It is projected that 7·7 % (439 million) of all adults in the world will have diabetes mellitus by 2030, with type 2 diabetes mellitus (T2DM) representing the major component of this epidemic( Reference Chen, Magliano and Zimmet 1 , Reference Passa 2 ). In Denmark, there are currently 10 000–20 000 new cases of T2DM each year, and the prevalence of known diabetes is 4·5 %( Reference Thomsen, Friborg and Nielsen 3 , Reference Carstensen, Kristensen and Ottosen 4 ).
T2DM is a complex group of metabolic disorders characterised by hyperglycaemia and impaired insulin action and/or insulin secretion( Reference Lin and Sun 5 , Reference Das and Elbein 6 ).
Human and animal studies have suggested an association between vitamin A deficiency and onset of T2DM, and vitamin A has also been demonstrated to impact β-cell production and insulin resistance( Reference Trasino, Benoit and Gudas 7 – Reference Brun, Grijalva and Rausch 9 ).
Vitamin A deficiency affects about nineteen million pregnant women, mostly from low- and middle-income countries. Vitamin A is mainly known for its role in vision among both children and adults. However, through its role in gene regulation, vitamin A is also involved in fetal development, organogenesis, limb formation and body symmetry(10, Reference Elmadfa and Meyer 11 ). Furthermore, data from animal studies suggest that vitamin A may be involved in fetal pancreatic cell development, and hence a low level of fetal vitamin A may be related to risk of T2DM in adult life( Reference Matthews, Rhoten and Driscoll 12 , Reference Öström, Loffler and Edfalk 13 ).
However, until now, there have been no studies exploring associations between gestational vitamin A exposure and T2DM risk in adulthood, as such studies require long-term follow-up of the offspring of mothers with different vitamin A statuses or intakes, and such studies are usually too difficult to conduct. On 1 March 1962, however, the amount of mandatory vitamin A added to margarine in Denmark was increased by 25 %, from 4·2 to 6µg and 3·6 to 3µg of retinol and β-carotene, respectively. Alongside access to information on health outcomes from all Danish individuals in national health registers, this change in fortification practice provided the possibility of examining whether gestational exposure to extra vitamin A may be related to later-onset diseases( 14 , 15 ). The aim of the present study was to analyse whether exposure during fetal life, to extra vitamin A from food fortification, was related to subsequent risk of developing T2DM in adulthood (before age 49 years).
Method
Study population
The study population, as extracted from the Danish Civil Registration System( Reference Pedersen 16 ), included individuals born in Denmark during similar time windows before and after the national vitamin A fortification policy change in 1962. Fortification of margarine with vitamins A and D became compulsory in Denmark in 1937. A ministerial order that increased the amount of vitamin A added to margarine by 25 % from 4·2µg/g of retinol and 3·6µg/g of β-carotene (equivalent to 0·6 % of the current RDA) to 6µg/g of retinol and 3µg/g of β-carotene (equivalent to 0·8 % of the current RDA) was introduced in 1961 and subsequently implemented from 1 March 1962( 14 , 15 ). (RDA for vitamin A during pregnancy: 770 retinol activity equivalents (RAE). 1µg RAE corresponds to 1µg retinol and 12µg β-carotene( 17 , 18 ).) Meanwhile, the mandatory vitamin D content was unchanged at 0·125µg /g margarine. Data from food disappearance show that an average of 18 kg margarine/person per year was bought in Denmark in the 1960s, suggesting that personal daily intake from fortified margarine increased from 207 to 296 µg of retinol and decreased from 178 to 148 µg of β-carotene( Reference Fagt and Trolle 19 ).
Thus, the less-exposed group included individuals born before the introduction of the ministerial order, born between 1 September 1959 and 31 December 1960. Individuals born in 1961 were excluded as it is unclear whether the increase in vitamin A added to margarine already became partially effective between 1961 and 1962. A washout period of 9 months from when the ministerial order became effective was applied to define the exposed group to include those born between 1 December 1962 and 31 March 1964. Using the Danish civil registration number, individuals were linked to the Danish National Diabetes Register (NDR)( Reference Carstensen, Kristensen and Marcussen 20 ) combined with the National Patient Register (NPR)( Reference Lynge, Sandegaard and Rebolj 21 ) in order to obtain information on their diabetes outcomes and were followed up until, at the latest, 31 December 2012. Diabetes diagnoses in the NDR registry are considered reliable from 1 January 1995( Reference Carstensen, Kristensen and Ottosen 22 ); therefore, as individuals in our study were earliest born on 1 September 1959, the age of 36 years was chosen as the start of follow-up.
