INTRODUCTION
Bacillus species often contaminate heroin [Reference McLauchlin1]. Bacillus anthracis affecting people who inject drugs (PWID) was first observed in 2000 [Reference Ringertz2]. No further heroin-related anthrax occurred until an outbreak in 2009 of injectional anthrax (IA) occurred predominantly in Scotland [3]. PWID with confirmed, probable and possible IA (Table 1) occurred along with many PWID with similar presentations for whom testing for anthrax was negative. Since June 2012 sporadic IA has occurred both in the UK and elsewhere in Europe [Reference Health4]. IA has several recognized clinical manifestations [3, Reference Jallali5–Reference Knox14]. Confirmed IA has a mortality rate of 28% [3].
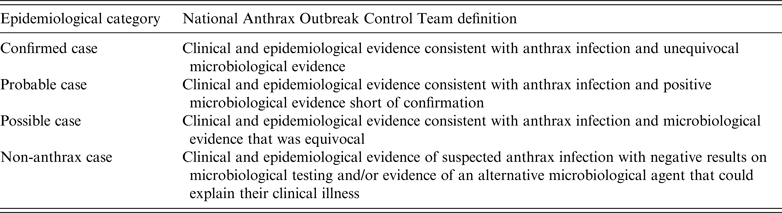
Table 1. Health Protection Scotland National Anthrax Outbreak Control Team case classification used in the 2009–2010 outbreak of IA [3]
METHODS
A database at Monklands Hospital used to store anthrax results issued by the Health Protection Agency Special Pathogens Reference Unit (SPRU) was consulted to identify confirmed, probable and possible cases of IA (as defined in Table 1) and then followed by case-note review and review of laboratory results.
RESULTS
Seven confirmed, five probable and nine possible IA cases were diagnosed; all of which survived. Seventy percent of the cases were men. The median age of females was 28 (range 21–33) years and for males 33 (range 24–54) years. The median duration of admission was 5 days (range 0–62 days).
Table 2 summarizes laboratory results obtained from SPRU and Table 3 summarizes details regarding the clinical features and bacterial co-infections. For only three confirmed and probable cases was the patient's bloodborne virus status known at presentation. During treatment six confirmed cases were identified as antibody positive for hepatitis C virus (HCV) although not all of these had polymerase chain reaction (PCR) evidence of active HCV infection.
Table 2. Summary of diagnostic microbiological test parameters used to identify cases of injectional anthrax
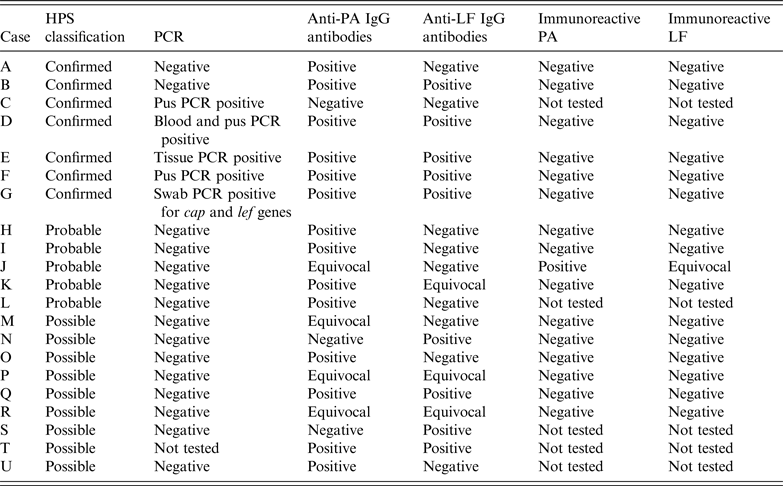
HPS, Health Protection Scotland; PA, Protective antigen; LF, lethal factor.
