INTRODUCTION
In the USA during the first half of the 20th century, population-level Tuberculosis (TB) mortality rates fell rapidly from their 19th-century heights [Reference Noymer and Garene1]. These declines have been attributed to improved living conditions and medical care [Reference McKeown and Record2, Reference D'Arcy Hart and Wright3]. However, as TB mortality rates fell, racial disparities in TB mortality in the nation's rapidly expanding cities widened. In some cities, the TB mortality rate of African-Americans in 1930 was as high as four or five times that of whites [Reference Roberts4]. One explanation for the durability of the disparity in TB mortality is that living and working conditions for African-Americans and whites diverged as US cities grew: From 1916 to 1930, during the First Great Migration [Reference Lee5], more than 1·5 million African-Americans migrated from the agrarian South into densely populated, segregated neighborhoods in the industrial North. This rapid influx of migrants, combined with discriminatory housing policies that artificially reduced the pool of available housing for African-Americans, resulted in overcrowding that, in turn, provided an ideal environment for TB transmission [Reference Acevedo-Garcia6].
The complicated natural history of TB infection makes it difficult to determine whether these divergent TB mortality rates reflect rising disparities in infection risk or in factors that differentially affected case-fatality rates, such as advances in TB treatment that were unequally distributed. Because the epidemiology of TB is characterized by long and variable delays between infection and disease [Reference Vynnycky and Fine7], changes in transmission rates are not immediately reflected in temporal trends of pulmonary TB mortality [Reference Blower, McLean and Porco8]. In addition, the trajectory of TB mortality in the USA was dramatically altered by the 1918 influenza pandemic. Noymer [Reference Noymer9] has demonstrated that people with TB were more likely to die during the flu pandemic than people without TB, leading to a rapid decline in TB mortality immediately after the pandemic. Noymer suggests that the flu hastened the decline of TB in the USA by removing infectious people from the population. But the flu's effect on racial disparities in TB mortality in US cities is unclear: increasing or stagnating transmission rates in increasingly segregated neighborhoods could have offset the effects of the reduction in the pool of infectious people.
To understand whether rising disparities in pulmonary TB mortality reflected changes in underlying transmission rates, we estimated the annual rate of TB infection (ARTI) for African-Americans and whites in large northern cities from 1910 to 1933. We used the method of Vynnycky and Fine to indirectly estimate ARTI using pediatric TB meningitis (TBM) mortality data [Reference Vynnycky and Fine7]. TBM is a form of extra-pulmonary TB that causes inflammation of the membranes surrounding the brain, known as the meninges. Although TBM is an extra-pulmonary infection, people with TBM typically acquire their infection through exposure to someone with infectious pulmonary TB. All TB disease among young children is the result of a recent infection of a susceptible individual. Consequently, it reflects short-term changes in the risk of TB in the population of susceptible people.
We focus on northern cities in the USA because we are interested in changing TB risks in cities that were growing rapidly in part because of the First Great Migration. In addition, research in historical demography has demonstrated that patterns of segregation were very different in northern and southern cities during this period, with northern segregation characterized by the emergence of large, isolated ghettoes that are particularly conducive to TB transmission [Reference Acevedo-Garcia6], and southern segregation characterized by lower residential density [Reference Grigoryeva and Ruef10].
DATA
We used city-specific TB mortality data compiled by the US Department of Commerce from 1910 to 1933 [11] that we digitized and cleaned. These data include counts of pulmonary TB mortality in all years, specific causes of extra-pulmonary TB mortality from 1910 to 1920, and aggregated counts of extra-pulmonary TB from 1921 to 1933. We focus on the period from 1910 to 1933 both because of its historical importance and because after 1933 our mortality data switches from reporting deaths for whites and African-Americans to reporting deaths for whites and nonwhites.
To calculate per-capita mortality rates, we obtained age-specific city population estimates for the census years 1910, 1920, 1930 and 1940 using census extracts provided by IPUMS [Reference Ruggles12]. To obtain population estimates for our panel of cities during intercensal years, we aggregated the population data for the relevant age groups in all cities and linearly interpolated these values across census years.
