INTRODUCTION
Influenza has long been recognized as a major cause of excess morbidity in the community, excess health-care utilization and excess deaths [Reference Serfling, Sherman and Houseworth1–Reference Fleming, Harcourt and Smith5]. The magnitude of the excess varies season by season according to the virulence of the strain type and the extent to which antigenic drift has taken place. Periodically, more radical changes in virus strain type occur and these give rise to pandemics most of which cause severe illness [Reference Simonsen6]. In the United Kingdom, influenza epidemics occur in most winters and usually last 8–10 weeks [Reference Fleming7].
The overall impact of respiratory syncytial virus (RSV) infection is well known in relation to its impact on young children both on morbidity and mortality, but the impact in adults is much less clear [Reference Mufson8–Reference Fleming, Pannell and Cross15]. Over the last 20 years an increasing number of publications have drawn attention to its impact on morbidity among the elderly, especially the frail elderly in institutional care [Reference Crowcroft, Cutts and Zambon12, Reference Agius16–Reference Falsey19]. It has been recognized as a major cause of excess winter mortality among the elderly [Reference Fleming and Cross10, Reference Nicholson11, Reference Thompson14]. More recently, RSV has been recognized as a cause of influenza-like illness (ILI) in persons of all ages [Reference Zambon13].
Estimation of the differential impact of influenza and RSV presents problems because both viruses so often co-circulate in mid-winter periods. These difficulties have been increased by the custom of many doctors to investigate young children with acute respiratory conditions for RSV, and older people for influenza. It has not been common practice to investigate patients routinely for both viruses. Recent developments in virological investigation procedures involving much more widespread use of polymerase chain reaction (PCR) technology have resulted in more extensive investigation of adults with respiratory illness [Reference Zambon13, Reference Stockton20, Reference Aymard21]. Many of the European primary care-based surveillance networks for monitoring influenza also report on the incidence of RSV infection. However, although these networks investigate for both viruses, they do so in patients presenting with what are generally described as ILI [Reference Meerhoff22]. Whilst not all networks collect data on the basis of strict diagnostic criteria, there is a general consensus of investigating patients with illnesses which include acute onset of respiratory symptoms such as cough accompanied by fever.
This study investigates the respiratory diagnostic profiles reported in routine medical practice during influenza and RSV active periods. It extends work previously reported in young children to now include adults and a broader spectrum of respiratory diagnoses [Reference Fleming23]. It is particularly relevant to the prevention and management of epidemics of respiratory infection in winter which are widely different for the two viruses and to the economic analysis of the value of interventions for these conditions.
METHODS
Virological data
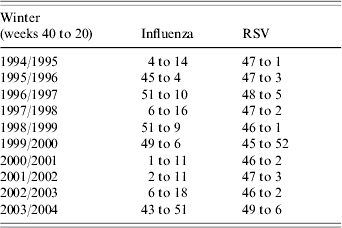
Virological data were used to define virus active periods; laboratory reports of influenza and RSV, submitted to the Health Protection Agency over the winters of 1994/1995 to 2003/2004, were examined. These laboratory reports (based predominantly upon tests using culture and molecular methods) were obtained from samples submitted from both hospital laboratories and primary-care sources in England and Wales. For the purpose of this study, virus active periods were defined as the weeks surrounding the peak week of the respective virus laboratory reports which encompassed a minimum of 70% of the total number of reports submitted to the Health Protection Agency during that winter (weeks 40 to 20; Table 1). Winter weeks outside this period were described as weeks of baseline virus activity.
Table 1. Influenza and respiratory syncytial virus (RSV) active periods by week number
Clinical incidence data
The clinical incidence data used for this study were obtained from the Weekly Returns Service (WRS) of the Royal College of General Practitioners collected over the ten winters 1994/1995 to 2003/2004. This surveillance information system collects clinical diagnostic data from a population of approximately 650 000 monitored by around 400 doctors on a twice-weekly basis [Reference Fleming24]. Doctors record each clinical diagnosis in Read code which is assembled into the disease categories and rubrics of the International Classification of Disease Version 9 (ICD-9) for analysis. New episodes of illness are distinguished from ongoing consultations. New episodes of recurring or chronic conditions such as asthma are deemed to occur when exacerbations occur or when the condition is out of control. The data are obtained by gender in eight age groups (<1, 1–4, 5–14, 15–24, 25–44, 45–64, 65–74, ⩾75 years). Diagnostic data were examined for common cold (ICD-9 460, 465), otitis media (ICD-9 381.0, 382.0, 382.9), ILI (ICD-9 487), acute bronchitis/bronchiolitis (ICD-9 466, 490), and for upper respiratory tract infection (URTI; ICD-9 381.0, 382.0, 382.9, 460–463, 465) and lower respiratory tract infection (LRTI; ICD-9 464, 466, 480–487, 490, 511). Incident episodes were counted in each group of Read codes. Multiple new episodes within a single Read code grouping were treated as a single entry. New episodes in differing Read code groupings were counted separately (multiple entries of new episodes for similar conditions are infrequent).
