INTRODUCTION
Clostridium difficile (CD) is a Gram-positive anaerobic, spore-forming bacillus, part of the normal intestinal microbiota in a wide range (1–15%) of the healthy adults [Reference Galdys1, Reference Kato2]. Colonization is much more common in infants (prevalence 2–75%), during the first 2 years of life and usually is asymptomatic [Reference Rousseau3, Reference Sandora4]. There are few studies regarding CD epidemiology in Poland, which are mostly focused on molecular aspects, regarding children, or in the case of one study, part of a total European study. Pituch et al. assessed prevalence (8·6%) of binary-toxin-positive strains in Polish patients [Reference Pituch5]. The same group reported the first case of CD PCR-ribotype 027/toxinotype III in Poland in 2005 [Reference Pituch6]. Duleba et al. analysed a group of 16 children hospitalized due to Clostridium difficile infection (CDI) in 993 (1·6%) children hospitalized due to acute diarrhoea. All children with CDI received antibiotics before illness; a correlation between hospitalization and the development of CDI was found in 56% of children. There were no deaths in children with CDI, recurrent infection was present in one (6%) child [Reference Duleba, Smukalska and Pawłowska7]. In the study by Bauer et al., a hospital-based survey in 34 European countries, three Polish hospitals were also represented. According to data from this study, 263 individuals from hospitals in Poland with diarrhea were tested, and CDI was confirmed in 102 cases. The weighted mean healthcare-associated CDI incidence rate per hospital was 12·5/10 000 patient-days and 76/10 000 admissions [Reference Bauer8].
The aim of our study was to estimate the incidence and epidemiology of patients with CDI hospitalized in the University Hospital, Krakow, Poland and also to evaluate whether the number of beds and annual number of hospitalizations reflect the risk of CDI.
METHODS
We performed a retrospective study based on the records of patients hospitalized with CDI at the University Hospital, Krakow, Poland, between January 2008 and June 2014. CDI was suspected in patients experiencing diarrhoea (defined as the passage of ⩾3 unformed stools in 24 h). The infection was then confirmed by detection of the CD antigen and toxins in faeces using the C. diff Quick Check Complete test kit (TechLab Inc., USA). When the test was positive for the antigen but negative for the toxin, the test for the CD toxin was repeated with the CD toxin ELISA A/B II test kit (TechLab Inc.) or by genetic methods using Illumigene CD (Meridian Bioscience Inc., USA). Primary CDI was defined as the first episode of CDI during the previous 3 months. Recurrent CDI was defined as a new episode of diarrhoea with a confirmation of the presence of the CD antigen and toxin using methods mentioned above following initial resolution of the prior episode for at least 24 h after the discontinuation of CDI treatment.
Statistical analysis
In order to perform statistical analysis of the data obtained, we summarized data to analyse the incidence, while quantitative data were presented as arithmetic means and their standard deviations. Moreover, epidemiological ratings, including crude mortality calculated per patient with 95% confidence intervals were used. The mortality of patients infected only once and those infected more than once were compared using the difference between two proportions. Student's t test was used to compare the age within specific groups. Pearson's correlation coefficients were used to study the relationship between epidemiological parameters connected with the hospital load and CDI parameters. P values ⩽0·05 were regarded as statistically significant. The data were processed using Statistica v. 10 software (StatSoft Inc., USA).
RESULTS
Demographic characteristics of the patients infected with CD between January 2008 and June 2014 are presented in Table 1. In the study period, CDI occurred in 1009 individuals. In the analysed group, 790 (78%) individuals developed infection only once, whereas 219 (22%) developed infection more than once with an average number of CDI episodes in the latter group of 2·5 ± 1·0. The frequency of infection has been increasing since 2008, reaching its peak in 2012 (6·5-fold increase compared to 2008), and then decreasing. The incidence rate for the entire study period was 2·87/1000 hospitalizations for all hospital patients, 4·04/1000 hospitalizations for medical wards, 0·59/1000 hospitalizations for surgical wards and 24·4/1000 hospitalizations for intensive care units (ICUs). The average age of patients with CDI was 63·1 years. There were 408 females and 382 males (mean age 63·2 and 60·5 years, respectively) in the group of patients with only one episode of CDI and 119 females and 100 males (mean age 65·2 and 63·6 years, respectively) in the group of patients with more than one episode of CDI. There were 474 (47%) individuals aged ⩽65 years and 535 (53%) people aged >65 years. Changes regarding age in the entire analysed population and separately for surgical, medical, and ICU wards are specified in Table 2. The age trends of patients with CDI in the analysed group are shown in Figure 1.