In the Department of Commerce data, there are 16 cities (and counties in the case of boroughs within New York City), for which age-specific counts of pulmonary TB and extra-pulmonary TB deaths are available. Of these, 11 were in the northern USA: Baltimore, MD; Brooklyn, NY; Chicago, IL; Cincinnati, OH; Indianapolis, IN; Kansas City, MO; Manhattan, NY; Philadelphia, PA; Pittsburgh, PA; St. Louis, MO; Washington, DC. The remaining five were in the South: Atlanta, GA; Birmingham, AL; Louisville, KY; New Orleans, LA; Richmond, VA. We consider cities in states that belonged to the former Confederacy to be southern cities. We classify the rest as northern cities.
METHODS
Age-standardized pulmonary TB mortality rates
Our estimates of pulmonary TB mortality are age-adjusted to minimize the risk that they are confounded by the changing age composition of these cities from migration or changing birth and non-TB mortality rates. For more information on our approach to age-standardization, see the Supplementary Materials.
Estimating annual risk of TB infection (ARTI)
Vynnycky and Fine [Reference Vynnycky and Fine7] used TBM mortality data for children less than 5 years old to estimate the ARTI in England and Wales in the pre-chemotherapy era. They based their analysis on results from the Netherlands indicating that the mortality rate for TBM among children under 5 was roughly equivalent to 1% of the population-level ARTI [Reference Styblo, Meijer and Sutherland13], a pattern which was also found in Sweden for the period from 1925 to 1935 [Reference Sjorgen and Sutherland14]. We denote the ARTI in year t for group g as
![]() $\lambda _{\rm t}^{\rm g} $
.
$\lambda _{\rm t}^{\rm g} $
.
For the years 1910–1920, our data include counts of deaths from TBM as well as other categories of extra-pulmonary TB, including Pott's Disease and ‘White swelling’. Because total TB mortality rates fell during the 1910s, extra-pulmonary causes of death in the raw data were aggregated into a single category called ‘Other forms of Tuberculosis’ from 1920 onward. To estimate ARTI using these aggregated data, we used a Poisson model to predict the expected number of TBM deaths in group g (
![]() $D_{{\rm g,t}}^{{\rm TBM}} $
) as a function of the number of observed extra-pulmonary TB deaths (
$D_{{\rm g,t}}^{{\rm TBM}} $
) as a function of the number of observed extra-pulmonary TB deaths (
![]() $D_{{\rm g,t}}^{{\rm EP}} $
), where α
g is the ratio of TBM deaths to all extra-pulmonary deaths for group g. We used this information to estimate the ARTI (
$D_{{\rm g,t}}^{{\rm EP}} $
), where α
g is the ratio of TBM deaths to all extra-pulmonary deaths for group g. We used this information to estimate the ARTI (
![]() ${\hat \lambda} _{\rm t}^{\rm g} $
) for each racial group, g, from the aggregate extra-pulmonary TB mortality data, where
${\hat \lambda} _{\rm t}^{\rm g} $
) for each racial group, g, from the aggregate extra-pulmonary TB mortality data, where
![]() ${\hat \gamma} _{{\rm g,t}} $
is the expected number of extra-pulmonary TB deaths in group g during year t:
${\hat \gamma} _{{\rm g,t}} $
is the expected number of extra-pulmonary TB deaths in group g during year t:
Per-case infection risk
We can use the ARTI estimates derived from Eq. (3) to approximate the number of new infections generated by a single infectious pulmonary TB case in a given year among African-Americans (
![]() $\beta _{\rm t}^{\rm B} $
) and whites (
$\beta _{\rm t}^{\rm B} $
) and whites (
![]() $\beta _{\rm t}^{\rm W} $
), respectively. We denote the total number of infectious TB cases in the population during year t to be I
t, and the total population size to be N
t. The ARTI for a specific group (i.e. black, white) in a given year can be decomposed into the product of: (1) the population prevalence of smear-positive TB (I
t/N
t) and (2) the group-specific per-case infection rate at time t,
$\beta _{\rm t}^{\rm W} $
), respectively. We denote the total number of infectious TB cases in the population during year t to be I
t, and the total population size to be N
t. The ARTI for a specific group (i.e. black, white) in a given year can be decomposed into the product of: (1) the population prevalence of smear-positive TB (I