Analysis
Weekly incidence rates were averaged in each winter season (week 40 of one year to week 20 of the next) for each disease and each age group, in four periods determined according to virus activity. These periods were classified as: influenza active weeks; RSV active weeks; weeks when there was either influenza or RSV activity (combined); and those weeks outside the virus active period (baseline).
The average weekly incidence rate in each virus active period (influenza, RSV, combined influenza/RSV) over the average weekly incidence rate of the period of baseline virus activity was calculated and multiplied by the number of weeks of respective virus activity to estimate the cumulative winter excess rate. As periods of activity for these viruses overlap, the sum of the individual estimates exceeded the combined estimate. The estimates finally attributed to each virus were calculated from the combined estimate by allocation in the proportion of the individual virus estimates. Thus, if the combined estimate was C and the estimates for individual viruses influenza and RSV were I and R, the apportioned estimates were C×I/[I+R] and C×R/[I+R] for influenza and RSV active weeks respectively. All calculations were made separately for each condition, each winter and in each age group.
For each morbid condition and age group the resulting annual cumulative excess incidence rates for influenza and RSV were then treated as two paired samples, the 10 paired differences being the observations of main interest. Their analysis was undertaken by application of the non-parametric method for paired differences as described by Campbell & Gardner [Reference Campbell, Gardner, Gardner and Altman25]. The difference between two population medians, referable to influenza and RSV, was estimated by calculating all the 55 (10×11/2) possible averages of two of these differences taken together (including each difference with itself) and then selecting their median, i.e. the 28th ranked observation. The confidence interval for the difference between the two population medians for a given morbid condition and age group was also derived using these average differences. The approximate 95% confidence intervals (95% CIs) around the sample median were obtained using tabulated values based on the Wilcoxon matched-pairs signed-rank sum distribution: for two paired samples of size 10, the 95% CIs are given by the 9th and 47th ranked average values [Reference Campbell, Gardner, Gardner and Altman25].
Results
Weekly incidence rates of LRTI were contrasted with laboratory reports for influenza and RSV over the 10 years 1994/1995 to 2003/2004; results for age groups 45–64 and 65–74 years are presented (Fig. 1). Periods of RSV activity were consistent throughout the study period, starting at approximately week 40, peaking at week 52 and ending at approximately week 14. Periods of influenza activity were less consistent than RSV, with periods of early (1995/1996) and late (1998/1999) activity both recorded. The peak activity of influenza and RSV occurred simultaneously in some winters (1995/1996), whilst in others, there was clear distinction between the peaks of virus activity (1997/1998). Rates of LRTI were consistently higher in the 65–74 years age group than in the 45–64 years age group. There were well-defined peaks of LRTI each winter, although in some years (1997/1998 and 2000/2001) these were less pronounced. In some winters, the peak of LRTI in both age groups was associated with the peak of RSV (1996/1997 and 2003/2004) and influenza (1999/2000) virus reports.

Fig. 1. The incidence rate of lower respiratory tract infections in the 45–64 years (–––) and 65–74 years (·······) age groups contrasted with laboratory reports of RSV (

The estimates of cumulative excess rates of URTI and LRTI in three illustrative age groups (1–4, 25–44 and 65–74 years) in each winter during influenza and RSV active periods are given in Figure 2 (note different scale on vertical axis for 1–4 years). There was considerable inter-year variation of cumulative excesses, although in the 1–4 years age group there was some suggestion of a reducing trend for both URTI and LRTI. As age increased, the relative balance between URTI and LRTI changed, from a greater excess for URTI than LRTI in children for both viruses, to a relatively equal distribution in the 25–44 years group, to the reverse in the 65–74 years age group.