In case G, although the full three genes required for PCR diagnosis were not present on the swab obtained, anthrax was confirmed from debrided tissue when the patient had surgery in another health board. In case C serology was tested on blood taken on day 8 after admission which might account for the negative serology despite the case being confirmed by PCR if seroconversion had not yet taken place. Cases A and B were classed as confirmed on the basis of rising titres of anti-PA IgG several days apart. For case A, the negative PCR result relates to peripheral blood PCR as no tissue or pus was received for PCR. For case B a single wound swab was received and was negative by PCR but as the patient had multiple sites of soft tissue infection at the time it is possible that a lesion was swabbed that did not have B. anthracis detectable whereas if sampling had been performed for all the lesions a different one may have tested positive by PCR.
Table 3. Summary of clinical features of cases of injectional anthrax
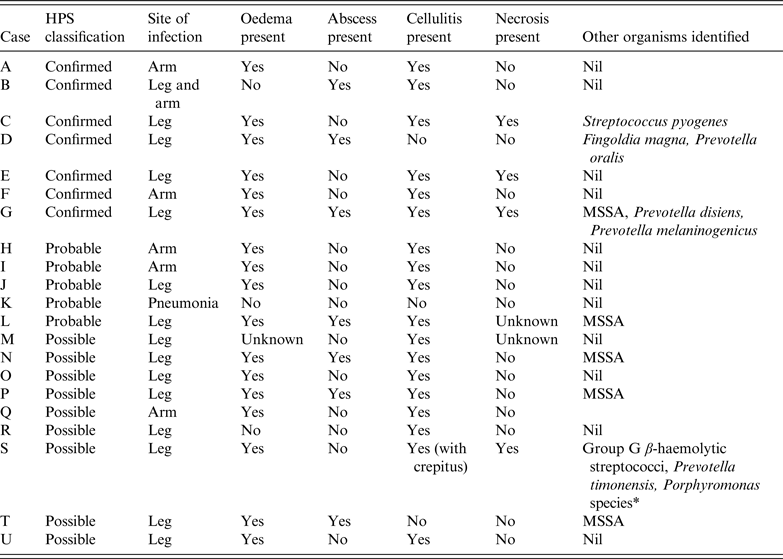
HPS, Health Protection Scotland; MSSA, methicillin-sensitive Staphylococcus aureus.
* Identified by Public Health Wales Anaerobe Reference Laboratory using 16 s rRNA sequence analysis but sequence analysis was unable to determine between Porphyromonas asaccharolytica and Porphyromonas uenonis.
Table 4 summarizes haematological and biochemical results of significance including parameters which proved helpful in identifying cases with more severe infection requiring greater levels of intensive care support and surgical intervention and which may help to elucidate the pathogenesis of IA and its spectrum of presentations.
Table 4. Summary of haematological and biochemical parameters for injectional anthrax cases
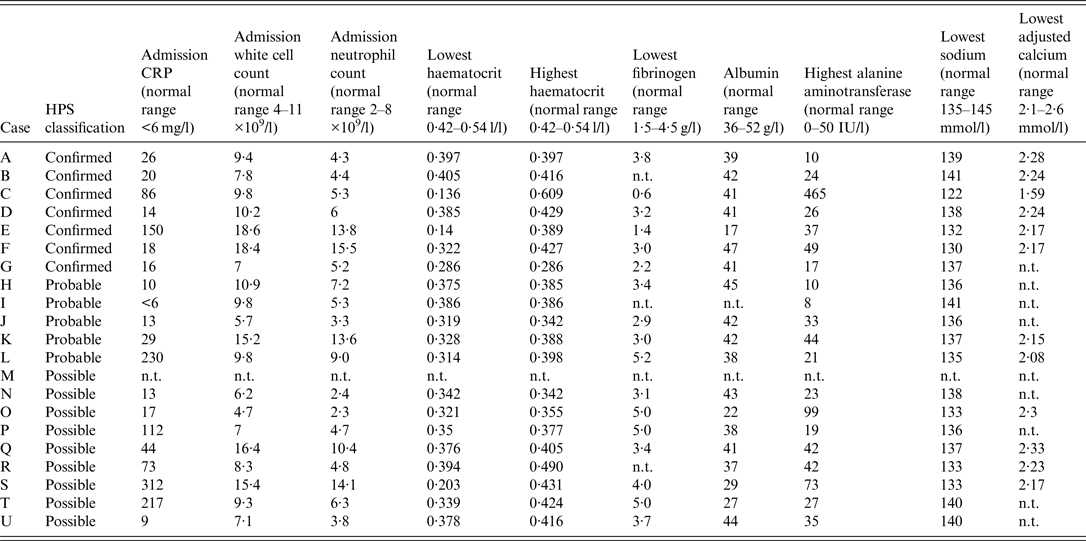
HPS, Health Protection Scotland; CRP, C-reactive protein; n.t. not tested.
