Background
The tobacco epidemic remains a major global public health threat and requires multiple strategies for tobacco control that includes not only the enactment of public policies, but also the promotion of smoking cessation services at the individual level (WHO, 2003). These strategies are intended to reduce the number of present and future smokers and related morbidity and mortality (Konfino et al., Reference Konfino, Ferrante, Mejia, Coxson, Moran and Goldman2012).
Efficacy of a clinical smoking cessation intervention is evaluated 6 to 12 months after implementation by the Russell Standard criteria that were established to guide outcome assessments. These criteria require self-report of smoking status and biochemical verification of abstinence among others (West, Hajek, Stad & Stapleton, Reference West, Hajek, Stad and Stapleton2005). However, obtaining biochemical verification of abstinence is challenging in community studies and especially costly in low- and middle-income countries.
In public health surveys, self-reported status is widely used to report on population smoking prevalence (Wong, Leatherdale, Malaison & Hammond, Reference Wong, Shields, Leatherdale, Malaison and Hammond2012; Yeager & Krosnick Reference Yeager and Krosnick2010). Self-report smoking status has been used as the principal population metric and misclassification was reported to be only 2% in a large US sample (Caraballo et al., Reference Caraballo, Giovino, Pechacek, Mowery, Richter and Strauss1998; Yeager & Krosnick, Reference Yeager and Krosnick2010) and 8.4% in a Canadian study (Wong et al., Reference Wong, Shields, Leatherdale, Malaison and Hammond2012). Self-report smoking status has also been used successfully in internet-based surveys (Ramo, Hall & Prochaska, Reference Ramo, Hall and Prochaska2011), among pregnant women (Kvalvik et al., Reference Kvalvik, Nilsen, Skjaerven, Vollset, Midttun and Ueland2012), and patients with chronic medical conditions (Ismail, Gill, Lawton, Houghton & MacFarlane, Reference Ismail, Gill, Lawton, Houghton and MacFarlane2000; Wilson, Elborn, Fitzsimons & McCrum-Gardner, Reference Wilson, Elborn, Fitzsimons and McCrum-Gardner2011).
The use of self-report smoking status alone has been questioned as a definitive cessation outcome in a systematic review (Connor Gorber, Schofield-Hurwitz, Hardt, Levasseur & Tremblay, Reference Connor Gorber, Schofield-Hurwitz, Hardt, Levasseur and Tremblay2009), among patients with medical conditions (Hilberink et al., Reference Hilberink, Jacobs, van Opstal, van der Weijden, Keegstra and Kempers2011; Pell et al., Reference Pell, Haw, Cobbe, Newby, Pell and Oldroyd2008), and among participants from low- and middle-income countries (Fakhfakh, Klouz, Lakhal, Belkahia & Achour, Reference Fakhfakh, Klouz, Lakhal, Belkahia and Achour2011). There is widespread belief that participants will often misreport their smoking status so as not to disappoint the clinician or researcher evaluators (Perez-Stable, Marin, Marin, Brody & Benowitz, Reference Perez-Stable, Marin, Marin, Brody and Benowitz1990).
The use of proxies to compare smoking status to self-report in population surveys have been described outside of Latin America (Barnett, O’Loughlin, Paradis & Renaud, Reference Barnett, O’Loughlin, Paradis and Renaud1997; Chen, Rennie & Dosman, Reference Chen, Rennie and Dosman1995; Gilpin et al., Reference Gilpin, Pierce, Cavin, Berry, Evans and Johnson1994; Kolonel, Hirohata & Nomura, Reference Kolonel, Hirohata and Nomura1977), and these varied from less than 1% to 13.6% among student/father pairs in a school-based program. One study from Hong Kong reported that spousal proxy report of smoking status was valid and reliable, but in that study, proxy reports were not collected following a cessation intervention (Mak, Loke, Lam & Abdullah, Reference Mak, Loke, Lam and Abdullah2005). With the aim of providing information about the use of proxies in Latin America, we sought to compare self-reported cessation to that reported by the participant's spouse or other household member in a sample of patients who completed a smoking cessation intervention in Buenos Aires, Argentina.
Methods
Setting
This study was based in the smoking cessation clinic at a university hospital primary care program and in selected internal medicine private practices located in Buenos Aires, Argentina. Participants were evaluated 12 months after they had completed the cessation intervention.