t/N
t) and (2) the group-specific per-case infection rate at time t,
![]() $\beta _{\rm t}^{\rm g} $
. Because I
t for this period cannot be observed directly, we approximate it from pulmonary TB mortality: the ‘Styblo rule’ suggests that prior to the advent of effective TB antibiotics in the 1940s, there were approximately four prevalent TB cases for every one death from pulmonary TB [Reference Styblo15]. A recent model-based analysis suggests that this approximation of pulmonary TB prevalence from mortality data is sensible for data from the pre-chemotherapy era [Reference Begun16], which includes our entire study period. Consequently, we approximate the count of infectious pulmonary TB cases as I
t = 4 × D
t, where D
t is the number of pulmonary TB deaths. We can then rearrange terms to estimate β
t for each group as follows, as in [Reference Vynnycky and Fine17]:
$\beta _{\rm t}^{\rm g} $
. Because I
t for this period cannot be observed directly, we approximate it from pulmonary TB mortality: the ‘Styblo rule’ suggests that prior to the advent of effective TB antibiotics in the 1940s, there were approximately four prevalent TB cases for every one death from pulmonary TB [Reference Styblo15]. A recent model-based analysis suggests that this approximation of pulmonary TB prevalence from mortality data is sensible for data from the pre-chemotherapy era [Reference Begun16], which includes our entire study period. Consequently, we approximate the count of infectious pulmonary TB cases as I
t = 4 × D
t, where D
t is the number of pulmonary TB deaths. We can then rearrange terms to estimate β
t for each group as follows, as in [Reference Vynnycky and Fine17]:
We also modeled the relationship between city population density and
![]() $\beta _{\rm t}^{\rm g} $
for each northern US city during the census years 1910, 1920, and 1930, when group-specific population estimates were most reliable. Our measure of population density is the average number of people living in a dwelling, which allows us to distinguish between cities characterized by low-density housing and cities characterized by high-density housing like apartments and tenements. We include dummy terms for each census year to adjust for trends not explained by changing city population density. For more information on this analysis, see the Supplementary Materials. We completed all analyses and data cleaning in R 3.3.1; we generated figures using ggplot2 0.9.3 and we fit models through MCMC using Stan 2·9 [18].
$\beta _{\rm t}^{\rm g} $
for each northern US city during the census years 1910, 1920, and 1930, when group-specific population estimates were most reliable. Our measure of population density is the average number of people living in a dwelling, which allows us to distinguish between cities characterized by low-density housing and cities characterized by high-density housing like apartments and tenements. We include dummy terms for each census year to adjust for trends not explained by changing city population density. For more information on this analysis, see the Supplementary Materials. We completed all analyses and data cleaning in R 3.3.1; we generated figures using ggplot2 0.9.3 and we fit models through MCMC using Stan 2·9 [18].
RESULTS
Disparities in pulmonary TB mortality
Figure 1 shows age-adjusted pulmonary TB mortality rates for African-Americans and whites in our panel from 1910 to 1933. Panel (A) shows that age-adjusted mortality for African-Americans and whites fell, with a persistent gap between black and white pulmonary TB mortality rates. For whites, the age-adjusted pulmonary mortality rate fell from 158 cases/100 K population (95 % CI = 155, 160) in 1910 to 37/100 K (95 % CI = 36, 38) in 1933. For African-Americans, the age-adjusted TB mortality rate fell from 495/100 K (95 % CI = 479, 513) in 1910 to 196/100 K (95 % CI = 188, 203) in 1933. However, over this same period, the relative risk of death from TB rose for African-Americans as compared to whites, from 3·1 (95 % CI = 3·0, 3·3) in 1910 to 5·3 (95 % CI = 5·0, 5·5) in 1933. Panel (B) illustrates the growth in this ratio over time.