DISCUSSION
Clinical observations
Spectrum of presentations and clinical features
All confirmed IA cases presented with soft tissue infection and features distinct from classical cutaneous anthrax and necrotizing fasciitis (NF) [Reference Booth15]. No cases of intracranial haemorrhage occurred. Some descriptions of IA note necrosis [Reference Jallali5–Reference Powell7] while others do not [Reference Knox14]. Pleural effusions have been described as present [Reference Jallali5, Reference Grunow13] or absent [Reference Parcell6, Reference Johns8, Reference Knox14]. The presence [Reference Parcell6, Reference Powell7, Reference Grunow13] or absence of fever [Reference Johns8, Reference Grunow13, Reference Knox14] may be seen.
In case C, despite thigh fasciotomies there remained gross limb oedema and skin petechiae. Wounds were affected by blistering golden vesicles and desquamation. Thigh oedema worsened with new suprapubic and genital oedema and new vesicles over 24 h associated with worsening coagulopathy and thrombocytopenia. After further debridement and prolonged intravenous antibiotics, the fasciotomies healed with limb salvage although this took over 40 days.
Vesicles are characteristic features of cutaneous anthrax [Reference Sweeney16] and have been described in IA [Reference Grunow13, Reference Knox14] despite a review suggesting they do not occur [Reference Sweeney16]. In case C, vesicles manifested with substantial oedema and resembled impetigo [Reference Knox14] although culture was sterile. Microscopy of vesicular fluid demonstrated no bacilli and vesicle PCR was negative for herpes zoster and herpes simplex.
Cases B and K did not have oedema. Case K did not have soft tissue infection but had pneumonia although serology suggested recent exposure to anthrax. Oedema is a common feature of IA [3] but is not always present.
Case E had a swollen, tender, dusky injection site with central oedematous tissue but no drainable collection and no crepitus. Other injection sites on the limb were necrotic but not oedematous. Case F had significant limb oedema with few surface changes except cellulitis.
The site of infection is not always the site of injection [Reference Knox14]. The oedema and abscess in case D was where concealed heroin was in contact with skin under clothing and not at a site of injection. IA was confirmed by PCR performed on a swab taken at a General Practice surgery. Of note, case D avoided admission for 6 days and was managed cautiously in the community with oral clindamycin. When admitted, incision and drainage of a small abscess was performed. There was minimal oedema, no necrosis but an impetigo-like appearance.
Neither case A nor case B required debridement and were managed almost entirely with oral antibiotics when continued admission was declined.
Most deaths in confirmed IA are before day 10 after presentation [3, Reference Johns8]. Confirmed and probable cases usually have an incubation period of <1 day [3]. Protective immunity may develop 14 days after vaccination [Reference Hicks17]. The most severe patients we managed improved by day 15 so protective immunity in IA patients may also develop around this time.