Participants and Recruitment
Potential participants for this study were selected from (i) 520 smokers who attended a smoking cessation clinic and (ii) 1,378 smoking patients seen by private-practice physicians who had participated in an educational program to help their patients quit from March 2009 to July 2011. Patients treated at the smoking cessation clinic were referred by their physicians and consented for follow-up telephone calls. The patients recruited from private practices were recruited from lists of patients seen by the physicians participating in the study, were called to ascertain smoking status and confirmed smokers were randomly selected and invited to participate in the study by responding to the surveys. Eligibility criteria for the current investigation included reporting continuous abstinence non-smoking status at 12 months after the cessation intervention and having a proxy (spouse or other household member) who could be phoned to answer questions about the participant's quitting process and smoking status. All proxies of participants recruited from the tobacco cessation clinic and a 10% random sample of proxies for the private practice patients were selected for telephone interviews.
Interventions
The intervention at the clinic consisted of 8 to 12 weeks of individual treatment based on a cognitive behavioural approach, options for available pharmacotherapy, and support by clinicians. The patients recruited from private practices were seen by physicians who took part in a study aimed to test the effectiveness of an educational program to teach them cessation counselling techniques, referrals to services, and use of pharmacotherapy (University of California San Francisco, 2004).
Procedures
All patients received a telephone call 12 months after the date on which they had completed the cessation intervention or visited their physician. Continuous smoking abstinence was ascertained and only self-reported non-smokers were included in the current study. The spouses or persons who lived with the participant from the tobacco cessation clinic and a 10% random sample of patients from the private practices, who reported having quit smoking, were interviewed by telephone. Contacted proxies were asked about the smoking status and quitting process of their partner/spouse. The study protocols were approved by an NIH (National Institutes of Health) approved Institutional Review Board Centro de Investigación Clinica y Educacion Medica (CEMIC).
Data Analysis
We compared the responses of the proxy respondents (mostly spouses) to the responses of the study participants. Data were analyzed using SAS and descriptive statistics reported means and standard deviations. Tobacco use was dichotomized as smoker or non-smoker with continuous abstinence. We reported response percentages of participants and their proxies.
Results
At 12 months, 1,423 of 1,898 participants were contacted (75% of those eligible) to assess their smoking status and 346 reported being non-smokers. Of those 346 participants, 172 were called again to confirm their smoking status and 161 reported being non-smokers. We asked to interview a proxy for each of these 161 non-smokers and 140 were interviewed. We were unable to reach the other 21. The 140 self-reported non-smoking participants averaged 51 years of age, 69% were women, and 49% had 12 years or more of education (see Table 1). The mean number of cigarettes per day at baseline survey was 20.1 (SD = 9.9) and the average number of quit attempts in the previous year was 2.4 (SD = 1.2). Reported cessation methods included physician advice or behavioural intervention only (21%), bupropion (56%), nicotine replacement therapy (20%), and varenicline (3%).
Table 1 Characteristics of 140 smokers reporting abstinence 12 months after cessation intervention, 2009–2011, Buenos Aires, Argentina
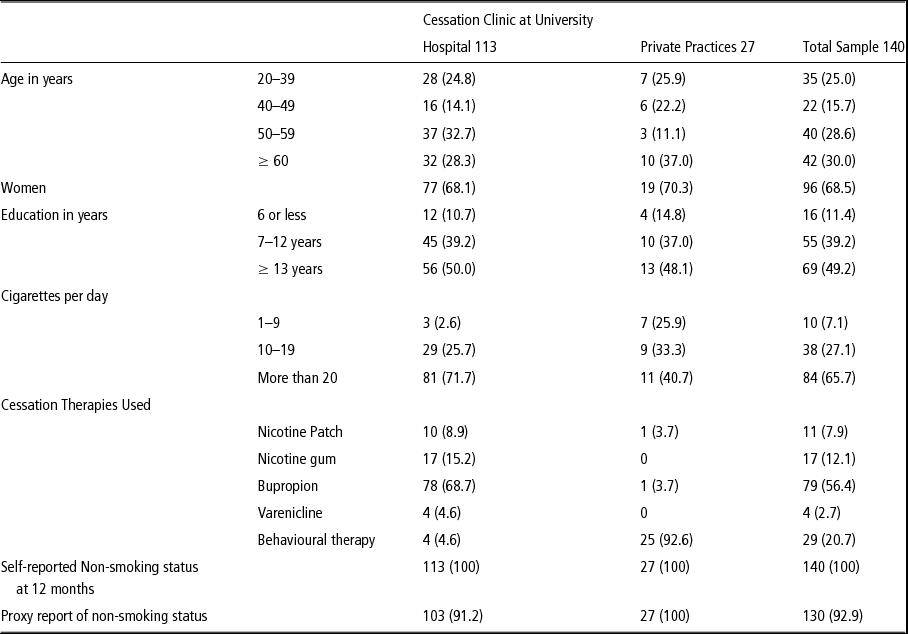
At 12 months, these 140 participants reported that they remained continuously abstinent of smoking but of the 140 proxies interviewed, 10 (7.1%) reported that their partner/household member was a current smoker. For all 10 discordant responses between participant and proxy, the participant was from the cessation program in the university hospital. These 10 patients averaged age of 61 years and smoked an average of 21 cigarettes per day at baseline; four of them had completed 7 years of education, six had 12 or more years of education, and six were women.