Fig. 2. Cumulative excess incidence of upper respiratory tract infection (URTI) and lower respiratory tract infection (LRTI) in influenza (■) and RSV (□) active periods for persons aged (a) 1–4 years, (b) 25–44 years and (c) 65–74 years.
We next examined the major components of LRTI (acute bronchitis and ILI) and URTI (common cold and otitis media). Excess incidence rates of ILI occurred in both influenza and RSV active periods in the same three age groups (also in those age groups not presented in Fig. 3) in each winter although the cumulative excesses were generally higher in the influenza active periods (Fig. 3; note different scale on vertical axis for 1–4 years). There was again some suggestion of a reducing trend for both ILI and acute bronchitis over the 10-year period in the 1–4 and 25–44 years age groups. There was a greater excess of acute bronchitis compared to ILI in the 1–4 years age group where the excess of acute bronchitis occurred predominately in the RSV active periods. In the 65–74 years age group there was a greater excess of acute bronchitis compared to ILI and in the 2001/2002, 2002/2003 and 2003/2004 winter seasons an overall predominance of acute bronchitis in the RSV active periods.

Fig. 3. Cumulative excess incidence of influenza-like illness (ILI) and acute bronchitis (AB) in influenza (■) and RSV (□) active periods for persons aged (a) 1–4 years, (b) 25–44 years and (c) 65–74 years.
In Figure 4 we summarize the median winter estimates over the 10 years of the cumulative excess episode incidence rates of selected respiratory syndrome diagnoses in the virus active periods for each age group (note the scale difference between Fig. 4a and Fig. 4b). To determine the significance of differences between excesses in virus active periods, sample median differences (with 95% CIs) were calculated between either RSV and influenza (RSV-FLU) or influenza and RSV (FLU-RSV) population medians (Table 2). In children aged <1 year (Fig. 4a) the estimates of population excesses in RSV active periods were significantly higher than those in the influenza active periods for acute bronchitis [RSV-FLU (2702, 95% CI 929–4867)]; there was no significant excess for otitis media [RSV-FLU (−119, 95% CI −272 to 260)] in this age group; as regards ILI, there was a small estimated population excess during the influenza compared with RSV active periods [FLU-RSV (119, 95% CI 18–218)]. Excess estimates for acute bronchitis and common cold in children aged <1 year far exceeded those estimates in children aged 1–4 years. Even so, for the latter age group the estimated population median difference between RSV and influenza active periods was highly significant for acute bronchitis [RSV-FLU (994, 95% CI 338–1747)]; for ILI there was a significant estimated excess during the influenza active period [FLU-RSV (216, 95% CI 115–475)].

Fig. 4. Median winter cumulative excess incidence (1994/1995 to 2003/2004) of acute bronchitis (AB), influenza-like illness (ILI), otitis media (OM) and common cold (CC) during influenza (■) and RSV (□) active periods, in (a) children aged <1, 1–4 and 5–14 years and (b) adults aged 15–24, 25–44, 45–64, 65–74 and ⩾75 years.
Table 2. Differences (95% confidence intervals) between estimated population medians of cumulative excess incidence (per 100 000) of acute bronchitis, influenza-like illness, otitis media and common cold, during influenza (FLU) and RSV active periods during winters 1994/1995 to 2003/2004
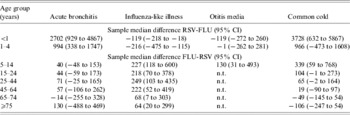
n.t., Not tested; excess rates of otitis media were low in all age groups ⩾15 years therefore sample median differences were not calculated.
Estimates of excesses for otitis media in the <1 and 1–4 years age groups were similar in both virus active periods; for children aged 5–14 years the estimate for the FLU active period exceeded that for RSV [FLU-RSV (130, 95% CI 31–493)].
In the age groups ⩾15 years (Fig. 4b and Table 2) estimated population excesses were higher in influenza than RSV active periods for ILI; for acute bronchitis there were no differences between the estimates which were significant at the 95% level; and for the common cold there were excess population estimates for the influenza over RSV active periods for the 15–24 and 25–44 years groups, and thereafter no differences between the two active periods.