Co-infections
Staphylococci may inhibit growth of B. anthracis in cutaneous lesions [Reference Brentall18]. Co-infection may stimulate the host immune system and oppose immunosuppressive effects of anthrax toxins [3]. Cases G, L, N, P and T were co-infected with Staphylococcus aureus and had mild IA. Case C had severe IA co-infected with Streptococcus pyogenes yet survived. Cases D, G and S had co-infection with anaerobes which could account for the clinical features more like NF of case S. It is possible that bacterial co-infection may improve survival from IA.
Co-infection with HCV generally did not cause hepatic decompensation although fatal liver failure during IA and HCV co-infection has been described elsewhere [Reference Grunow13, Reference Holzmann19].
Injecting partners
We investigated individuals who knew they injected the same infected heroin (some from the same syringe) as two severe, confirmed IA cases. One contact had soft tissue abscesses but tested negative for anthrax, highlighting the difficulty in differentiating anthrax and non-anthrax infection even after exposure to infected heroin. Contacts who tested negative had not taken antibiotics, suggesting there may be a protective host factor, but antibodies to anthrax were not detected. These individuals did not receive post-exposure prophylaxis with ciprofloxacin as indicated in inhaled anthrax [Reference Hart and Beeching20], yet they did not develop any anthrax even after 60 days. The risk of infection from injected anthrax may be less than from inhaled anthrax.
Cases B and I both injected the same batch of heroin and both developed signs of IA. Had it been possible to obtain follow-up serology from case I they might have been classifiable as confirmed.
Laboratory diagnosis
Culture
No specimens cultured B. anthracis despite procedures identical to laboratories that grew anthrax. For several patients antibiotics were necessary prior to debridement. Sometimes antibiotics were administered prior to blood culturing. The lack of sampling prior to antibiotics may explain negative blood cultures. One patient had been self-medicating with amoxicillin prior to presentation. It is unclear if antibiotic self-medication was widespread locally in PWID but this could explain the less severe clinical presentations and the absence of B. anthracis bacteraemia.
PLET (polymixin, lysozyme, EDTA, thallous acetate) agar selects B. anthracis and inhibits growth of contaminating organisms. It proved difficult to obtain and was not used. However, overgrowth with contaminating organisms did not occur and cultures using Columbia blood agar were often sterile.
PCR
PCR was considered positive if three B. anthracis genes were detectable – lef (lethal factor on plasmid pXO1), capA (capsular gene on plasmid pXO2) and bagC (a genomic gene). Blood, body fluids, swabs or tissue were tested. This became the mainstay of confirming IA when cultures were negative.
Serology
In cases A and B paired sera showing rising anti-PA IgG titres suggesting recent anthrax exposure enabled classification as confirmed rather than probable cases. Both cases had soft tissue infection but had minimal contact with medical services. It proved easier in these circumstances to obtain blood for follow-up serology than blood cultures, tissue, pus or swabs for culture and PCR.
Histopathology
Debrided tissue was available from three confirmed IA cases. For two, abundant necrosis was identified within the deep subcutaneous tissue. Although non-specific, these features were similar to conventional NF. In the remaining case, superficial tissue was preferentially debrided. Small amounts of necrosis were seen but the prominent feature was oedema in the superficial dermis (Fig. 1) similar to that reported in cutaneous anthrax [Reference Mallon and McKee21]. Gram-positive bacilli or spores were not identified in any cases suggesting toxin-mediated tissue damage although Gram-positive bacilli have been visible in tissue from other IA cases [Reference Parcell6]. The different manifestations may relate to the relative proportions of oedema and lethal toxin that are produced.

Fig. 1. Haematoxylin and eosin stained section of debrided skin from a patient with confirmed injectional anthrax, illustrating significant subepidermal oedema. No Gram-positive bacilli were visible in the tissue.
Antibiotic management
A five antibiotic empirical regimen was advocated [22] based on previous experience of polymicrobial soft tissue infections in PWID [Reference Brett23–Reference McLauchlin25]. The identification of polymicrobial infections (Table 3) supported this strategy.
