Fe deficiency is a common nutritional problem for piglets raised indoors during the growing period. Fe deficiency reduces the rate of Hb formation and erythrocyte count, and furthermore, it results in pale mucous membranes, enlarged heart, skin oedema, listlessness and spastic breathing (thumps) in suckling piglets( Reference Victor and Mary 1 ). Confinement-reared suckling piglets are given access to Fe from two main sources: sows’ milk and exogenous Fe supplementation( Reference Perri, Friendship and Harding 2 ). Sows’ milk could not satisfy the daily requirements of Fe for the sucking piglets. It is also a routine practice to administer an intramuscular injection of an organic Fe complex such as iron dextran within the 1st week of life for neonatal piglets( Reference Loh, Leong and Too 3 ).
Fe absorption mainly occurs in the duodenum, where Fe is taken up by enterocytes via the divalent metal transporter 1 (DMT1) pathway in mammals( Reference Martini, Tchack and Wood 4 , Reference Foot, Dalton and Shearwin-Whyatt 5 ). Once inside the enterocytes, Fe can be stored as ferritin and exported by ferroportin (FPN)( Reference Donovan, Lima and Pinkus 6 , Reference Theurl, Aigner and Theurl 7 ) into the systemic circulation via the basolateral membrane. Systemic Fe homoeostasis is mediated by hepcidin, which is exclusively synthesised by the liver and targets the DMT1( Reference Brasse-Lagnel, Karim and Letteron 8 ) and FPN( Reference Qiao, Sugianto and Fung 9 ) of enterocytes to reduce intestinal Fe transport. The intestinal Fe transport could be influenced by several dietary factors including amino acids, vitamins, plant components and so on( Reference Zijp, Korver and Tijburg 10 – Reference Xin, Xugang and Xie 12 ).
It has been noted that dietary protein affects Fe absorption and metabolism in a dose-dependent manner( Reference Klavins, Kinney and Kaufman 13 – Reference Thomas, Gaffney-Stomberg and Sun 15 ). Previous research demonstrates that a diet composed of 15–18 % protein is necessary for adequate Fe absorption in rats( Reference Thomas, Gaffney-Stomberg and Sun 15 , Reference Kone, Kinney and Kaufman 16 ). The protein-free or low-protein (LP) diet significantly decreases Fe absorption in rats( Reference Klavins, Kinney and Kaufman 13 , Reference Thomas, Gaffney-Stomberg and Sun 15 ). Klavins et al. ( Reference Klavins, Kinney and Kaufman 13 ) reported that Fe absorption was impaired in rats fed a protein-free diet compared with those provided a diet containing 10 % casein. A 5 or 10 % protein diet given over 4 weeks leads to lower liver Fe concentrations in rats compared with rats fed 18 % dietary protein, whereas there is no difference in hepatic Fe storage in rats provided diets containing 15, 18 or 25 % protein( Reference Klavins, Kinney and Kaufman 13 ). Besides, the colostrum, milk and serum Fe levels do not differ when sows are fed diets containing 12 or 16 % protein during 1–21 d postpartum( Reference Spruill, Hays and Cromwell 14 ).
Numerous studies indicate that maternal LP diet during gestation and/or lactation leads to altered carbohydrate metabolism, glucose metabolism or metabolic disorders in offspring( Reference Menendez-Castro, Fahlbusch and Cordasic 17 , Reference Metges, Görs and Lang 18 ). In addition, maternal LP diet (5 % protein) could suppress the Fe absorption in rats at weaning compared with those of female rats fed a high-protein diet (18 % protein) throughout pregnancy and lactation( Reference Enwonwu, Monsen and Jacobson 19 ). Fe is an essential trace element and is important for cell functions at biological, chemical and molecular levels( Reference Perri, Friendship and Harding 2 ). To date, a limited number of studies have focused on offspring Fe metabolism exposed to maternal protein restriction. Therefore, the present study, using a pig model, was aimed, first, to assess the influence of maternal protein restriction on offspring Fe metabolism at birth and weaning and, second, to analyse the possible mechanisms underlying such an effect.