Type 2 diabetes mellitus definition
As NDR does not contain disease codes that would allow type 1 diabetes mellitus (T1DM) and T2DM differentiation, and NPR only contains T2DM cases that were identified via patient discharges from hospitals, we merged the information from the two registers. Individuals were defined as T2DM cases if they were alive, at risk and diabetes free until age 36 years and had a first diagnosis of diabetes corresponding to International Classification of Diseases (ICD) 8 code 250 or ICD 10 code E11 between 36 years and 48 years and 9 months (48·75 years) of age. In addition, individuals were also classified as having T2DM when one of the following criterion as first diagnosis, combined with a following diagnosis of ICD 10 code E11, was met( Reference Carstensen, Kristensen and Marcussen 20 ):
-
∙ chiropody for diabetic patient;
-
∙ date of fifth blood glucose measurement within a year;
-
∙ second purchase of oral glucose-lowering drugs; and
-
∙ second purchase of insulin.
Censoring at the age of 48·75 years was fixed throughout, for all applicable individuals, to have comparable ages-at-risk spans in both exposed and less-exposed groups.
Sensitivity analyses using slightly different T2DM definitions were performed.
Statistical methods
Logistic regression analyses were performed. Analyses were adjusted for sex. We tested for interactions between exposure and sex by Wald’s test. OR and 95 % CI, corresponding to a level of significance as defined using P≤0·05, were used. Additional analyses with different age of inclusion cut-offs were also performed to assess the effect of the fortification among different age groups: 40 and 44 years old (Fig. 2; online Supplementary Table S1).
Furthermore, Cox regression analyses were also performed, as sensitivity analyses, with age as the underlying time-scale, and hence, incorporating the exact age-of-diagnosis, facilitating comparisons of hazards of T2DM between exposed and less-exposed individuals (online Supplementary Table S3).
The statistical software package Stata 13 (StataCorp LP www.stata.com) was used for all data management and analyses.
Results
Among the 193 803 included individuals, 101 178 and 92 625 were exposed and less-exposed, respectively, to the higher extra levels of vitamin A prenatally. Among the exposed, 1273 developed T2DM, whereas among the less-exposed 1322 developed T2DM (Table 1; Fig. 1). There were slightly more male (52 %) than female (48 %) cases in both the exposure groups. The mean age for developing T2DM was 43·2 (sd 3·5) and 43·6 (sd 3·4) years in the exposed and less-exposed groups, respectively (P=0·15).

Fig. 1 Flow chart logistic regression. T2DM, type 2 diabetes mellitus.
Table 1 Characteristics of individuals born in Denmark between September 1959–December 1960 (less-exposed) and December 1962–March 1964 (exposed) (Numbers and percentages)
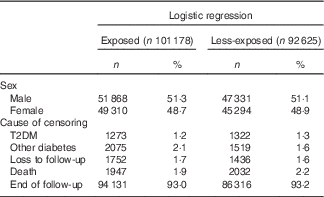
T2DM, type 2 diabetes mellitus.
The individuals exposed to higher vitamin A from fortification were less likely to develop T2DM (OR 0·88; 95 % CI 0·81, 0·95, P=0·001) (Table 2). Furthermore, the effect of the fortification was stronger for higher age (Fig. 2; online Supplementary Table S1). Women were generally at a lower risk of developing T2DM than men (OR 0·70; 95 % CI 0·65, 0·76, P<0·001). However, there was no interaction between exposure status and sex (OR 0·94; 95 % CI 0·81, 1·10, P=0·47), suggesting that substantial effect modification by sex was unlikely. Results from the supplementary survival analyses showed similar patterns (online Supplementary Table S3). The majority of sensitivity analyses, using slightly different T2DM definitions, also showed similar results (online Supplementary Table S2).

Fig. 2 OR of developing type 2 diabetes mellitus between 36, 40, 44 and 48·75 years of age among individuals exposed to extra vitamin A in utero.
Table 2 Logistic regression of fetal exposure to extra vitamin A and type 2 diabetes mellitus (Odds ratios and 95 % confidence intervals)

Discussion
This study suggests that fetal exposure to extra vitamin A from fortified margarine may have lowered the risk of developing T2DM in adulthood, and that this effect was similar for men and women and stronger for those developing T2DM at an older age.
To our knowledge, this study is the first ever to report long-term influences of fetal exposure to extra vitamin A on the risk of developing T2DM in adulthood. Our findings are supported by one previous longitudinal study conducted in Nepal, which reported that in utero exposure to extra vitamin A ensured higher natural antibodies concentration among preadolescent boys and girls, a finding that may have implications for inflammatory diseases development, including T2DM in later life( Reference Palmer, Schulze and Khatry 23 ). Indeed, previous studies have shown that elevated levels of inflammatory markers IL-6 and C-reactive protein are involved in the development of T2DM( Reference Spiegler, Kim and Wassef 24 , Reference Ng, Ma and Leung 25 ).