Optimal treatment [26] was not always achieved due to patient non-compliance. In such cases, 14 days of oral clindamycin and ciprofloxacin was offered. In mice, clindamycin and ciprofloxacin combined may increase mortality in anthrax [Reference Brook27] but we experienced no adverse events using this combination in humans.
Avoiding debridement risks relapse if spores are retained. None of the patients who did not have debridement relapsed despite only 14 days treatment. One confirmed case had continued wound oozing after 14 days treatment and wound fluid remained PCR positive for B. anthracis on day 15. As a precaution, the duration of treatment was extended.
Case C reported a penicillin allergy and had severe disease that progressed despite intravenous (IV) ciprofloxacin and clindamycin. They received 6 mg/kg IV daptomycin rather than IV vancomycin. Daptomycin is advocated for septic PWID [Reference Seaton28] and evidence in mice supports its use treating anthrax [Reference Heine29]. It is licensed for use in complicated skin and soft tissue infection due to Gram-positive organisms. This patient was co-infected with B. anthracis and S. pyogenes and risked amputation which they were unlikely to survive. A combination of daptomycin, ciprofloxacin, clindamycin, meropenem and metronidazole in addition to cautious surgery resulted in full recovery both of the patient and the infected limb. Daptomycin is bacteriocidal through calcium-dependent insertion of pores into Gram-positive cell membranes resulting in potassium efflux and membrane depolarization without lysis of the bacteria resulting in minimal exotoxin release [Reference Wiedemann30]. Penicillins cause cell lysis. The minimizing of exotoxin release may be relevant in anthrax infection which is predominantly driven by two exotoxins. Daptomycin also crosses the blood–brain barrier and could therefore treat IA with meningeal involvement [26], albeit it is inactive in pulmonary infections.
Non-antibiotic adjunctive treatment
Some non-antimicrobial therapies have activity against anthrax toxins [Reference Artenstein31]. None of these drugs were used. Indomethacin and other anti-platelet drugs were considered inappropriate as patients were often thrombocytopenic and haemorrhagic.
Possible exacerbation of haemodynamic instability was felt to be too great a risk for calcium channel antagonists to be used. Additionally, case C was hypocalcaemic throughout the first week of treatment [lowest calcium measured was 1·64 mmol/l on day 6 (normal range 2·1–2·6 mmol/l)] and was receiving infusions of 10% calcium gluconate almost daily (11 bolus infusions of 10 ml of 10% calcium gluconate administered in the first 7 days). It was counterintuitive to replace calcium and administer calcium antagonists concurrently. Calcium replacement was considered important while daptomycin was being administered. No cases received anthrax immunoglobulin.
Surgical management
Initial expert advice was to excise affected skin with a >2 cm margin, remove any areas of subdermal fat that appeared altered and to widely excise any needle track identified in muscle [26].
Cases C and F exhibited compartment syndrome and had fasciotomies to improve tissue perfusion and antibiotic delivery. Review was performed at 12 h and 24 h post-operatively and then on a daily basis for worsening oedema or new skin changes. In case E despite an initial 10 cm excision margin, further debridement was required and wound oozing was substantial in cases C, E and F.
Because IA does not always present as NF, radical debridement may not always be necessary. Latterly a more cautious surgical approach was promoted when necrosis was absent [Reference Jallali5]. Where there was obvious tissue necrosis, we opted for debridement and frequent wound inspection. Repeat debridement in case C was purposefully superficial as deeper tissue appeared healthy. Wounds in case C were dressed with non-adherent materials [oxidized cellulose polymer to promote haemostasis (Surgicel®, Ethicon Inc., USA), paraffin gauze (Jelonet®, Smith & Nephew Healthcare, UK), viscose and rayon absorbent pad with adhesive border (Primapore®, Smith & Nephew Healthcare)] and wool and crepe bandage pressure dressings were used to reduce limb oedema. In case D, where there was no significant oedema, Kaltostat® (ConvaTec, UK) dressings were used. Alginate and antiseptic dressings have been used in other places with good results [Reference Grunow13]. Similarly to other cases [Reference Powell7, Reference Grunow13], two of our patients had negative pressure wound therapy using a Venturi™ (Talley Group, UK) wound sealing kit. It was used after 14 days treatment had elapsed to minimize the risk of aerosolizing viable B. anthracis if the system was dislodged in the first 14 days.