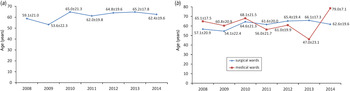
Fig. 1. Average age of patients with Clostridium difficile infection (CDI) hospitalized in (a) a tertiary hospital and (b) medical and surgical wards of a tertiary hospital in Krakow, Poland in consecutive years (mean ± s.d.).
Table 1. Demographic characteristics of patients infected with Clostridium difficile hospitalized between January 2008 and June 2014 in a tertiary hospital in Krakow, Poland

CDI, Clostridium difficile infection; IR, incidence rate per 1000 patients; s.d., standard deviation.
Table 2. Mean age of patients with Clostridium difficile infection hospitalized in a tertiary hospital in Krakow, Poland depending on the ward of hospitalization
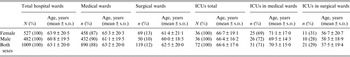
ICU, Intensive care unit; s.d., standard deviation.
We performed analysis of the frequency of infections depending on the location of the patient (i.e. medical vs. surgical wards) (Table 2). This analysis revealed that most CDI was found in patients hospitalized in medical wards: as much as 88% of CDI whereas only 12% was in surgical wards (Table 2). The frequency of CDI in ICUs at medical wards was also larger than in surgical wards (71% vs. 29%). The mean ages of CDI patients in analysed wards are presented in Table 2. Moreover, we analysed data from the 492 cases showing the time span between hospital admission and positive CDI test results, taking into consideration only the first episode of infection for each of the patients. In 178 (36·2%) cases CDI was confirmed during first 72 h of admission, while in 314 (63·8%) cases confirmation took longer. To confirm the predominance of medical vs. surgical wards in CDI development we calculated CDI incidence in these wards, but we eliminated cases that likely developed an infection prior to hospitalization. In further calculations we took into account only the group which developed CDI >72 h after admission (medical wards, n = 266; surgical wards, n = 48) and compared them with the total number of all hospital admissions in these wards. We found that the incidence of CDI, which developed >72 h after admission, was statistically higher in medical wards than surgical wards (P < 0·001).
The correlation between number of CDI and characteristics of specific hospital wards (the number of beds, annual number of hospitalized cases, and average annual percentage occupancy) was established. There was a direct proportion between the number of beds and the number of CDI for medical wards (Table 3).
Table 3. The correlation between number of patients with Clostridium difficile infection hospitalized in a tertiary hospital in Krakow, Poland and the characteristics of wards

r, Correlation coefficient.
* P < 0·05.
Mortality analysis of patients with CDI is shown in Table 4, the periods for analysis were 14 and 30 days post-CDI diagnosis. The time span between diagnosis and death was shorter for patients who experienced the infection only once and also the percentage of deaths was higher in this group, irrespective of the time period in which the deaths were analysed. Moreover, the comparison (one-tailed test) between the age of the patients who died and the age of the whole group of infected patients showed that the mean age of the patients who died was significantly higher than the mean age of the infected patients in each of the analysed time periods (P < 0·01). The mortality rate was significantly higher in patients infected only once (Table 4).
Table 4. Mortality characteristics of patients with Clostridium difficile infection hospitalized in a tertiary hospital in Krakow, Poland
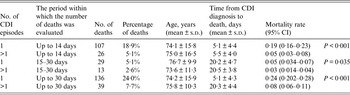
CDI, Clostridium difficile infection; CI, confidence interval; s.d., standard deviation.