Methods
Animals and samples
The animal experiment was carried out in the Shanghai Haifeng farm in Yancheng City, Jiangsu Province, People’s Republic of China. At 1 week after artificial insemination, twenty-four second-parity cross-bred sows (Landrace×Yorkshire) were randomly allocated to standard-protein (SP) and LP groups. All sows were housed in individual gestation stalls (2·1×0·60×0·97 m) with half-slatted concrete floors, individual feeders and drinkers made of stainless steel. Sows were transferred to the farrowing pens (2·1×3·0 m, stainless steel stall and plastic floor) 1 week before the predicted farrowing date. The warm-air blower, wetted-pad and air-exhaust fan were used to maintain room temperature (17–25°C) and humidity (70–80 %) during gestation and lactation. Sows in the SP group were fed diets containing 15 and 18 % crude protein, whereas those in the LP group were fed diets containing 7·5 and 9 % crude protein during gestation and lactation, respectively (Table 1). During the early, middle and late gestation periods, sows were fed three times (at 05.00, 10.00 and 17.00 hours)/d and given a ration of 1·2, 1·8 and 2·4 kg/d, respectively. Sows were fed the lactation diet twice daily (05.00 and 17.00 hours) at 4·2 kg/d during lactation. Sows were provided ad libitum access to tap water (containing Fe at 2·5 mg/l) throughout pregnancy and lactation. Newborn piglets were allowed free access to their mothers until weaning. Piglets were given 100 mg of supplementary Fe (iron dextran) by intramuscular injection at 3 d of age.
Table 1 Composition and nutrient content of the experimental diets
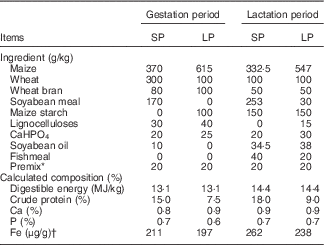
SP, standard-protein; LP, low-protein.
* All additives added in the complete diet (per 100 kg): retinol, 0·66 g cholecalciferol, 17·5 mg vitamin K3, 0·8 g; vitamin B1, 0·8 g; vitamin B2, 3280 mg; vitamin B6, 1·30 g; vitamin B12, 8·8 mg; lysine, 144 g; niacin, 9·0 g; pantothenic acid, 5·0 g; d-pantothenic acid, 4·0 g; folic acid, 10·4 g; biotin, 60 mg; d-biotin, 32 mg; choline chloride, 60 g; vitamin C, 40 g; Mn (as MnSO4.H2O), 1·6 g; Zn (as ZnSO4.7H2O), 14 g; ferrous iron (as FeSO4.7H2O), 14 g; Cu (as CuSO4.5H2O), 4·0 g; Se (as NaSe2O3), 40 mg; sodium chloride, 6·0 g; β-xylanase, 16000 kIU.
† Actual measured level.
Piglets were individually weighed immediately after parturition and at weaning on day 28 without fasting. Maternal blood samples were collected from the jugular vein at day 10 after parturition. One male and one female piglet per litter were euthanised for sampling at birth and weaning, respectively. Blood samples were centrifuged at 4°C at 1000 g for 10 min, and serum was subsequently collected and stored at –20°C until analysis. The duodenal mucosa and liver samples were collected within 20 min postmortem, snap-frozen in liquid N2 and stored at −80°C until further analysis. The experimental protocol was subject to approval by the Animal Ethics Committee of Nanjing Agricultural University, with the project no. 2012CB124703. Slaughter and sampling procedures complied with the ‘Guidelines on Ethical Treatment of Experimental Animals’ (2006) no. 398 set by the Ministry of Science and Technology, China.
Determination of serum, liver and tap water iron concentrations
Serum Fe level was measured using the 7020 Automatic Biochemical Analyzer (Hitachi High-Technologies Corp.) with reagents provided by Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Serum ferritin concentration was determined with a commercial RIA kit (Beijing North Institute of Biological Technology). Serum transferrin (TF) level was measured with an ELISA kit according to the manufacturer’s instruction (Abcam). Serum concentrations and liver levels of hepcidin were determined using a hepcidin ELISA kit (Nanjing Jiancheng Bioengineering Institute) based on a competitive binding method. Tap water and liver samples were digested by Microwave Digestion System (EHD36 electrothermal hotblock digester; LabTech) according to the procedures described previously( Reference Ma, Gu and Lu 20 ). Fe concentrations in tap water and liver were measured by graphite furnace atomic absorption spectroscopy using a Graphite Atomic Absorption Spectrometer (Z-2000; Hitachi High-Technologies Corp.).