Both patients managed in an intensive care unit (ICU) with confirmed anthrax had pleural effusions and one had significant ascites. These can be reservoirs of anthrax toxins [Reference Booth15, Reference Hicks32]. However drainage was not performed. In one patient because the infection was managed prior to guidance advising drainage being issued and in the other, chest drain insertion was considered too great a risk given the degree of coagulopathy and haemodynamic instability.
Intensive care management
Management of shock
Two of the confirmed cases had shock requiring fluid resuscitation and transfusion of blood products. Case C responded to fluids alone (with 6 l of colloid and crystalloid) whereas case E required noradrenaline infusions for 4 days. Both had increased cardiac output despite lethal toxin reducing cardiac output in monkeys and dogs [Reference Hicks32–Reference Sweeney34]. This was unusual as many cases of IA requiring ICU support described elsewhere had vasopressor-resistant shock [Reference Booth15] postulated to be a consequence of oedema toxin [Reference Hicks17]. Our patients had significant limb oedema and pleural effusions (likely due to oedema toxin), but did not experience vasopressor-resistant shock. This was fortunate as its development appears to be fatal [Reference Grunow13, Reference Booth15].
Management of coagulopathy and haemorrhage
Cases C and E required substantial blood products replacement. Within the first 14 days, case C required 23 U packed red cells, 22 U platelets, 30 U fresh frozen plasma (FFP) and eight pools of cryoprecipitate. Within the first 17 days, case E required 25 U packed red cells, 6 U platelets and 4 U FFP (Fig. 2). These requirements are similar to other cases where up to 37 U blood were transfused [Reference Parcell6]. A drop in fibrinogen was noted in cases C and E (Table 4) possibly from dilution secondary to fluid resuscitation and consumption from disseminated intravascular coagulopathy.
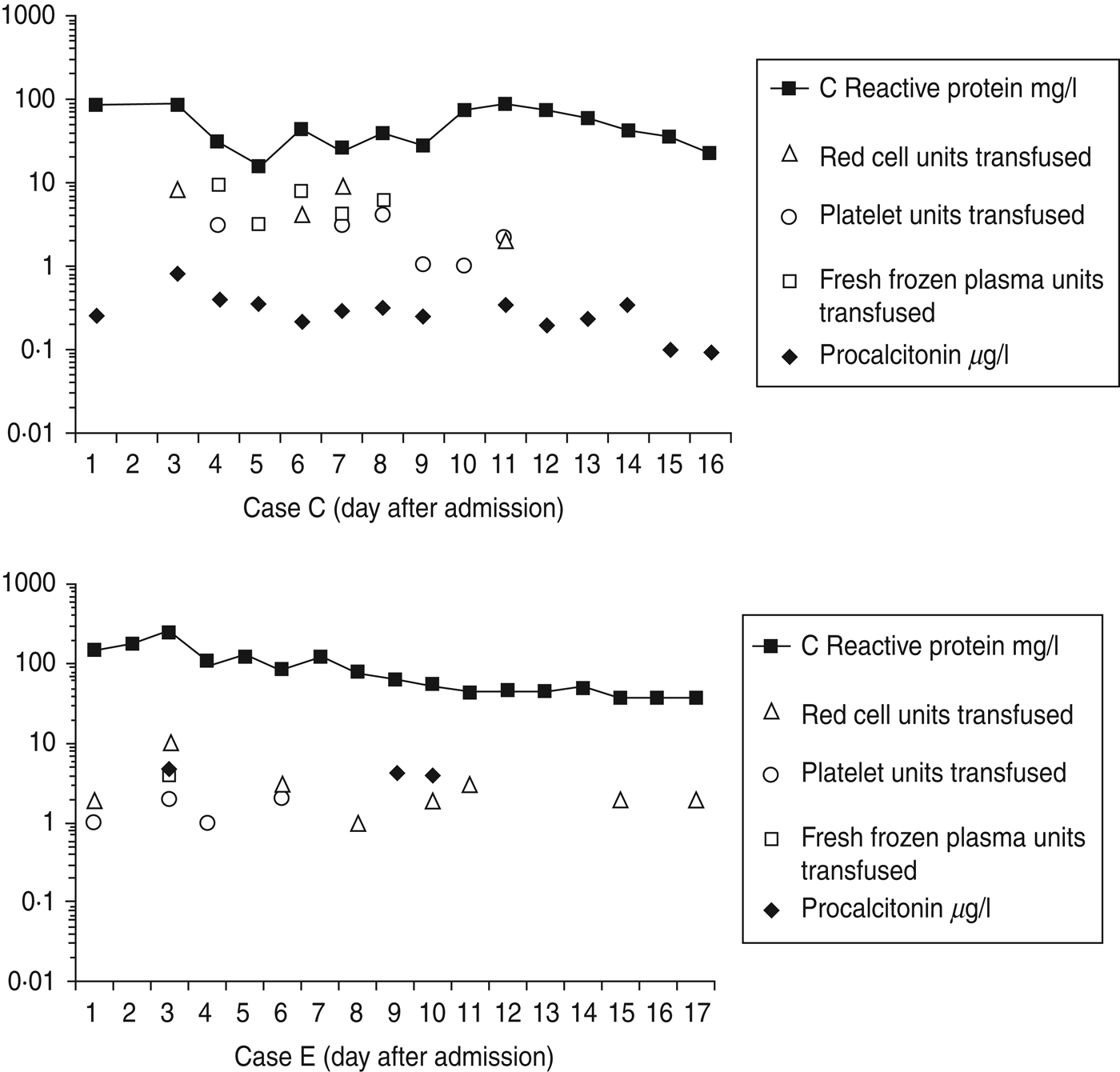