DISCUSSION
CDI epidemic
The first reports of increasing CDI incidence came from North America [Reference McDonald, Owings and Jernigan9–Reference Pepin11]. Between 1996 and 2003, the rate of CDI in US hospitals remained stable at a rate of approximately 30–40 discharges/100 000 population, but nearly doubled to 60 discharges/100 000 population in 2003 [Reference McDonald, Owings and Jernigan9]. According to data provided by the Healthcare Cost and Utilization Project (HCUP) in the United States, based on the information from the disease list upon discharge, the prevalence of CDI increased from 3·82 to 8·75/1000 discharges between 2000 and 2008 [10]. A similar trend began to be observed in many European countries. In Austria, the incidence of CDI increased by 255% between 2003 and 2007 [Reference Indra12] and in Spain by more than 300% [Reference Asensio13]. In Korea, the prevalence of CDI grew from 1·7/1000 to 2·7/1000 adult admissions between 2004 and 2008 [Reference Kim14], a similar trend has been observed in China [Reference Huang15, Reference Huang16]. In Australia, Slimings et al. reported that the annual incidence of CDI increased from 3·25/10 000 patient-days in 2011 to 4·03/10 000 patient days in 2012 [Reference Slimings17].
In our study, a significant 6·5-fold increase of CDI in the period between 2008 and 2012 was observed. Sex and age distribution as well as the percentage of recurrent infections were comparable to the data from other regions of Europe and the United States. The incidence rate increased from 0·88/1000 admissions in 2008 to 5·12/1000 admissions in 2012, and then decreased to about 2·5–3·0/1000 admissions in subsequent years. Similar data are recorded from other centres, where, after an initial increase in incidence, appropriate procedures were implemented which resulted in decreased incidence of CDI. The average age of the patients with one CDI episode was lower than in patients with more than one episode (62·5 vs. 65·4 years, P = 0·048), which implies that age is a risk factor for CDI recurrence.
Age >65 years is one of the most important risk factor for CDI development. In a study conducted as part of the European Centre for Disease Prevention and Control (ECDC) project, Bauer et al. analysed data from 97 hospitals in 34 European countries; 63% of patients were aged >65 years and 56% were women [Reference Bauer8].
In our study, the majority of patients were aged >65 years; however, this preponderance was very small. Forty-seven percent of patients with CDI (n = 474) were aged ⩽65 years, which reinforces the fact that we cannot ignore the possibility of CDI in younger populations. Our study confirmed the age-related correlation between infection and death. The percentage of people aged >65 years who died was very high at 78·9%. The average age of the patients who died was significantly higher than in whole group of infected patients (P < 0·01).
The hospital problem
According to data provided by the Centers for Disease Control and Prevention (CDC, USA), as many as 94% of CDI patients have come into contact with healthcare services; this number includes hospitalized patients, outpatients, as well as nursing home residents [18]. Of all forms of contact, hospital treatment is identified as the greatest risk factor for the development of CDI. According to analysis of the HCUP database, sponsored by the Agency for Healthcare Research, nearly 1% of all hospitalizations in the United States were linked to CDI in 2009 [Reference Lucado, Gould and Elixhauser19]. The colonization rates of CD in adult hospitalized patients varies geographically. It is reported as 4·4% at admission in Canada, 7·9 % in the UK, 2·1–16·7% in the United States, and 20·0% in Taiwan [Reference Hung20–Reference Marciniak24]. However, the methodology of the studies are sometimes different, so it is not easy to compare their results. One of the most important factors is the moment of hospitalization when the specimen was diagnosed. Hung et al., in a prospective study, reported that prevalence of CD was 20·0% in hospitalized patients at an initial screening, with an additional 25·4% of patients testing positive during follow-up [Reference Hung25]. A similar observation was reported by Clabots et al., CD prevalence rate rose from 2·1% at initial screening to 50% after 1 month during follow-up [Reference Clabots23]. Johnson et al. showed that the rate of colonization was ~1% for patients hospitalized for up to 1 week and increased to ~50% in those hospitalized for >4 weeks [Reference Johnson26]. In Germany, CDI colonization in elderly patients in the community was just 0·8% but increased almost sixfold, to 4·6%, in nursing home residents [Reference Arvand27].
