from peripheral artery disease (PAD). If the patient has any concerns, they should be instructed to call 911 (or the local number for an ambulance) or go to the nearest emergency department.
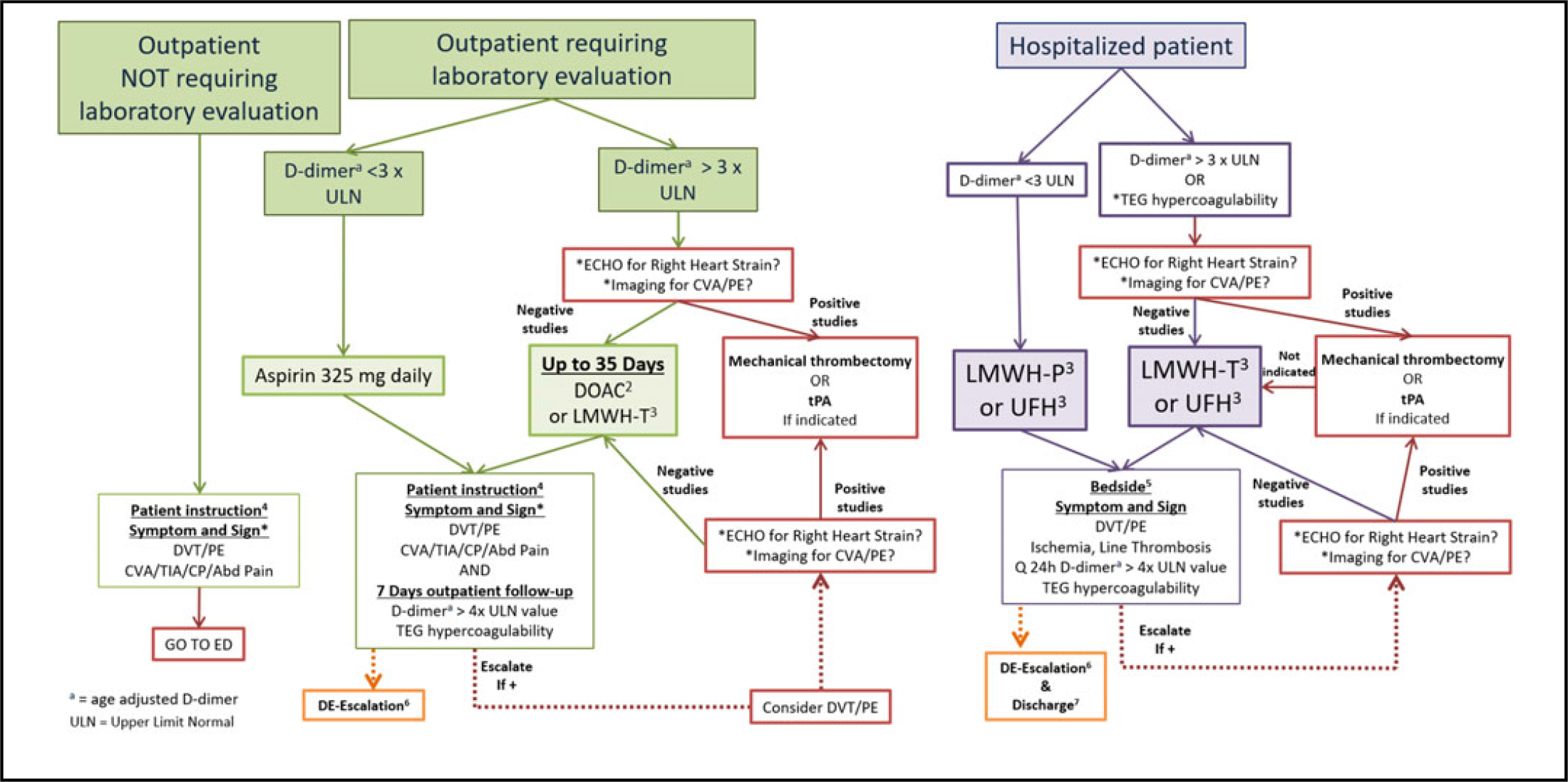
FIGURE 2 VTE/PE Thromboprophylaxis Clinical Guidelines for SARS-nCoV-2 PCR+/IgM+ (COVID+) or Person Under Investigation1
TABLE 5 Key to Figure 2

a Well’s Criteria for DVT. https://www.mdcalc.com/wells-criteria-dvt. Accessed May 1, 2020.
b Well’s Criteria for Pulmonary Embolism. https://www.mdcalc.com/wells-criteria-pulmonary-embolism. Accessed May 1, 2020.
c PERC Rule for Pulmonary Embolism. https://www.mdcalc.com/perc-rule-pulmonary-embolism. Accessed May 1, 2020.
d Gibson C, Spyropoulos A, Cohen A, et al. The IMPROVEDD VTE Risk Score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;01(01):e56-e65. doi: 10.1055/s- 0037-1603929.
e People Who Are at Higher Risk for Severe Illness. CDC. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html). Accessed May 28, 2020.
f Sepsis-Induced Coagulopathy (SIC) Score. https://www.mdcalc.com/sepsis-induced-coagulopathy-sic-score. Accessed April 29, 2020.
g ISTH Criteria for Disseminated Intravascular Coagulation (DIC). https://www.mdcalc.com/isth-criteria-disseminated-intravascular-coagulation-dic#pearls-pitfalls. Accessed April 29, 2020.
The CG advocates for clinicians to be keen on these symptoms during the post-acute COVID-19 phase as the period of hypercoagulability has yet to be determined. While admitted, all providers, including nurses, should be hyperacute to any signs of thrombosis, of hemodialysis or other catheters, as well as during extracorporeal membrane oxygenation (ECMO). Rapid decrease in oxygenation or an increase in other parameters that indicate a PE should trigger an escalation in anticoagulation treatment before formal diagnosis. The decision to obtain initial and serial imaging for DVT or echocardiogram for right heart strain or other easily obtainable studies for PE is not easily determined by this scoping review and is left up to the individual treatment team. All treating providers and nurses need to be on the alert for any clinical symptoms or signs of end-organ ischemia as an indication of thrombosis: TIA/CVA, angina equivalent, mesenteric ischemia, limb ischemia, etc. The most effective process to develop a local CG is to initiate a Clinical Guideline Team of Scientists (Table 6) to study and maintain a focus on the evolving science.
TABLE 6 Clinical Guideline Team of Scientists

CONCLUSIONS
This study stands on the shoulders of all the clinical scientists who knew that their observations were important for other scientists to study and will continue to study. Clinicians have abrogated their role in the translational science process that uses evidence-based medicine in the development of the CG that they follow in their daily practice. In the midst of the COVID-19 pandemic, response clinicians have had to create their own CGs for many aspects of the COVID-19 clinical spectrum while they practice, acquiring information from their own institution, networks, and from available on-line publications ahead of print, in essence building the plane (CGs) as they fly (practice).
These CGs reflect the current available information obtained by a scoping review with the full expectation that refinements, particularly the use of the D-dimer as a hypercoagulable marker and the timing and dosages of LMWH, DOACs, and aspirin (and other medications) will reflect on-going study waiting for the randomized controlled trials and subsequent systematic literature reviews and meta-analysis. The cautious process outlined in the creation of these guidelines remains at the forefront to address the COVID-19 morbidity and mortality.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/dmp.2020.195