In summary, consideration of the material from the perspective of the syndrome diagnoses shows that: (i) the estimated population median rates of ILI were greater during the influenza active periods than the RSV active periods in all age groups; (ii) the excess of acute bronchitis was significantly higher in the RSV active periods in the two youngest age groups, but for the other age groups there were no differences between the estimated median rates for the active periods which were significant at this level; (iii) an estimated excess median rate of common cold was higher in RSV active periods for children aged <1 year, but between the ages of 5 and 44 years estimated excess rates were higher in influenza active periods (of borderline significance for the 15–24 and 25–44 years age groups), otherwise there were no differences between estimates for the two active periods; (iv) for otitis media in children aged <5 years there was little difference between estimated medians for the two active periods, although in the 5–14 years age group the estimated median was greater during influenza periods. It is worthy of note that we performed similar analyses for excess rates of asthma in all ages (data not shown); these excesses were small in comparison to those for the respiratory diagnoses here considered and were confined to RSV active periods.
In order to validate the sensitivity of the results presented above, we repeated the analysis limiting the estimation of excess to those winters in which periods of influenza and RSV activity were separable, or at the minimum did not overlap by more than 3 weeks (winters 1995/1996 and 1996/1997 were excluded). The conclusions drawn from the interpretation of the original results presented above were very similar to those derived from this restricted analysis. The general point about the magnitude of illness due to RSV was reinforced.
Discussion
Over the past few decades, the improved surveillance of influenza in the community has led to a good understanding of the epidemiology of influenza viruses and the disease they cause. There are still, however, very limited data pertaining to the clinical burden of disease caused by RSV, especially in the adult population. This study demonstrates that in patients presenting to doctors in primary care, the clinical burden of RSV infection is likely to be as great, if not greater, than influenza and is apparent in clinical data for several respiratory conditions and in all age groups.
The methods applied in this study rely upon the reporting of the clinical incidence of respiratory conditions in general practice, and the capture of laboratory reports for influenza and RSV to the Health Protection Agency. In the WRS, doctors are required to report every consultation with its episode type but are given flexibility in their choice of the most appropriate diagnostic syndrome. Although there is inter-doctor variation in the way diagnostic terms are used, there is consistency in the way that individual doctors use them over time. The requirement to assign a morbidity code to every consultation ensures that no omissions occur.
The excess morbidity diagnosed as acute bronchitis was greater in RSV active periods as opposed to influenza active periods in the <1, 1–4 and ⩾75 years age groups, and a near equal burden in other age groups. Whilst there has been a strong emphasis on the burden of illness due to influenza, it should be borne in mind that acute bronchitis is diagnosed by general practitioners more frequently than influenza even when influenza viruses are known to be circulating. In the millennium winter (1999/2000), the United Kingdom experienced a serious hospital bed crisis and at that time the rates of acute bronchitis reported in the elderly in the WRS exceeded any reported in the previous 15 years and were much higher than the rates of ILI. We have previously drawn attention to the relatively high rates of asthma and acute bronchitis in children in mid-December each year when RSV is particularly prevalent [Reference Fleming23, Reference Sunderland and Fleming26].
Many previous studies have documented RSV infection in the young and old, especially the frail elderly, and the problems that the virus causes in terms of serious disease [Reference Mufson8–Reference Falsey19]. Our data suggest that RSV is associated with a greater excess of respiratory disease than influenza in other age groups as well. We have not measured the severity of the infections but the similarity in relative distribution of the estimated excesses across such a wide range of diagnoses would imply little difference between the two viruses. Nicholson and colleagues estimated 60–80% more mortality associated with RSV than influenza each winter [Reference Nicholson11]. Although this estimate exceeds the difference in the morbidity estimates which we report, both studies emphasize the importance of adequate surveillance of both viruses. The shift from URTI to LRTI in the 1–4 and 65–74 years age groups respectively is interesting and represents the large contribution made primarily by the common cold in children aged 1–4 years and by acute bronchitis in the elderly. These differences stress the importance of age-specific data when considering the impact of respiratory infections.