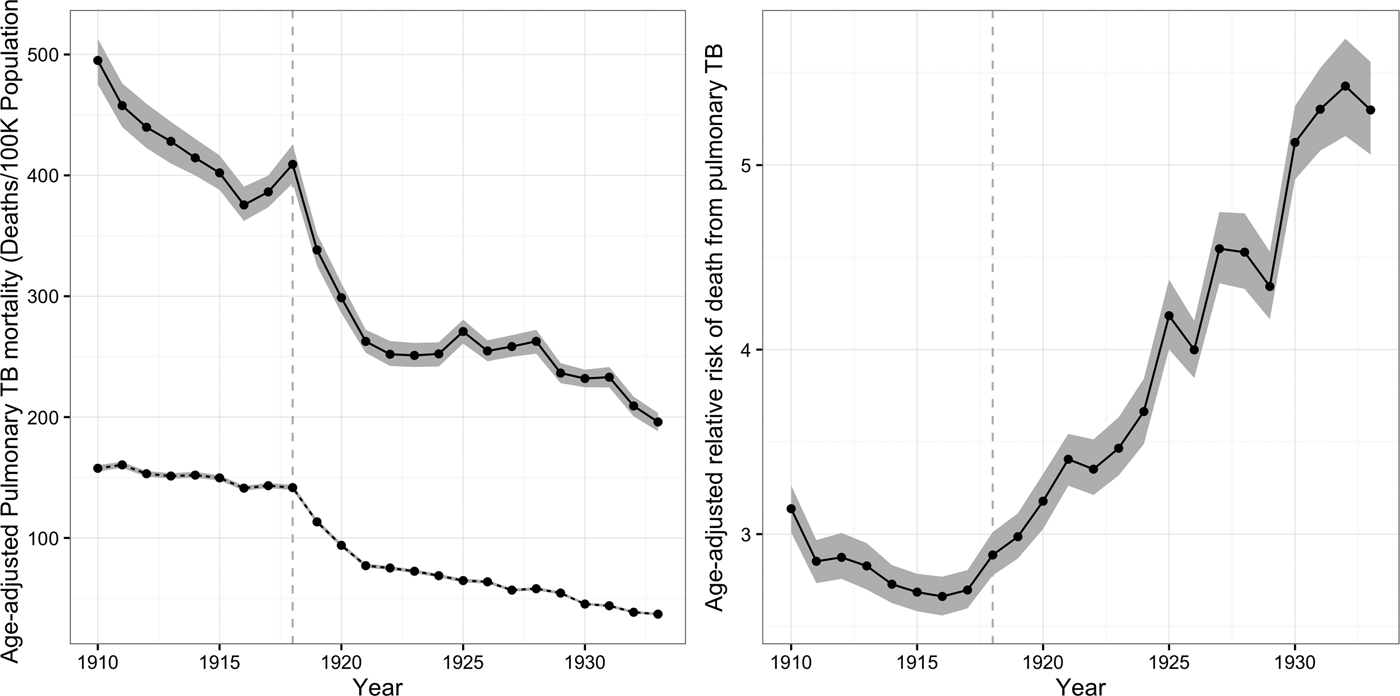
Fig. 1. Age-standardized TB mortality rates for African-Americans and whites. The left-hand panel shows age- standardized TB mortality rates in the panel of cities from 1910 to 1933 in deaths per 100 K population. Black mortality rates are indicated by the solid line and white TB mortality rates by the dashed line. The right-hand panel shows the adjusted relative risk (ARR) of death from pulmonary TB for African-Americans vs. whites from 1910 to 1933. The vertical dashed line indicates the timing of the 1918 influenza pandemic. Shaded regions in both panels indicate 95% posterior credible intervals.
Annual risk of TB infection
Figure 2 depicts the ARTI for African-Americans and whites from 1910 to 1933. The figure shows that for whites ARTI fell from 8·4% (95% CI = 7·8, 8·9) in 1910 to 1·7% (95% CI = 1·6, 2·0) in 1933. For African-Americans, the decline was less dramatic, falling from 17·3% (95% CI = 14·5, 20·7) in 1910 to 7·4% (95% CI = 6·2, 8·9) in 1933. The ratio of ARTI for African-Americans as compared with whites reached its lowest level in 1919 after the initial wave of the flu pandemic (RR = 2·1, 95% CI = 1·8, 2·5), but grew again as ARTI among African-Americans rebounded, even as successive waves of pandemic flu hit some of the cities in our sample as late as 1920 [Reference Olson19]. By 1933 relative ARTI for African-Americans as compared with whites was at its highest level (RR = 4·2, 95% CI = 3·4, 5·2) during our observation period.