Mechanisms
Multiple factors affecting early growth have been suggested to operate changes in the structure and function of certain organs and tissues, such as the pancreas( Reference Nielsen, Haase and Jaksch 26 , Reference Vaag, Brøns and Gillberg 27 ). Vitamin A is essential for mammalian reproduction and is transferred to the fetus through the placenta( Reference Debier and Larondelle 28 ). Animal studies suggest that vitamin A is required for endocrine function, β-cell neogenesis and replication as well as maintenance of normal pancreatic islet architecture during both fetal life and adulthood( Reference Trasino, Benoit and Gudas 7 , Reference Matthews, Rhoten and Driscoll 12 , Reference Christian and Stewart 29 ). Indeed, Öström et al.( Reference Öström, Loffler and Edfalk 13 ) showed that fetal pancreatic development requires retinoid acid (RA) receptor, and that the expression of RA-synthesising enzyme (Raldh 1) coincides with the stages when β-cells are normally generated in embryonic mammalian pancreas. Another animal study previously suggested that vitamin A (retinol) deficiency during gestation decreases offspring β-cell mass and contributes to impaired glucose tolerance in adulthood( Reference Matthews, Rhoten and Driscoll 12 ). Furthermore, vitamin A has antioxidant and anti-inflammatory functions, and through its role in gene regulation it is involved in fetal development and internal organ formation. Evidence also suggests that, during fetal life, vitamin A is essential for the development of B lymphocytes that have a role in immune-mediated and inflammatory diseases such as T2DM( Reference Palmer, Schulze and Khatry 23 ).
There was a change in both the absolute amount of vitamin A added to margarine as well as the retinol: β-carotene ratio – with an increase of retinol from 4·2 to 6µg/g and a decrease of β-carotene from 3·6 to 3µg/g between the two exposure groups( Reference Fagt and Trolle 19 ). Although maternal–fetal transfer of retinoids and carotenoids is not yet well understood, studies suggest that retinol crosses the placenta, whereas β-carotene may be a precursor of retinol in the placenta( Reference Spiegler, Kim and Wassef 24 ). Hence, it can be postulated that retinol may be more readily available to the fetus. Whether the retinol:β-carotene ratio change may have contributed to lowering the risk of T2DM in adulthood requires further basic studies.
Vitamin A was added to margarine together with vitamin D. Both vitamin A and D can pass through the cell membrane by binding with RA receptor and vitamin D receptor, respectively, and both receptors can heterodimerise with the retinol X receptor. Therefore, vitamin A and D may interact in an additive, synergistic or antagonist manner. Indeed, in relation to the development and functioning of human fetal pancreas, a study from 2011 suggested that both vitamins may be involved in pancreatic progenitor cell development and viability( Reference Ng, Ma and Leung 25 ). Thus, it is possible that the protection from the 25 % increase in vitamin A from vitamin A- and D-fortified margarine on the development of T2DM reported in this study may be due to a more beneficial vitamin A:D ratio. However, further investigation is needed to uncover the regulatory mechanisms of the effect of both vitamins in human fetal pancreatic development. Indeed, most of these mechanisms are still speculative and more studies are needed to unveil the role of gestational vitamin A, and its interaction with vitamin D, on the development of T2DM in humans.
Owing to its teratogenicity, intake of retinol during pregnancy should be limited and not exceed the current RDA of 770 RAE. Indeed, studies have shown an increased risk at intake levels from ≥3000 RAE/d of vitamin A, mainly from medication (i.e. acne treatment with isotretinoin) or inadequate supplementation( 17 , Reference Browne, Mason and Tang 30 , Reference Dolk, Nau and Hummler 31 ). Margarine was fortified with 6µg/g of retinol and 3µg/g of β-carotene only, and it is unlikely that such small amounts could cause any teratogenic risk.
The current daily intake of vitamin A among adults in Denmark is estimated to be 1326 (sd 994) µg RAE, which is above the recommended daily intake of 770 µg RAE/d for pregnant women( Reference Pedersen, Christensen and Matthiessen 32 ). Therefore, fortification of margarine with vitamin A in the current Danish setting might not be necessary. However, our results might be useful in prevalent vitamin A deficiency contexts such as some low- and middle-income countries were T2DM incidence is raising.
Strengths and limitations
The main strengths of this study lie in its longitudinal design with the possibility of following-up individuals into midlife and the inclusion of more than 190000 individuals. This study is also the first of its kind to examine health effects of vitamin A fortification, made possible by both the well-defined adjacent time window of margarine fortification changes with vitamin A in Denmark and the complete registration of every citizen via a civil registration number into the Danish national health registries. Furthermore, to our knowledge, this study is also the first to examine whether prenatal vitamin A may influence the risk of T2DM in adulthood.