Real-time PCR for mRNA quantification
Total RNA was isolated from duodenal mucosa and liver samples using TRIzol reagent (Invitrogen). RNA samples (2 μg) were treated with DNase and reverse transcribed to complementary DNA (cDNA) using random hexamer primers (Promega). A quantity of 2 μl of diluted cDNA (1:25) was utilised in real-time PCR performed in Mx3000P (Stratagene). Peptidylprolyl isomerase A (PPIA) was chosen as a reference gene to normalise the mRNA abundance of target genes, because its mRNA abundance was not affected by maternal dietary treatment. Real-time PCR data were analysed by using the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
21
). The mRNA levels were expressed as the fold change relative to the mean value of the SP group. Primers for real-time PCR were synthesised by Generay Biotech Co., Ltd. and are presented in Table 2.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
21
). The mRNA levels were expressed as the fold change relative to the mean value of the SP group. Primers for real-time PCR were synthesised by Generay Biotech Co., Ltd. and are presented in Table 2.
Table 2 Nucleotide sequences of specific primers

Dcytb, duodenal cytochrome b; DMT1, divalent metal transporter 1; Ferritin H, ferritin heavy chain; Ferritin L, ferritin light chain; FPN, ferroportin; HP, hephaestin; TF, transferrin; TFR1, TF receptor 1; TFR2,TF receptor 2; PPIA, peptidylprolyl isomerase A.
Protein extraction and Western blot analysis
Total protein was extracted from frozen duodenal mucosa and liver using lysis buffer (150 mm NaCl, 10 mm TRIS-HCl, 5 mm EDTA, 1 % Triton X-100 and 0·1 % SDS). The protease inhibitor cocktail (Roche Applied Science) was added according to the manufacturer’s instruction. The protein concentration was measured by a Pierce BCA Protein Assay Kit (Thermo Scientific). Western blot analysis of DMT1 (M10; Abnova), FPN (SC49668; Santa Cruz Biotechnology), ferritin heavy chain (Ferritin H) (ab81444; Abcam), TF (HPA001527; Sigma), Transferrin receptor 1 (TFR1) (10084-2-AP; Proteintech Group Inc.) and β-actin (ab8227; Abcam) was carried out according to the protocols provided by the primary antibody suppliers. The band density of target proteins was normalised with that of β-actin, and the content of target proteins is presented as fold change relative to the SP group.
Statistical analysis
Results are reported as mean values with their standard errors. All data were analysed using independent-samples t test with SPSS 20.0 to examine differences between the two groups. Individual sows or piglets served as the experimental unit. A value of P<0·05 was regarded as statistically significant.
Results
Effect of maternal low-protein diet on growth performance of piglets
Maternal LP diet did not affect the birth weight, but significantly inhibited the early postnatal growth of offspring piglets (Table 3). Piglets in the LP group had lower body weight (P=0·03) at weaning with decreased average daily gain (ADG, P=0·01) as compared with their counterparts in the SP group. When male and female piglets were separately analysed, the body weight did not differ markedly at birth and weaning regardless of sex, whereas female piglets in the LP group exhibited drastically decreased (P=0·03) ADG.
Table 3 Effect of maternal low-protein (LP) diet on growth performance of piglets (Mean values with their standard errors)
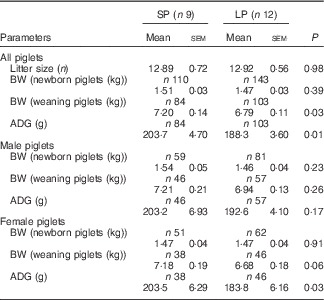
SP, standard protein diet; BW, body weight; ADG, average daily gain.
Effect of maternal low-protein diet on serum iron levels in sows and piglets
No meaningful differences were observed in serum Fe levels of sows or newborn piglets between the SP and LP groups (Table 4). However, serum Fe concentration was significantly lower in weaning piglets (P=0·01), especially for males (P=0·03) of the LP group. No significant (P=0·14) reduction in serum Fe level was observed in protein-restricted female weaning piglets.