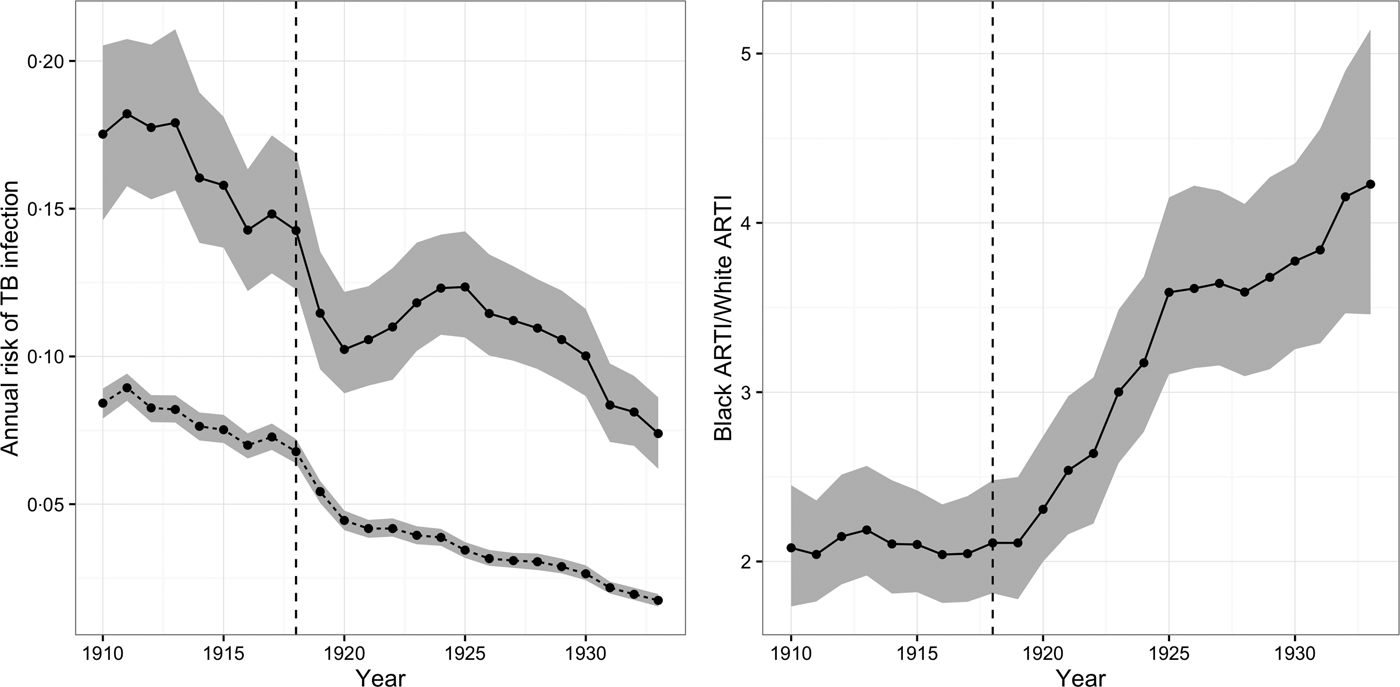
Fig. 2. Annual risk of TB infection (ARTI) for African-Americans and whites, 1910–1933. The left-hand panel illustrates the ARTI for African-Americans (solid line) and whites (dashed line) from 1910 to 1933. The right-hand panel illustrates the ratio of the ARTI for African-Americans vs. whites during this period. The gray-shaded area in both panels illustrates the 95% posterior credible intervals (CIs) for these quantities.
For African-Americans, TBM deaths accounted for 68% of the total extra-pulmonary TB deaths during the period from 1910 to 1920 (95% CI = 56, 83). For whites, TBM accounted for a larger share of extra-pulmonary deaths at about 82% (95% CI = 78, 86). These ratios and uncertainty in their estimation are reflected in the parameters α W and α B used to estimate λ g,t for African-Americans and whites as in Eq. (3). Figure S1 shows the correspondence between the total number of extra-pulmonary TB deaths recorded in the dataset and the number of TBM deaths in a given year. Additional models including an interaction term for group × year indicated that the value of α g was stable over time for both groups.
Per-case infection risk
The ARTI for African-Americans and whites illustrated in Fig. 2 is a product of the population prevalence of infectious (i.e. smear-positive) pulmonary TB and the group-specific rate of contact between susceptible individuals and infectious cases. Using the crude population prevalence of pulmonary TB in our cities (Figure S2) we can approximate the risk of TB infection posed by each prevalent case separately for African-Americans and whites, as described by Eq. (4).