Fig. 2. Timelines showing serial C-reactive protein measurements and serial procalcitonin measurements for two different cases of confirmed injectional anthrax, requiring intensive care management, during the first 17 days of treatment. Daily requirements of blood products (units of packed red cells, platelets and fresh frozen plasma) are also included to illustrate disease severity and response to treatment with regard to progression and resolution of coagulopathy.
Haemoconcentration has been observed in anthrax-associated sepsis when the route of infection has been cutaneous, gastrointestinal or inhalational [Reference Hicks32], but was seen only initially in case C during the first 48 h. It then fell, possibly due to dilution from the volume of fluid required to manage shock or as a consequence of anthrax toxins. Paradoxically, lower than normal haematocrit levels were observed in all cases during treatment even although IV fluids were not administered to all cases (Table 4).
Case C received other measures to improve their coagulopathy, including three doses of 10 mg IV vitamin K within the first 4 days and six doses of 1 g IV tranexamic acid within the first 6 days. In view of significant peri- and post-operative bleeding, 10 μg IV desmopressin was also given on days 2 and 6.
Hyponatraemia
Case C was hyponatraemic [sodium 134 mmol/l (normal range 135–145 mmol/l)] on admission and this fell to 124 mmol/l prior to the commencement of fluids. Hyponatraemia in inhalational anthrax has been described but it was not known how often this occurred prior to fluid resuscitation [Reference Hicks32]. Hyponatraemia may help differentiate inhalational anthrax from community-acquired pneumonia [Reference Kuehnert35]. Moreover, hyponatraemia has been associated more with NF than non-necrotizing soft tissue infection [Reference Wall36]. All our confirmed cases that required high dependency or intensive care support had hyponatraemia prior to fluid resuscitation or had hyponatraemia without receiving fluid resuscitation. Of the less severe IA cases, hyponatraemia was less common.