In this study we concentrated on the analysis of the incidence of infections in relation to the type of hospital ward. CDI concerns almost all hospital wards, yet such convincing predominance of infections in medical wards (88% vs. 12%) was a surprise to the authors of this study. The analysis of potential causes of this difference, allowed us to formulate three potential hypotheses. Patients of surgical wards are less mobile, which reduces their contact both with the ward and with other patients, who are often a source of spores. Moreover, patients who have recently undergone surgical interventions do not use the common toilets and the time period spent in such wards is usually shorter than in medical wards. The data showing the time span between hospital admission and a positive CDI test result are extremely important. According to the data analysed, 2/3 patients in our population developed CDI during their hospitalization even though they were admitted for different reasons.
Available data on the prevalence of CDI based on patient-days and discharges vary from one country to another and differ even between individual hospitals in the region. Barbut et al. reported a mean incidence of nosocomial CDI in 23 European hospitals as 2·45/10 000 patient-days [Reference Barbut28], whereas Bauer et al. reported it as 4·1/10 000 patient-days [Reference Bauer8]. Dubberke et al. reported that the incidence of CDI was 4·6 cases/1000 adult admissions between 2002 and 2007 in Canada [Reference Dubberke29]. Lupse et al. reported 37 cases/10 000 patient-days, and 26/1000 admissions were reported in Romania [Reference Lupse30]. In another Spanish study, Rodriguez-Pardo reported an average annual incidence of 1·93 episodes/10 000 patient-days and 1·22 episodes/1000 hospital discharges. The incidence rate ranged from 8·1/100 000 patients in the <15 years age group and 12·3/100 000 in the 15–65 years age group to 67/100 000 for patients aged >65 years [Reference Rodríguez-Pardo31].
One of the objectives of our analysis was the evaluation of whether the number of beds and annual number of hospitalizations reflect the risk of CDI, as, hypothetically, the larger number of patients in a ward, the larger the number of antibiotic therapies and potentially greater probability that individuals colonized with CD might introduce the spores into the hospital environment. Patient concentration in hospital might be important factor in antimicrobial selection pressure. It is known that antibiotic pressure has an impact on intestinal colonization [32.]. Boyer et al. observed that Pseudomonas aeruginosa acquisition was related to the proximity of a patient colonized with P. aeruginosa in the area (same room) with a chronological component (the previous day) along with selective antibiotic pressure [Reference Boyer33].
In our study the incidence rate was almost seven times higher for medical wards compared to surgical wards, yet it was highest for ICUs. The average annual proportion of bed occupancy reflects the patient concentration in the ward. First we assumed that the patient concentration in the ward would be a very important factor for the development of CDI. Our analysis revealed that there was a significant correlation between CDI and the number of beds in a ward, but there was no such correlation with the number of hospitalized patients and the patient concentration in the ward. The higher number of beds in a ward, the larger the ward is, so if there was no correlation with the number of hospitalizations, there had to be another factor which could explain the higher probability of CDI developing in large wards. We assumed that this factor was the higher number of staff, typical for large wards with a large number of beds. This can explain why there is a higher probability of CDI developing in larger wards. Every contact with a patient with CDI requires the use of disposable personal protective equipment – not only gloves, but also a gown. This could help decrease the likelihood of transmission of CD spores to other patients, even if they stay in a different room than the infected patient. Perhaps such management is even more important than complete isolation of patients infected with CDI in separate rooms, and the above correlations seem to confirm this fact.