Figure 3 shows that, for African-Americans, the number of new TB infections for every prevalent pulmonary TB case increased dramatically during the 1920s, whereas for whites this ratio continued to fall. On average, the risk of TB infection per pulmonary case fell by 2% per year for whites (95% CI = −2, −1), whereas for African-Americans, the number of new TB infections per prevalent case increased on average by 2% per year (95% CI = 1, 3).
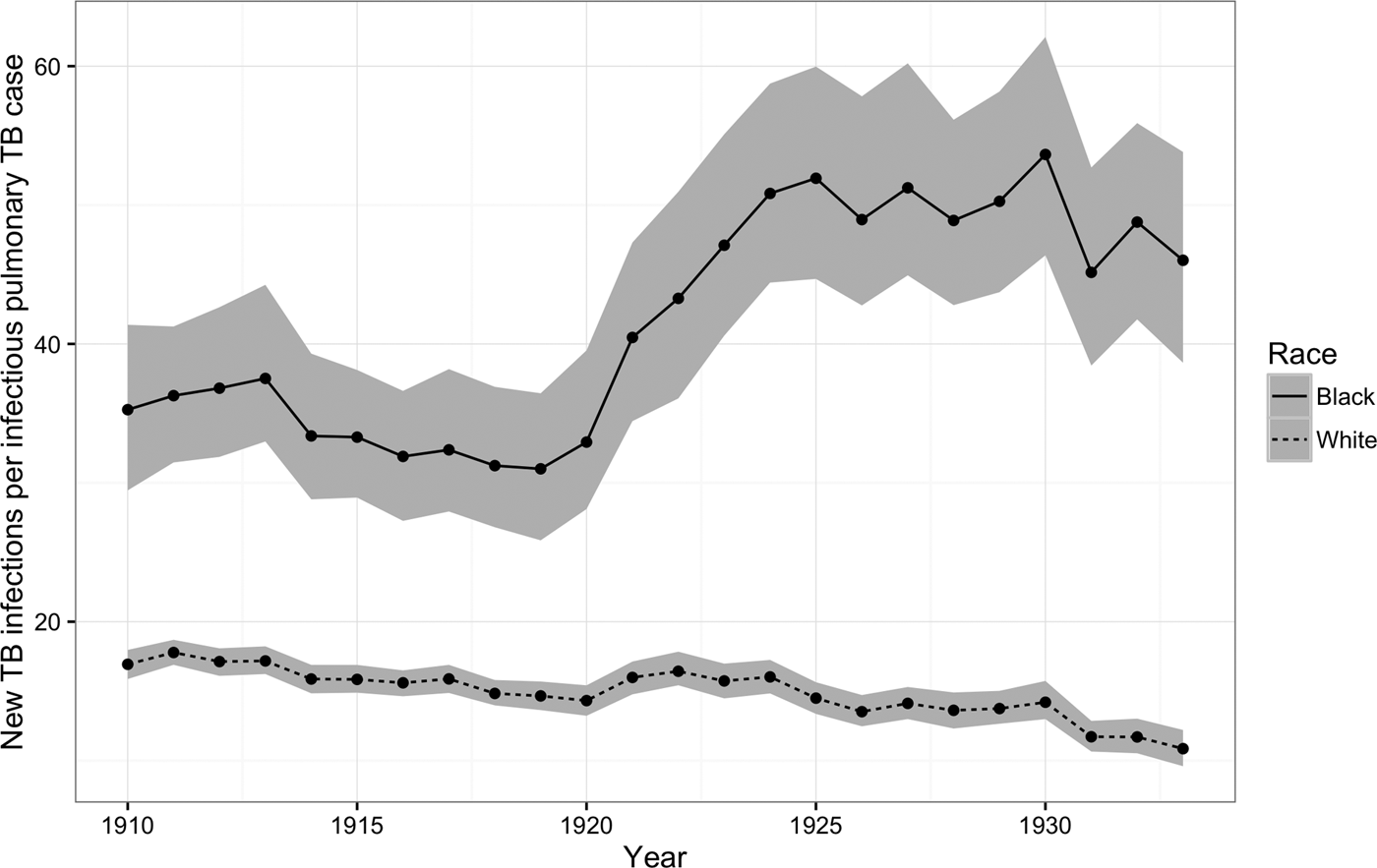
Fig. 3. New TB infections per prevalent pulmonary TB case, 1910–1933. The figure illustrates trends in the number of new TB infections among African-Americans (solid line) and whites (dashed line) for every prevalent pulmonary TB case during the period from 1910 to 1933. Areas shaded in gray indicate 95% posterior credible intervals.