Table 4 Effect of maternal low-protein (LP) diet on serum iron levels in sows and piglets (Mean values with their standard errors)
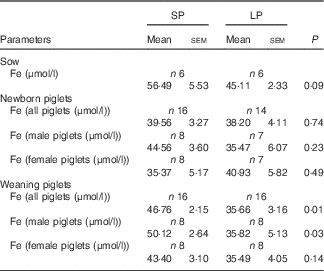
SP, standard protein diet.
Effect of maternal low-protein diet on serum iron metabolism-related parameters in male weaning piglets
Male piglets born to LP sows had a higher serum level of TF (P=0·01) compared with those born to SP sows (Table 5). Maternal LP diet caused a numerical decline of serum ferritin concentrations (P=0·08) in male weaned piglets. Maternal LP diet did not affect serum hepcidin level (P=0·74) in male piglets at weaning.
Table 5 Effect of maternal low-protein (LP) diet on serum iron metabolism-related parameters in male weaning piglets (Mean values with their standard errors)
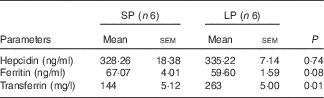
SP, standard protein diet.
Effect of maternal low-protein diet on expression of iron metabolism-related genes and proteins in the duodenum of male weaning piglets
Six genes involved in intestinal Fe absorption, storage and transport, including duodenal cytochrome b (Dcytb), DMT1, Ferritin H, Ferritin L, FPN and haphaestin, were quantified at the mRNA level in the duodenum of male weaning piglets and none of the genes exhibited a significant difference between the groups (Fig. 1(a)). However, the protein content of DMT1 and FPN was significantly down-regulated (P<0·05) in the duodenum of male weaning piglets in the LP group (Fig. 1(b)).
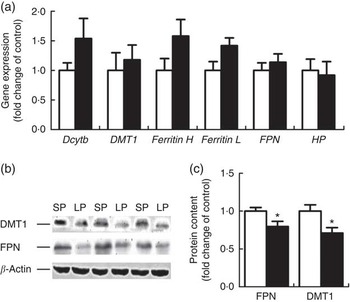
Fig. 1 Effect of maternal low-protein (LP) diet on iron metabolism-related gene (a) and protein (b, c) expression in duodenum of male weaning piglets. Values are means, with their standard errors represented by vertical bars. ![]() , Standard protein (SP);
, Standard protein (SP); ![]() , LP; Dcytb, duodenal cytochrome b; DMT1, divalent metal transporter 1; Ferritin H, ferritin heavy chain; Ferritin L, ferritin light chain; FPN, ferroportin; HP, hephaestin. * P<0·05, compared with control (n 6).
, LP; Dcytb, duodenal cytochrome b; DMT1, divalent metal transporter 1; Ferritin H, ferritin heavy chain; Ferritin L, ferritin light chain; FPN, ferroportin; HP, hephaestin. * P<0·05, compared with control (n 6).
Effect of maternal low-protein diet on iron content, hepcidin levels, iron metabolism-related gene and protein expression in the liver of male weaning piglets
Hepatic Fe content (Fig. 2(a)) did not differ between the LP and SP groups. Liver hepcidin levels tended to be higher (P=0·09) (Fig. 2(b)) in the LP piglets. TF and TFR1 were significantly down-regulated (P<0·01 or 0·05) at the mRNA levels within the liver of male weaning piglets of the LP group (Fig. 2(c)); yet, no alterations were detected for hepatic ferritin, TFR1 and TF protein expression (Fig. 2(d)).
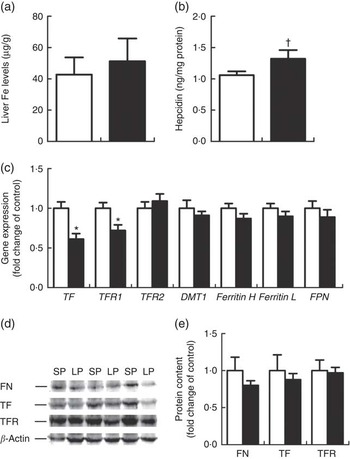
Fig. 2 Effect of maternal low-protein (LP) diet on iron content (a), hepcidin levels (b), iron metabolism-related gene (c) and protein (d, e) expression in liver of male weaning piglets. Values are means, with their standard errors represented by vertical bars. ![]() , Standard protein (SP);
, Standard protein (SP); ![]() , LP; TF, transferrin; TFR, TF receptor; DMT1, divalent metal transporter 1; Ferritin H, ferritin heavy chain; Ferritin L, ferritin light chain; FPN, ferroportin; FN, ferritin. † P=0·09, * P<0·05, compared with control (n 6).