ICUs
It is believed that that patients in ICUs, especially the elderly, are at significant risk of developing severe CDI. This infection also worsens the prognosis and hinders the treatment of other disorders, which were the original cause of admission to these wards [Reference Kazanowski34]. In a large cohort study (30-month study period, 2631 patients treated in the ICUs), Lawrence et al. reported that CDI was diagnosed in 4% of patients, 53% of whom had acquired CDI, with an incidence rate of 3·2 cases/1000 patient-days [Reference Lawrence35]. In Canada the mean incidence rate of CDI was 8·4 cases/1000 patient-days during a CD outbreak [Reference Beaulieu36]. In Brazil the average rate of CDI was reported as 1·8/1000 patient-days [Reference Balassiano37]. The incidence rate in Taiwan hospitals was found to be 0·45/1000 patients but 7·9/1000 patient-days in the ICU setting [Reference Lee38].
In our study 7·1% of all patients with CDI were hospitalized in ICUs. Our study confirmed that the ICU setting is particularly important in the epidemiology of CDI. The incidence and mortality rates in the ICU were several times greater than any other ward analysed.
Mortality
The methods of assessing CDI mortality differ from one study to another, the follow-up period after CDI diagnosis ranges between 14 and 90 days, whereas some studies do not specify the follow-up period at all. In the studies reviewed, a 14-day hospital mortality rate of 13%, 30-day hospital mortality rate of 10·5–30·9%, 60-day hospital mortality rate of 12·1%, and a 90-day hospital mortality rate of 29·5% are reported [Reference Huttunen39–Reference Cadena43]. CDI mortality in ICU patients is more than twice as high as in non-infected patients [Reference Vincent44, Reference Sidler45], and significantly higher than in patients treated in other wards. A 30-day hospital mortality rate of 29·5%, an in-hospital mortality rate of 25·1–33·9%, and an in-ICU mortality rate 19·7% have been reported [Reference Lawrence35, Reference Musa46–Reference Ang50].
In our study, when assessing mortality in patients with CDI, an attempt was made to evaluate from within what period from CDI confirmation, should the number of deaths be counted. In our study we selected 14- and 30-day periods for the analysis of mortality rates. It is difficult to state definitely within which period after CDI diagnosis, the infection is the factor leading directly or indirectly to death. The results of our analysis clearly show that the largest risk of death is specific to the patients infected for the first time when the time span between diagnosis and death is very short, amounting to 5·1 days on average. It seems therefore that the first 14 days from the diagnosis pose the highest risk of death and this is the period for which the mortality rate should be determined.
Moreover, CDI incidence is higher in medical wards, but it also comes with more than double the mortality rate. However, the highest mortality rate was observed in the ICU, twice as high as medical wards and four times as high as surgical wards. It is worthwhile to stress that the percentage of deaths was significantly higher in the group of patients who developed only one infection compared to those who had more than one infection, irrespective of the fact whether the deaths were evaluated within the period of 14 days (18·9% vs. 5·1%) or 30 days (24% vs. 7·7%).
A limitation of our study is that it is a retrospective study based on available data. We did not review data such as comorbidities or antibiotic treatments. The evaluation of CD strains using molecular methods is a recent introduction in our hospital. The strength of the study is the high number of assessed CDI cases. According to our PubMed search, our study is the first of its kind to present epidemiological data regarding the Polish adult population, especially mortality and characteristics of the studied population according to medical/surgical wards.
In conclusion, between 2008 and 2012 a 6·5-fold increase of CDI frequency was observed. Age is a risk factor for CDI recurrence. The majority of patients developed CDI during hospitalization. Medical wards pose a significantly higher risk of CDI than surgical wards. We suggest that the best period for the evaluation of the mortality rate related to CDI is the time within the first 14 days after diagnosis. In the case of patients who died, death occurred shortly after diagnosis (5·1 days on average in our study), hence delays in diagnosis must be eliminated with both causal and symptomatic treatment introduced as quickly as possible. The first CDI episode poses a much higher risk of death than consecutive ones.
ACKNOWLEDGMENTS
No funding was received beyond the usual salaries from our institutions.
DECLARATION OF INTEREST
None.