Our analysis of the city-level per-case risk of infection during census years 1910, 1920, and 1930 shows that for each doubling of city population density, the per-case risk of infection for African-Americans and whites increased by 1·24 times (95% CI = 1·19, 1·29). This is equivalent to a ratio of 2·33 for the densest vs. least dense northern city in our data. After adjusting for city population density, there is still a residual decrease in per-case infection risk for whites in 1930 as compared with 1910 (RR = 0·88, 95% CI = 0·78, 1·00), and an increase for African-Americans (RR = 1·56, 95% CI = 1·12, 2·08). Across cities, increasing population density was associated with increasing per-case risk against a higher baseline risk for African-Americans as compared with whites.
City population size was strongly correlated with our measure of population density (r = 0·79) and was omitted from the city-level analysis. As a result, it is difficult to determine whether the relationship between population size and ARTI reflects increasing population density alone or some other characteristic of city scaling. However, to understand the importance of city population density to the total burden of pediatric extrapulmonary TB in our data, we generated posterior simulations comparing the predicted number of pediatric extrapulmonary TB deaths in our dataset to a counterfactual scenario in which all cities had population density equal to the lowest value in the data. This allows us to account for the greater population size of the densely-populated, higher-risk cities in assessing the impact of this factor on total caseload. These simulations indicate that by 1930, city-level variation in population density accounted for approximately 40% (95% CI = 27, 53) of black ARTI and 40% of white ARTI (95% CI = 33, 46).
DISCUSSION
Our findings confirm that the persistent and increasing racial disparities in pulmonary TB mortality discussed by Roberts [Reference Roberts4] are reflected in patterns of age-adjusted mortality from a panel of large US cities over a period of more than 20 years (Fig. 1). More critically, we document that there were similar disparities for African-Americans as compared with whites in the ARTI, which more accurately measures short-term changes in risk than pulmonary TB mortality. By comparing our estimates of ARTI for each group to the burden of pulmonary TB mortality in the population at large, we documented that the number of new TB infections for every pulmonary case increased among African-Americans at the same time that it decreased for whites (Fig. 3).
Although mortality from pulmonary TB fell for both groups, the per-pulmonary-case risk of TB infection increased for African-Americans and decreased for whites. This suggests that, for African-Americans, the rapid decline in pulmonary TB mortality immediately after the 1918 flu may have been offset by increasing or stagnating risks of infection from the dwindling pool of prevalent TB cases. For whites, in contrast, the per-case rate of infection consistently fell over the same period. These diverging risks may reflect opposing trends in the quality of living conditions and medical care for African-Americans and whites in large cities during the First Great Migration. Our analysis of city-level variation in the per-case risk of infection during census years suggests that transmission rates for both African-Americans and whites were greatest in the largest, most densely-populated cities, such as Chicago, New York, and Philadelphia, which absorbed the largest numbers of black migrants during this period.
Historical census data from this period [Reference Logan20] indicate that racial residential segregation – as measured by the isolation index – grew dramatically in a subset of the northern cities in our panel during the study period. Isolation is a measure of segregation that accounts for both the geographic separation of African-Americans and whites and the black population share [21]. Scholars have proposed that it is a central explanation for the concentration of TB risk in black neighborhoods because it indicates a high within-group intensity of contact [Reference Acevedo-Garcia6]. This means that the results shown in Fig. 3 cannot be interpreted straightforwardly as transmission rates, since this would imply uniform mixing between black and white populations at a time when there is strong evidence that the rate of contact between African-Americans and whites was rapidly changing in large US cities. Future dynamic modeling studies should use age-specific pulmonary TB and TBM mortality data as well as geographic information on case locations to understand the causal impact of segregation on TB transmission in cities where such data are available. Our analysis represents a key step toward building these models.
A limitation of our findings is that we rely on aggregate extra-pulmonary TB mortality to estimate unobserved TBM mortality from 1920 onwards. However, the stability of the ratio of TBM to aggregate extra-pulmonary TB deaths from 1910 to 1920 suggests that this approximation is reasonable for the period we observe. Including separate terms for the proportion of extra-pulmonary TB cases among African-Americans and whites ensures that our estimates of disparities are conservative: pooling black and white extra-pulmonary TB mortality would result in a larger value of α B and greater disparities in the ARTI between African-Americans and whites.