, LP; TF, transferrin; TFR, TF receptor; DMT1, divalent metal transporter 1; Ferritin H, ferritin heavy chain; Ferritin L, ferritin light chain; FPN, ferroportin; FN, ferritin. † P=0·09, * P<0·05, compared with control (n 6).
Discussion
Maternal malnutrition during gestation and/or lactation is suggested to induce a programming of offspring growth and development in later life( Reference Eriksson 22 , Reference Fernandez-Twinn and Ozanne 23 ). Some data indicate that offspring born to dams fed a restricted protein diet throughout gestation were thinner at birth in pigs( Reference Davis, Fiorotto and Burrin 24 – Reference Shang, Jia and Sun 27 ) or rodents( Reference Zambrano, Bautista and Deás 28 – Reference Han, Li and Li 30 ). In the current study, feeding a diet containing LP (7·5 %) to gilts throughout gestation did not affect the birth weight of piglets, different from what was reported in previous studies. Reduced offspring birth weight caused by maternal protein restriction was presumably associated with the protein concentrations in maternal diets( Reference Davis, Fiorotto and Burrin 24 – Reference Shang, Jia and Sun 27 ) and feeding patterns (feed restriction or free feeding)( Reference Zambrano, Bautista and Deás 28 – Reference Han, Li and Li 30 ). Maternal protein restriction (0·5 %) throughout pregnancy resulted in lower birth weight among piglets compared with normal protein (13 %) level( Reference Davis, Fiorotto and Burrin 24 , Reference Pond, Maurer and Mersmann 25 ). Offspring birth weight was greatly reduced when gilts were fed a certain amount of LP (6·5 or 6·1 v. 12·1 % protein) diet/d during pregnancy( Reference Rehfeldt, Lang and Görs 26 , Reference Shang, Jia and Sun 27 ). Offspring birth weights of dams fed ad libitum an LP diet (8 or 10 % protein) were markedly lighter compared with those of progeny of mice fed a 20 % protein diet( Reference Zambrano, Bautista and Deás 28 – Reference Han, Li and Li 30 ). Maternal LP diet during gestation and lactation leads to lower body weight and ADG in weaning piglets, especially in female piglets. This study confirms former reports employing rodent models( Reference Han, Li and Li 30 – Reference Peixoto-Silva, Frantz and Mandarim-de-Lacerda 32 ). It is reported that maternal LP diet dramatically altered milk composition, resulting in a severe reduction in the concentrations of essential amino acids, non-essential amino acids, total fatty acids, and lipid content in the milk( Reference Peixoto-Silva, Frantz and Mandarim-de-Lacerda 32 ). The lowered milk quality induced by the maternal LP diet could be responsible for postnatal offspring growth( Reference Peixoto-Silva, Frantz and Mandarim-de-Lacerda 32 ).
Dietary protein restriction during pregnancy or/and lactation induces a reduction in serum Fe concentrations in dams( Reference Thomas, Gaffney-Stomberg and Sun 15 ) and in the offspring of rats( Reference Enwonwu, Monsen and Jacobson 19 ). In the current study, lowered serum Fe level and enhanced TF concentration indicate that maternal protein restriction affects the Fe metabolism in male weaned piglets( Reference Wang, Knovich and Coffman 33 , Reference Macedo and de Sousa 34 ). Serum TF levels increase with Fe reduction and reduce during Fe overload; thus, an enhanced serum TF level is used as a marker of Fe reduction( Reference Gkouvatsos, Papanikolaou and Pantopoulos 35 ). It is evident that certain amino acids including lysine, methionine, cystine, histidine, serine, proline, glutamine and so on( Reference Kone, Kinney and Kaufman 16 , Reference Martin Agnoux, Antignac and Boquien 36 ) are used as enhancers for Fe absorption in the intestine. A restricted protein diet during lactation decreases the essential amino acids (e.g. histidine, proline, lysine, methionine, valine and so on) and non-essential amino acids (e.g. glutamine, glycine, serine, tyrosine and so on) in the milk of sows( Reference Van Campen 37 ). It is suggested that maternal protein restriction might disturb Fe transport via reduced Fe-enhancer concentrations of amino acids.