Discussion
Results from this report imply that proxy respondents report on smoking status could be used to validate self-reported results following a smoking cessation intervention in low- and middle-income countries in place of more costly biochemical validation. Although only 7% of proxies reported discordant smoking status from that of study participants, the added effort of an additional follow-up with a proxy can provide additional methodological strength to a study.
Use of biochemical validation has been considered the gold standard for the past 30 years in smoking cessation studies because it lends a methodological rigour in ascertainment of outcomes. However, the operational challenge of collection and cost of testing samples inhibit formal evaluations of cessation programs. The strategy of asking the spouse or proxy, when available, offers a viable alternative to validate cessation status and may serve as a practical substitute for the more expensive biochemical validation that requires obtaining a biological sample. In our study, participants were selected after they reported having quit smoking in two previous telephone calls, so the strength of the strategy of asking proxies is that it is useful for identifying study participants who avoid saying they continue to smoke.
Our data were collected in the setting of a large urban centre and the results are only applicable to persons with a spouse who is willing to be contacted and respond to the study interviewer. However, one could possibly extend these findings by prospectively identifying a person who will know a participant's smoking status and contact that person as the proxy. Furthermore, most of the participants were heavier smokers reporting more than 20 cigarettes per day prior to the intervention. It is interesting that all discrepant proxy reports were on patients in the more intensive cessation program.
One limitation of this study is that the conclusions are based exclusively on the participants and proxy's self-reported answers without biochemical verification. However, the use of proxies has been used in public health studies and those results suggest that proxies can be used in smoking cessation studies. (Barnett et al., Reference Barnett, O’Loughlin, Paradis and Renaud1997; Gilpin et al., Reference Gilpin, Pierce, Cavin, Berry, Evans and Johnson1994; Kolonel et al., Reference Kolonel, Hirohata and Nomura1977; Mak et al., Reference Mak, Loke, Lam and Abdullah2005). The utility of proxies for validation of their household members smoking status rests on the assumption that proxies provide more truthful responses. (Chen et al., Reference Chen, Rennie and Dosman1995; McLaughlin, Dietz, Mehl & Blot, Reference McLaughlin, Dietz, Mehl and Blot1987). We were also unable to locate 21 proxies and if all had reported that the study participant was smoking, the rate of discordant results would be 19% (31 of 161).
In conclusion, our study suggests that proxy-reported data on smoking status could be used to confirm self-reported results in smoking cessation trials in low- and middle-income countries in place of biochemical verification but more research is needed, especially a study design that includes self-report, proxy-report, and biochemical validation.
Acknowledgement
We thank the medical and administrative directors of the respective clinical institutions that facilitated access to the participating physicians; we also thank Cecilia Populus-Eudave for administrative and research support and Jennifer Livaudais-Toman, PhD for statistical analyses at UCSF.
Financial Support
This research was funded by grant No.TW05935 from the Tobacco Research Network Program, Fogarty International Center, National Cancer Institute, National Institute of Drug Abuse, and National Institutes of Health and by grant No. 001726-037 from Research on International Tobacco Control, International Development Research Center, Canada.
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.