Because our estimates of ARTI and crude TB prevalence are derived from mortality data, they are necessarily approximate. However, this approximation of pulmonary TB mortality from mortality data has been shown to be sensible for data from the pre-chemotherapy era [Reference Begun16]. Another model-based analysis showed that the ARTI derived from TBM mortality data predicted age-specific patterns of pulmonary TB mortality [Reference Vynnycky and Fine17], suggesting that this is a good approximation of population-level risks. Further, our estimates of the relative ARTI for African-Americans compared to whites are not affected by this approximation because ARTI for each group was calculated by multiplying estimates of the TBM mortality rate by the same constant. Finally, tuberculin skin test results from black and white US Navy recruits in the late 1950s and early 1960s suggest that the disparities in infection risk that we documented here persisted into the chemotherapy era [Reference Edwards and Palmer22].
We also rely on the relative risk of infection and death from TB as a measure of inequality in infection risk, although the absolute difference in age-adjusted TB mortality and ARTI declined during the study period. We believe that this is the relevant measure of health disparity for the current analysis: across our panel of cities, ARTI and TB mortality among whites represents the best-case scenario for TB risk at any point in time and is the baseline against which gains in African-Americans' infection risk should be judged. In addition, the rate of infection and death from TB among whites was still great enough during the study period that our results are not susceptible to inflated relative-risk estimates due to a denominator rapidly approaching zero. Further, our analysis of per-case infection risk clearly indicates that both the absolute and relative per-case risk of infection increased during this period.
The finding that the number of new TB infections for every prevalent pulmonary TB case rose for African-Americans and declined for whites from 1910 to 1933 is consistent with the claim that increasing transmission rates may have undercut reductions in TB mortality among African-Americans following the influenza epidemic. There are, however, other potential explanations for the disparities in pediatric TBM mortality that underlie these findings. First, the potential for differential reporting of TB-related mortality for African-Americans and whites is an inherent limitation of any study of this type. Second, differential rates of extrapulmonary TB among African-Americans and whites with TB have been documented. For example, analyses of national data from the USA in the 1960s–1970s [Reference Farer, Lowell and Meador23] and 1980s [Reference Rieder, Snider and Cauthen24] showed that extrapulmonary TB accounted for a greater proportion of TB cases among African- Americans than whites, although these differences were relatively small. The age- and sex-adjusted proportion, for instance, was 18·9% for African-Americans and 16·1% for whites [Reference Rieder, Snider and Cauthen24]. However, rather than differences in susceptibility, these differences may instead reflect differential exposure [Reference Ong, Creasman and Hopewell25], disparities in SES, and access to health care. Moreover, even if racial differences in susceptibility to extrapulmonary TB affected the disparities observed in our data, they should not affect changing patterns of disparity over time.
Another potential explanation for the increasing relative risk of TBM mortality for African-Americans may be differing TB exposure profiles during the period of the Great Migration. In particular, African-American migrants from the agrarian South may have had greater exposure to Mycobacterium bovis than non-migrants. As a considerably larger proportion of M. bovis than Mycobacterium tuberculosis infections result in extrapulmonary disease [Reference Müller, Dürr and Hattendorf26] it is possible that the trend of increasing disparty reflects, at least in part, these exposure differences. However, our reliance on pediatric TBM mortality makes this explanation less likely, as there is no evidence of persistent human-to-human M. bovis transmission [Reference Grange27].
Despite its historical focus, our study is part of a larger effort to develop a comprehensive framework for fighting TB and other infectious diseases in the present day: Migration into densely-populated, transitory urban neighborhoods is an important cause of TB and other infectious diseases in the crowded cities of the developing world [Reference de Vries28, Reference Alirol29]. Rural-to-urban migration following economic development can affect the physical environment in ways that make communities more susceptible to infectious diseases like TB and diarrheal disease [Reference Zelner30, Reference Eisenberg, Cevallos and Ponce31], and such migration is expected to increase dramatically in the coming decades [Reference Goldstone32]. Historical data on reductions and disparities in TB mortality, paired with an understanding of the social and biological forces driving these patterns, are essential tools for developing theories and methods to more effectively combat present-day global inequalities in infection risk.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0950268817000802.