In addition, our results showed that maternal protein restriction throughout gestation and lactation reduced DMT1 and FPN protein expression but not DMT1 and FPN mRNA abundance in the duodenum of male weaned piglets. Fe is taken up mainly in the duodenum of pigs( Reference Blachier, Vaugelade and Robert 38 , Reference Lipinski, Starzyński and Canonne-Hergaux 39 ). DMT1, encoded by the SLC11A2 gene, is in charge of the transport of Fe2+ and other divalent metals from the lumen into the duodenal cells( Reference Mims and Prchal 40 , Reference Jiang, Garrick and Garrick 41 ). FPN, encoded by the SLC40A1 gene, is the only known Fe exporter in mammalian cells( Reference Donovan, Lima and Pinkus 6 , Reference Donovan, Brownlie and Zhou 42 ). The reduced protein expression of Fe influx transporter DMT1 and efflux transporter FPN inhibits intestinal Fe transport and decreases Fe levels in the circulation( Reference Griffiths, Sly and Cox 43 – Reference Ward and Kaplan 45 ). Unchanged transcript expressions and decreased protein expression of DMT1 and FPN suggest that DMT1 and FPN are regulated at the post-transcriptional level or translational level( Reference Galy, Ferring-Appel and Becker 46 , Reference Meister 47 ). In all, twenty-two microRNA targeting DMT1 or FPN were examined, but there were no noteworthy differences between the two groups (data not provided). Hepcidin, encoded by the HAMP gene, is a key regulator of Fe entry into the circulation by binding and down-regulating the DMT1 of enterocytes( Reference Brasse-Lagnel, Karim and Letteron 8 , Reference Mena, Esparza and Tapia 48 ) and FPN of the enterocytes, macrophages and hepatocytes in mammals( Reference Qiao, Sugianto and Fung 9 , Reference Nemeth, Tuttle and Powelson 49 ). However, there is no difference in serum hepcidin levels between the two groups. The mechanism of down-regulation of DMT1 and FPN in the duodenum of male weaning piglets needs further study.
Maternal protein restriction enhanced hepatic hepcidin levels, decreased liver TF and TFR mRNA levels, but did not affect the liver Fe concentrations, ferritin, TF and TFR protein abundance in male weaned piglets. TF is an Fe-binding blood plasma glycoprotein that delivers Fe to tissue cells via a receptor-mediated endocytotic process or internalisation( Reference Macedo and de Sousa 34 , Reference Leverence, Mason and Kaltashov 50 ). Almost all Fe in the circulation is transported by plasma TF( Reference Ponka, Beaumont and Richardson 51 ). Plasma TF loaded with Fe3+ encounters and binds to its receptors on the cell surface and being endocytosed in the liver. Furthermore, it is reported that intramuscular administration of iron dextran induces hepatic Fe accumulation in piglets( Reference Lipinski, Starzyński and Canonne-Hergaux 39 , Reference Starzyński, Laarakkers and Tjalsma 52 ). Hepatic Fe concentrations persist at 60–100 mg/kg at day 20 and 25–40 mg/kg at day 30 when piglets are injected with 100 mg of iron dextran at days 1–3( Reference Ness, Engle and Thompson 53 ). Rising levels of serum TF and injection of iron dextran could play a role in unchanged liver Fe deposition in male weaning piglets of the LP group.
In conclusion, our results indicate that the maternal protein restriction during gestation and lactation decreases female offspring growth performance at weaning. Maternal protein restriction throughout pregnancy and lactation significantly suppresses the duodenal expression of Fe transporters (DMT1 and FPN) and reduces serum Fe level in male weaning piglets.
Acknowledgements
The present study was supported by the National Basic Research Program of China (2012CB124703), the National Natural Science Foundation of China (31302053), the Fundamental Research Funds for the Central Universities (KJQN201404), the Innovation Project of Jiangsu Province Postgraduate Education (2013CXLX13_292), the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control.
R. Z. and W. M. conceived the project. J. L. performed most experiments. S. J. analysed the data. D. C. assisted the western blot assay. S. P. and Y. J. conducted the animal experiment. W. M. wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.