Our data suggest that excesses of both LRTI and acute bronchitis in the 65–74 years age group shifted from occurring predominantly in influenza active periods (1994/1995 to 2000/2001) to occurring in RSV active periods (2001/2002 to 2003/2004, Figs 2 and 3). The weekly incidence of ILI (as reported in the WRS in England and Wales) has been falling over the last 30 years, and rates in the winter seasons 2001/2002, 2002/2003, and 2003/2004 reached levels that were very close to the baseline threshold for winter activity [Reference Elliot and Fleming27]. Therefore, the apparent shift of acute bronchitis from influenza to RSV active periods may simply be a reflection of the relative change in burden of disease caused by influenza and RSV due to the reduced circulation and probably reduced virulence of influenza viruses in the community. The most serious influenza epidemics in recent winters have been caused by influenza A H3N2 variants which may have a waning impact because of residual immunity in persons as a result of repeated infections with antigenically similar viruses.
The findings of this study carry two important messages:
(1) RSV is a major respiratory pathogen in all age groups. The evidence against RSV in middle-aged adults as well as in children and the elderly mounts [Reference Zambon13, Reference Hall, Long and Schnabel28]. Hall and colleagues have recently shown that in healthy adults, RSV infection can result in absenteeism from work in 38% of cases infected with the virus and an average duration of illness 3 days greater than with influenza infection [Reference Hall, Long and Schnabel28]. Further studies of the epidemiology of this virus infection in adults are needed. In order to design these effectively we need to determine the most appropriate method of investigation, obtain much better information on the pattern of virus shedding and its relationship to symptoms.
We strongly suspect that RSV infection is an even greater cause of excess morbidity and mortality in underdeveloped countries than in the western world and is one of the conditions that account for the big differences in mortality which we need to address [Reference Bustamante-Calvillo29–Reference Stensballe, Devasundaram and Simoes32]. Our findings challenge the WHO recommendations of Epidemic and Pandemic Alert and Response (EPR) diseases for surveillance, which currently excludes RSV [33]. Although there is not yet an effective vaccine, it is also important to prioritize problems in which vaccine development is well advanced.
(2) The effectiveness of influenza vaccination requires further assessment. The second message concerns the significance of these findings for the assessment of the effectiveness of influenza vaccination. In their recent meta-analysis, Jefferson and colleagues referred to the confounding effect of non-influenza respiratory virus infections where outcomes include non-virological confirmation in individual cases [Reference Jefferson34]. Whilst in clinical trials it has been shown that acute respiratory infection with fever presenting early after illness onset during periods of influenza activity are highly likely to be true influenza infection [35–Reference Zambon37], this cannot be applied to the routine operational circumstances of diagnosing ILI (or other acute respiratory syndrome diagnoses) in primary care throughout winter. Furthermore, the similarity in timing of RSV and influenza activity is such that dual diagnosis is a potential confounder. The impact of both viruses is so variable from year to year that the results of studies based on one year are not necessarily applicable to another. In consequence, we believe the estimation of the impact of influenza needs to be conducted with concurrent and continuing evaluation of RSV and that in the assessment of both conditions, a much broader clinical diagnostic spectrum than ILI needs to be considered.
Exercises that attempt to model the economic burden of influenza often do not take sufficient account of the huge burden of disease imposed by RSV. Since the 1969 influenza pandemic, rates of ILI recorded in the community have been steadily falling [Reference Elliot and Fleming27]. Using data based on recent years therefore, the clinical and economic impact of RSV infection may be increased relative to that commonly attributed to influenza. However, a new pandemic strain of influenza may change the position for several years. Our data suggest that future modelling exercises of influenza and RSV infection need to be undertaken as a combined exercise and need to focus on the entire spectrum of acute respiratory syndrome diagnoses. Furthermore, we suspect many persons experience infection from both viruses during the course of the winter and we would like to see investigation of the effects of repeated virus respiratory infections and of dual infections [Reference Zambon13].
Acknowledgements
We gratefully acknowledge the contribution of the WRS sentinel practices and their staff in providing the general practitioner episode data. We are grateful to Mary Cooke (Health Protection Agency, Centre for Infections) for generously providing the virus datasets for influenza and RSV. The Birmingham Research Unit of the Royal College of General Practitioners is funded by the Department of Health. A.J.E. is jointly funded by the Royal College of General Practitioners and the Health Protection Agency.
DECLARATION OF INTEREST
D.M.F. has received financial support from the pharmaceutical industry in matters relating to influenza prevention and treatment.