This absence of hyponatraemia is in keeping with cases elsewhere that did not require ICU support [Reference Knox14] while cases requiring ICU support presented with hyponatraemia [Reference Johns8, Reference Booth37]. It has been observed that infusion of oedema toxin in dogs reduces sodium levels [Reference Sweeney34] so it is possible that the hyponatraemia we observed in our patients is an effect of oedema toxin independent of the dilutional effects of fluid resuscitation. Potentially, hyponatraemia in a septic PWID individual could be used as a marker to indicate possible IA requiring greater support.
Procalcitonin
Procalcitonin was tested in confirmed cases C and E (Fig. 2) and possible cases R and U. In case C procalcitonin remained low suggesting ongoing infection was unlikely despite uncontrolled coagulopathy, further debridements, rising C-reactive protein (CRP) and deterioration on day 9 from secondary bacterial infection. In case E procalcitonin was raised suggesting infection and did not change over the first 10 days of treatment despite a falling CRP, reducing requirements of blood products and improvement. Case R had an initial procalcitonin level of 4·4 μg/l and in case U it was < 0·05 μg/l. A German who died from IA with septic shock, coagulopathy and multi-organ failure had a single procalcitonin measurement reported of 1·05 ng/ml [Reference Grunow13, Reference Holzmann19]. Procalcitonin testing was not useful either in identifying infection or monitoring response to treatment.
Release of procalcitonin is triggered by pro-inflammatory cytokines interleukin (IL)-1β, tumour necrosis factor (TNF)-α and IL-6 during bacterial infections [Reference Shuetz, Albrich and Mueller38]. B. anthracis lethal toxin suppresses TNF-α in macrophages, T cells and dendritic cells, suppresses IL-1β in macrophages and dendritic cells and suppresses IL-6 in dendritic cells [Reference Baldari39]. Procalcitonin may be low in IA as the cytokine pathways which require stimulation to release procalcitonin may be being suppressed by anthrax lethal toxin.
CONCLUSIONS
We describe a series of patients with IA who presented to Monklands Hospital for diagnosis and management with 100% survival. Clinical presentations varied from mild soft tissue infection to multi-organ failure, shock and coagulopathy. These cases highlight the difficulty in differentiating IA from non-anthrax-related soft tissue infection. Our clinical management of IA at times, out of clinical necessity, deviated from expert opinion, or scenarios were encountered where there was no agreed expert opinion. For instance, whether to use a less aggressive approach to surgical debridement, optimal post-operative wound management, management of severe coagulopathy, management of mild cases with oral antibiotics and what we believe to be the first description of using daptomycin to treat human anthrax. Some cases had IA plus co-infection with other bacteria or HCV. Additionally we report observations regarding some routine laboratory tests, such the sodium level, which may be useful biomarkers of severe IA.
ACKNOWLEDGEMENTS
The authors thank all staff of Monklands Hospital who have been involved in the diagnosis and management of these patients. In particular from the Department of Infectious Diseases (Drs A. Todd, N. Kennedy, S. Dundas, C. McGoldrick), from the Intensive Care Unit (Drs S. Marshall, D. Clough, S. Chohan, M. MacKinnon, R. McKenzie), from the Department of Orthopaedics (Drs A. Campbell, K. Cheng, B. Singh, S. Sinha, D. Bramley), from the Department of General Surgery (Drs A. MacDonald, T. Salem), from the Department of Microbiology (Dr P. Hunter, Dr P. Robertson, H. Robertson, I. Winning, R. Yates, E. Kilgour, K. Gordon), from the Department of Pathology (Dr M. Agarwal) and the Infection Control Team (L. Thomas, R. Fox). The Anaerobe Reference Unit, National Public Health Service for Wales, Cardiff for identification of anaerobes and the Health Protection Team, Department of Public Health, NHS Lanarkshire.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.