In the present study, proper randomisation was mimicked by separating individuals from exposure groups by points in time with minimum selection bias. In this way, individuals were unselected and generally representative of the Danish population, in relation to both exposure status and development of T2DM. For this reason, we believe that confounding from other T2DM risk factors was equally distributed among individuals in the two exposure groups. Furthermore, we are unaware of any relevant periodicity factors that could have explained our findings. However, residual confounding cannot be excluded, and in the following sections several considered confounders are discussed.
Vitamin A and D supplementation in pregnancy
A report from the National Board of Health suggests that pregnant women in Denmark were recommended to take cod liver oil from the 1940s and cod liver oil or vitamins A, B, C and D during winter from the 1960s( Reference Rosenstand 33 ). Therefore, recommendations of vitamin A and D supplementation for pregnant women were similar between adjacent exposed and unexposed birth cohorts.
Bright sunshine hours as a source of vitamin D
Because of the potential interaction effect of vitamin A and D, differences in sunshine hours between exposure groups could potentially influence our results. However, analyses of data from the Danish Meteorological Institute indicated that there were virtually no differences in bright sunshine hours from 1959 to 1964 (data not shown). Furthermore, birth dates were largely equally distributed across seasons in the two groups.
Trends in type 2 diabetes mellitus incidence
We expected that the increase in T2DM incidence over time, mainly related to improvement in its diagnosis over years, will be reflected in the birth cohort effect (or secular trends) for T2DM incidence in our study population. Such a situation would have complicated the interpretation of the analyses, making it difficult to disentangle secular birth cohort effect from the effect of the vitamin A dose change, as it would attenuate the estimates in our study. However, no apparent or significant secular trends in birth cohort effects were observed (data not shown).
Age at type 2 diabetes mellitus onset
In Denmark, T2DM is mainly a mid- or late-adulthood disease. The stronger preventive effect of vitamin A fortification on T2DM risk at an older age therefore strengthens our results as an inverse trend would normally be expected.
Trends in birth weight
Secular trends showing an increase in birth weight, which is related to T2DM risk, between 1959 and 1988 in Copenhagen have previously been reported. Unfortunately, we did not have information about individual birth weight in the present study. However, an increase in birth weight over the years would most likely have attenuated our estimates.
To our knowledge, it is still unclear whether the intra-uterine level of vitamin A is associated with birth weight as studies report contradictive findings( Reference Gazala, Sarov and Hershkovitz 34 – Reference Christian, Klemm and Shamim 36 ). Birth weight, on the other hand, has been shown to be inversely related to T2DM risk( Reference Whincup, Kaye and Owen 37 ). Therefore, birth weight would be a mediator if it lies on the pathway between fetal vitamin A levels and T2DM and a confounder if it is both associated with fetal vitamin A levels and T2DM.
Other factors in adjacent birth cohorts
It is well-known that T2DM is largely under-diagnosed and that only about half of the true cases can be found in the Danish registries( Reference Carstensen, Kristensen and Marcussen 20 ). We also recognise that the use of ICD codes may not be fully medically accurate for diagnosing T2DM. Finally, it is also well-known that there is a clinical overlap between the phenotypes of T1DM and T2DM, leading to higher probability of misclassification of T2DM, particularly in the younger age groups. Therefore, the number of T2DM cases in the present study might have been underestimated. In addition, there were gradual changes in the use of ICD codes over time with a decrease in the use of ICD 8 code 250 and an increase in the use of ICD 10 code E11. However, the potential misclassification this may have caused is expected to have occurred at random in the two exposure groups because of the adjacent birth cohorts they came from. Random misclassification would be expected to have attenuated our results, and the fact that we still find significant differences speak in favour of true differences.
Conclusion
Our results indicate that exposure to vitamin A early in life may have beneficial health effects related to the risk of T2DM development many decades later in life, and that small extra amounts of vitamin A from food fortification consumed by pregnant women may be sufficient to reduce the risk of T2DM in their offspring.
Even though vitamin A intake or fortification recommendations cannot be provided on the basis of our results, these results may have public health relevance, especially in vitamin A-deficient populations, as they demonstrate that one of twenty-first century’s most costly chronic diseases may be prevented by increased vitamin A intake through food fortification, which is a simple and affordable public health nutrition intervention.
Acknowledgements
The authors thank Birgitte Marie Skogstad for proofreading the manuscript.
The study is a part of the four-year project ‘D-tect’ funded by the Programme Commission on Health, Food and Welfare under the Danish Council for Strategic research (grant no. 0603-00453B). Financial support has further been granted by the Danish Diabetes Academy supported by the Novo Nordisk Foundation and The Lundbeck Foundation (grant no. R170-2014-643). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
B. L. H. designed the study. A. K., R. J., A. V. and B. L. H. formulated the research question. A. K. and L. A. analysed the data. A. K. wrote the manuscript. All authors read and provided written feedback on the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451700037X







